Improvement in Atrioventricular Conduction Using Cardioneuroablation Performed Immediately after Pulmonary Vein Isolation
Abstract
1. Introduction
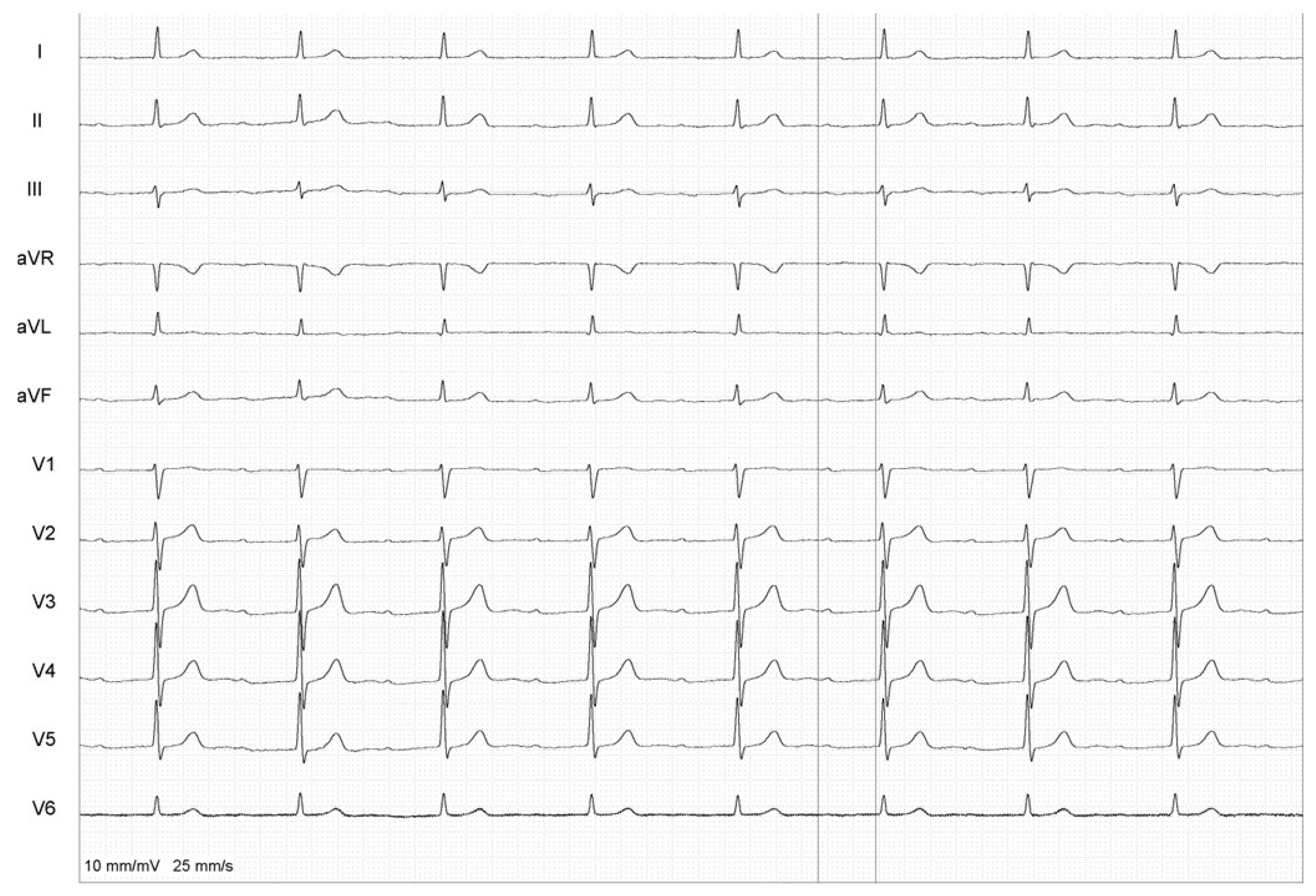
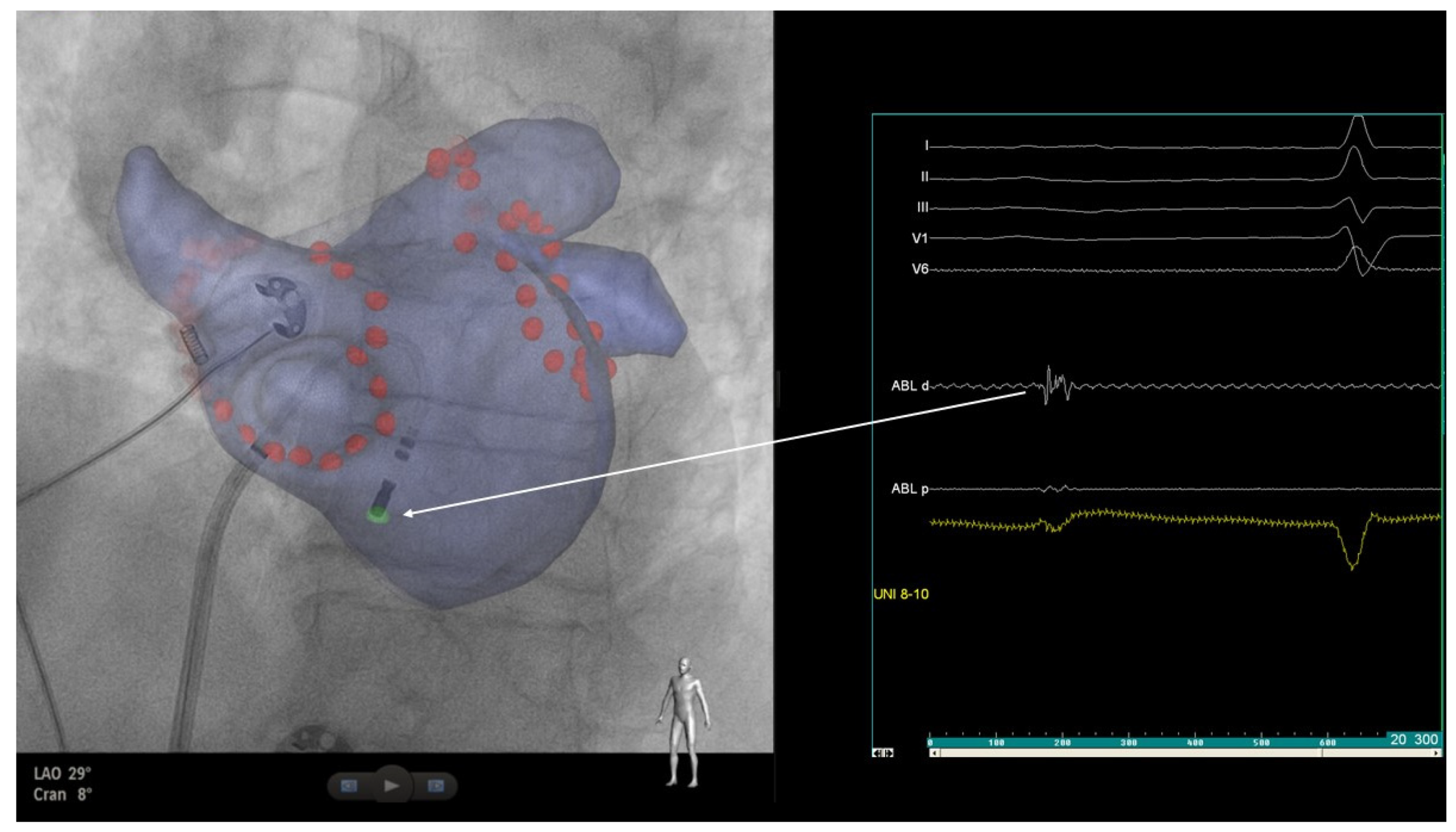
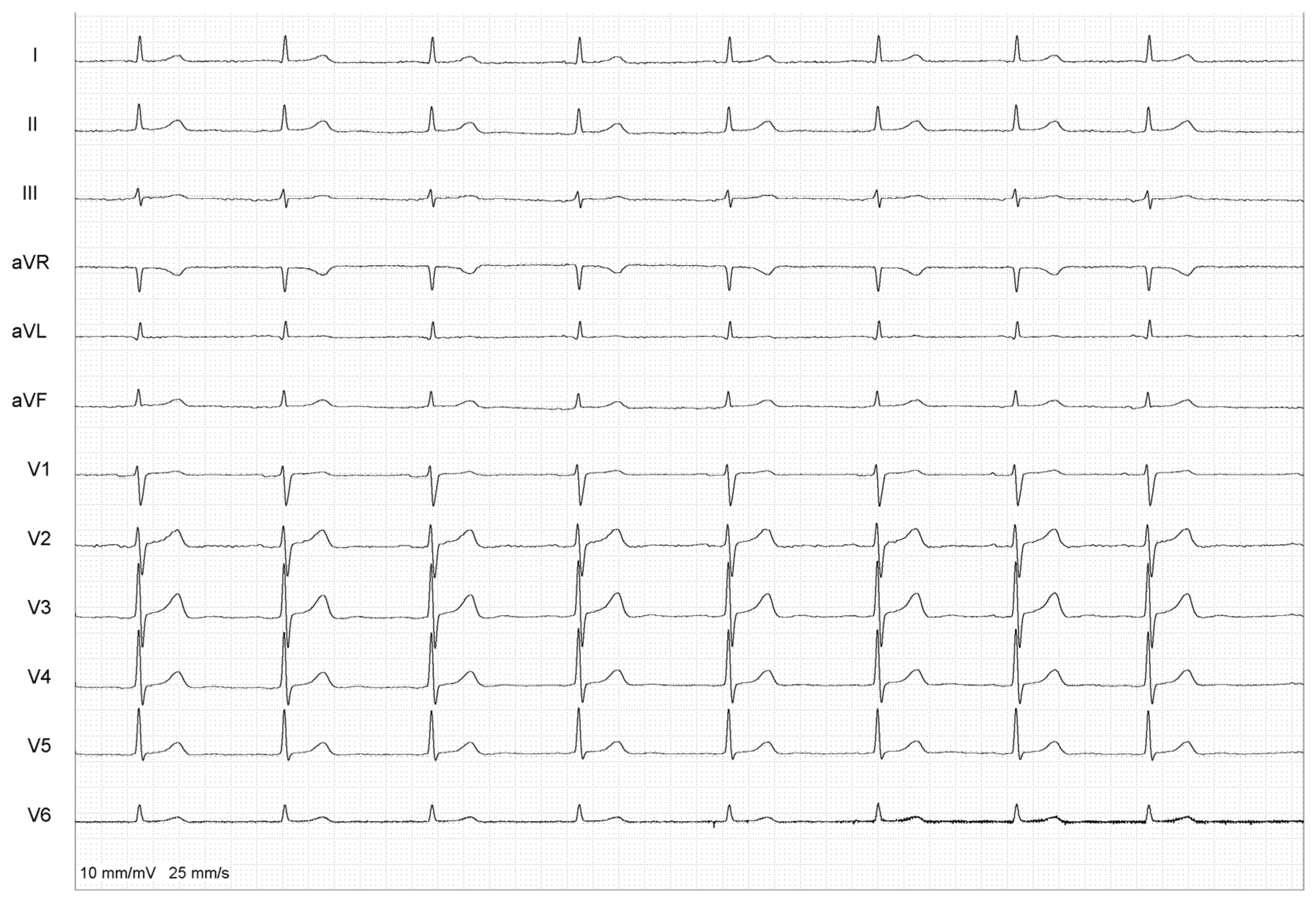
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Duytschaever, M.; Demolder, A.; Phlips, T.; Sarkozy, A.; El Haddad, M.; Taghji, P.; Knecht, S.; Tavernier, R.; Vandekerckhove, Y.; De Potter, T. PulmOnary vein isolation with vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): Results from a multicentre randomized trial. Eur. Heart J. 2018, 39, 1429–1437. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Giazitzoglou, E.; Zografos, T.; Pokushalov, E.; Po, S.S.; Camm, A.J. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm 2011, 8, 672–678. [Google Scholar] [CrossRef]
- Lemery, R.; Birnie, D.; Tang, A.S.; Green, M.; Gollob, M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006, 3, 387–396. [Google Scholar] [CrossRef]
- Pachon, J.C.; Pachon, E.I.; Pachon, J.C.; Lobo, T.J.; Pachon, M.Z.; Vargas, R.N.; Jatene, A.D. “Cardioneuroablation”—New treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 2005, 7, 1–13. [Google Scholar] [CrossRef]
- Aksu, T.; Gopinathannair, R.; Bozyel, S.; Yalin, K.; Gupta, D. Cardioneuroablation for Treatment of Atrioventricular Block. Circ. Arrhythm. Electrophysiol. 2021, 14, e010018. [Google Scholar] [CrossRef]
- Baysal, E.; Guler, T.E.; Gopinathannair, R.; Bozyel, S.; Yalin, K.; Aksu, T. Catheter Ablation of Atrioventricular Block: From Diagnosis to Selection of Proper Treatment. JACC Case Rep. 2020, 2, 1793–1801. [Google Scholar] [CrossRef]
- Brignole, M.; Aksu, T.; Calò, L.; Debruyne, P.; Deharo, J.C.; Fanciulli, A.; Fedorowski, A.; Kulakowski, P.; Morillo, C.; Moya, A.; et al. Clinical controversy: Methodology and indications of cardioneuroablation for reflex syncope. Europace 2023, 25, euad033. [Google Scholar] [CrossRef]
- Futyma, P.; Zarębski, Ł.; Wrzos, A.; Futyma, M.; Kułakowski, P. Cardioneuroablation of Right Anterior Ganglionated Plexus for Treatment of Vagally-mediated Paroxysmal Atrial Fibrillation: A Pilot Study. J. Am. Coll. Cardiol. Clin. Electrophysiol. 2024, in press. [Google Scholar]
- Kim, M.-Y.; Coyle, C.; Tomlinson, D.R.; Sikkel, M.B.; Sohaib, A.; Luther, V.; Leong, K.M.; Malcolme-Lawes, L.; Low, B.; Sandler, B.; et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm 2022, 19, 516–524. [Google Scholar] [CrossRef]
- Calò, L.; Rebecchi, M.; Sciarra, L.; De Luca, L.; Fagagnini, A.; Zuccaro, L.M.; Pitrone, P.; Dottori, S.; Porfirio, M.; de Ruvo, E.; et al. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012, 5, 22–31. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, W.; Liu, S.; Liang, E.; Du, Z.; Guo, J.; Wu, L.; Asirvatham, S.J.; Yao, Y. The Diagnostic Value of Cardiac Deceleration Capacity in Vasovagal Syncope. Circ. Arrhythm. Electrophysiol. 2020, 13, e008659. [Google Scholar] [CrossRef]
- Avazzadeh, S.; McBride, S.; O’brien, B.; Coffey, K.; Elahi, A.; O’halloran, M.; Soo, A.; Quinlan, L.R. Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation. J. Clin. Med. 2020, 9, 3081. [Google Scholar] [CrossRef]
- Tang, L.Y.W.; Hawkins, N.M.; Ho, K.; Tam, R.; Deyell, M.W.; Macle, L.; Verma, A.; Khairy, P.; Sheldon, R.; Andrade, J.G.; et al. Autonomic Alterations after Pulmonary Vein Isolation in the CIRCA-DOSE (Cryoballoon vs. Irrigated Radiofrequency Catheter Ablation) Study. J. Am. Heart Assoc. 2021, 10, e018610. [Google Scholar] [CrossRef]
- Futyma, P.; Kułakowski, P. Reinnervation after cardioneuroablation: When on the run for best intraprocedural endpoints, be aware of possible ablation overdose. HeartRhythm Case Rep. 2022, 8, 469–470. [Google Scholar] [CrossRef]
- Piotrowski, R.; Zuk, A.; Baran, J.; Sikorska, A.; Krynski, T.; Kulakowski, P. Ultrasound-guided extracardiac vagal stimulation-New approach for visualization of the vagus nerve during cardioneuroablation. Heart Rhythm 2022, 19, 1247–1252. [Google Scholar] [CrossRef]
- Aksu, T.; Guler, T.E.; Yalin, K. There are still debates on cardioneuroablation strategy despite increasing evidence. Commentary to the article: “Cardioneuroablation using an anatomical approach: A new and promising method for the treatment of cardioinhibitory neurocardiogenic syncope”. Kardiol. Pol. 2019, 77, 65–66. [Google Scholar] [CrossRef]
- Stavrakis, S.; Nakagawa, H.; Po, S.S.; Scherlag, B.J.; Lazzara, R.; Jackman, W.M. The role of the autonomic ganglia in atrial fibrillation. JACC Clin. Electrophysiol. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Quan, K.J.; Lee, J.H.; Van Hare, G.F.; Biblo, L.A.; Mackall, J.A.; Carlson, M.D. Identification and characterization of atrioventricular parasympathetic innervation in humans. J. Cardiovasc. Electrophysiol. 2002, 13, 735–739. [Google Scholar] [CrossRef]
- Lazzara, R.; Scherlag, B.J.; Robinson, M.J.; Samet, P. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ. Res. 1973, 32, 393–401. [Google Scholar] [CrossRef]
- Ardell, J.L.; Randall, W.C. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am. J. Physiol. 1986, 251, H764–H773. [Google Scholar] [CrossRef]
- Hong, M.; Hwang, I.; Yu, H.-T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Jee, S.H.; Pak, H.-N. Potential causal association of a prolonged PR interval and clinical recurrence of atrial fibrillation after catheter ablation: A Mendelian randomization analysis. J. Hum. Genet. 2020, 65, 813–821. [Google Scholar] [CrossRef]
- Nikolaidou, T.; Pellicori, P.; Zhang, J.; Kazmi, S.; Goode, K.M.; Cleland, J.G.; Clark, A.L. Prevalence, predictors, and prognostic implications of PR interval prolongation in patients with heart failure. Clin. Res. Cardiol. 2018, 107, 108–119. [Google Scholar] [CrossRef]
- Everett, T.H., IV; Olgin, J.E. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm 2007, 4 (Suppl. S3), S24–S27. [Google Scholar] [CrossRef]
- Pfeufer, A.; van Noord, C.; Marciante, K.D.; Arking, D.E.; Larson, M.G.; Smith, A.V.; Tarasov, K.V.; Müller, M.; Sotoodehnia, N.; Sinner, M.F.; et al. Genome-wide association study of PR interval. Nat. Genet. 2010, 42, 153–159. [Google Scholar] [CrossRef]
- van Setten, J.; Brody, J.A.; Jamshidi, Y.; Swenson, B.R.; Butler, A.M.; Campbell, H.; Del Greco, F.M.; Evans, D.S.; Gibson, Q.; Gudbjartsson, D.F.; et al. PR interval genome-wide association meta-analysis identifies 50 loci associated with atrial and atrioventricular electrical activity. Nat. Commun. 2018, 9, 2904. [Google Scholar] [CrossRef]
- Zhou, Q.; Hou, Y.; Yang, S. A meta-analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin. Electrophysiol. 2011, 34, 1687–1694. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Pokushalov, E.; Romanov, A.; Giazitzoglou, E.; Siontis, G.C.; Po, S.S.; Camm, A.J.; Ioannidis, J.P. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J. Am. Coll. Cardiol. 2013, 62, 2318–2325. [Google Scholar] [CrossRef]
- Pokushalov, E.; Romanov, A.; Katritsis, D.G.; Artyomenko, S.; Shirokova, N.; Karaskov, A.; Mittal, S.; Steinberg, J.S. Ganglionated plexus ablation vs. linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: A randomized comparison. Heart Rhythm 2013, 10, 1280–1286. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Nakagawa, H.; Jackman, W.M.; Yamanashi, W.S.; Patterson, E.; Po, S.; Lazzara, R. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J. Interv. Card. Electrophysiol. 2005, 13 (Suppl. S1), 37–42. [Google Scholar] [CrossRef]
- Chung, W.-H.; Masuyama, K.; Challita, R.; Hayase, J.; Mori, S.; Cha, S.; Bradfield, J.S.; Ardell, J.L.; Shivkumar, K.; Ajijola, O.A. Ischemia-induced ventricular proarrhythmia and cardiovascular autonomic dysreflexia after cardioneuroablation. Heart Rhythm 2023, 20, 1534–1545. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarębski, Ł.; Futyma, P.; Sethia, Y.; Futyma, M.; Kułakowski, P. Improvement in Atrioventricular Conduction Using Cardioneuroablation Performed Immediately after Pulmonary Vein Isolation. Healthcare 2024, 12, 728. https://doi.org/10.3390/healthcare12070728
Zarębski Ł, Futyma P, Sethia Y, Futyma M, Kułakowski P. Improvement in Atrioventricular Conduction Using Cardioneuroablation Performed Immediately after Pulmonary Vein Isolation. Healthcare. 2024; 12(7):728. https://doi.org/10.3390/healthcare12070728
Chicago/Turabian StyleZarębski, Łukasz, Piotr Futyma, Yashvi Sethia, Marian Futyma, and Piotr Kułakowski. 2024. "Improvement in Atrioventricular Conduction Using Cardioneuroablation Performed Immediately after Pulmonary Vein Isolation" Healthcare 12, no. 7: 728. https://doi.org/10.3390/healthcare12070728
APA StyleZarębski, Ł., Futyma, P., Sethia, Y., Futyma, M., & Kułakowski, P. (2024). Improvement in Atrioventricular Conduction Using Cardioneuroablation Performed Immediately after Pulmonary Vein Isolation. Healthcare, 12(7), 728. https://doi.org/10.3390/healthcare12070728





