Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction
2.4. Assessment of Methodological Quality
2.5. Grouping of Omega-3 and Control Participants
2.6. Primary Outcome: (Improvement in Depression in Patients with Dementia)
2.7. Subgroup Outcomes
2.8. Publication Bias
3. Results
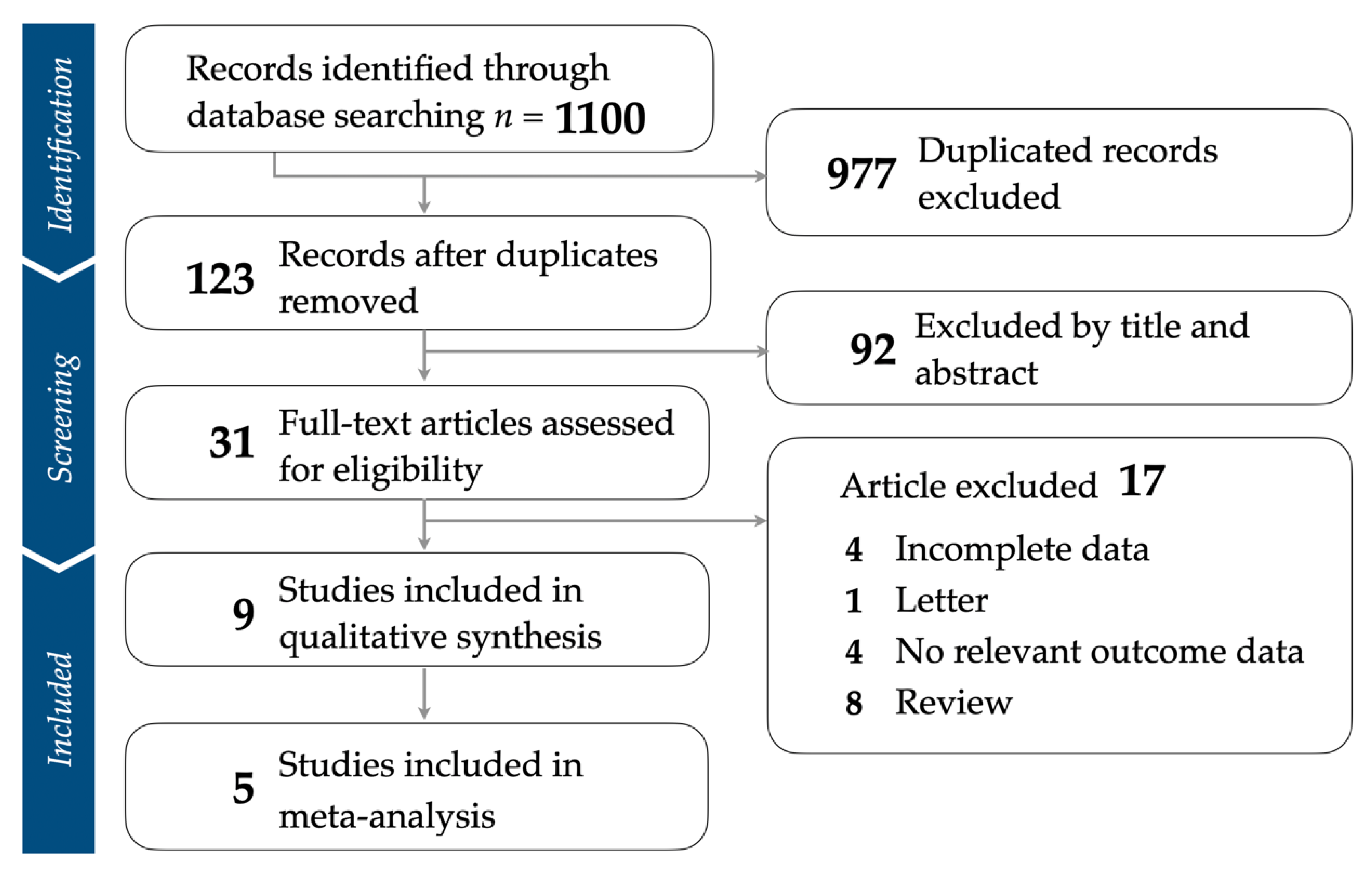
3.1. Identification of Eligible Studies
3.2. Study Characteristics and Patient Population
3.3. Quality Assessment of Included Articles
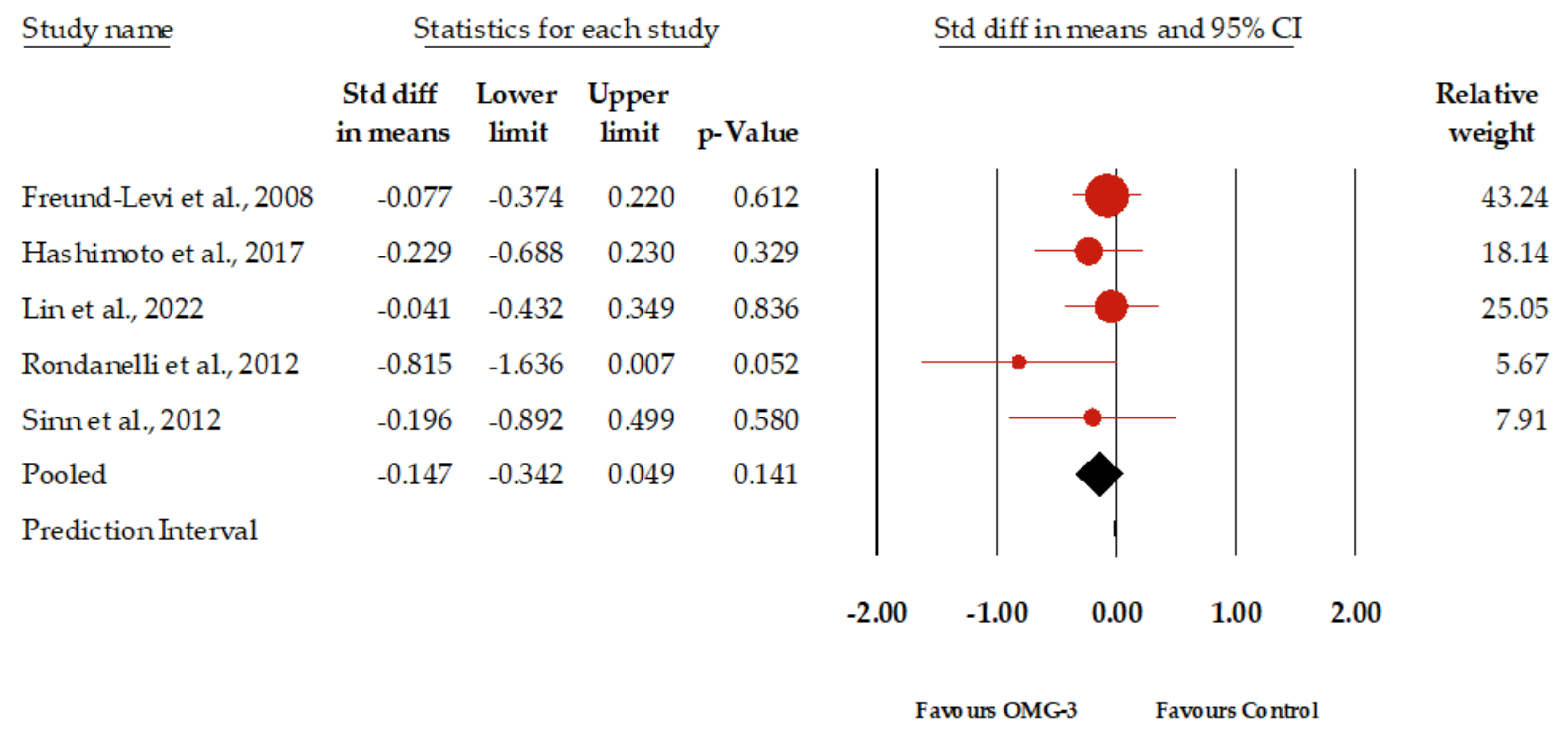
3.4. Primary Outcome: Effect of Omega-3 Fatty Acids for Depression in Elderly Patients with Dementia
3.5. Subgroup Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendricksen, M.; Thomas, A.J.; Ferrier, I.N.; Ince, P.; O’Brien, J.T. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am. J. Psychiatry 2004, 161, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D.; Halperin, I. Depression and dementia. J. Neurol. Sci. 2009, 283, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, A.; Bourke, J.; Rivera-Hernández, R.; Tsolaki, M.; Woźniak, J.; Kaźmierski, J. Depression in dementia or dementia in depression? Systematic review of studies and hypotheses. Curr. Alzheimer Res. 2020, 17, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; Cummings, J.L. Dementia: A Clinical Approach; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- World Health Organization. Global Status Report on the Public Health Response to Dementia; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Smith, K.; De Torres, I. A world of depression. Nature 2014, 515, 10–1038. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.D. Depression in the elderly. N. Engl. J. Med. 2014, 371, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Blazer, D.G. Depression and cognition in the elderly. Annu. Rev. Clin. Psychol. 2015, 11, 331–360. [Google Scholar] [CrossRef]
- Overshott, R.; Burns, A. Treatment of dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, v53–v59. [Google Scholar] [CrossRef]
- Tisher, A.; Salardini, A. A comprehensive update on treatment of dementia. Semin. Neurol. 2019, 39, 167–178. [Google Scholar]
- Zekry, D.; Hauw, J.J.; Gold, G. Mixed dementia: Epidemiology, diagnosis, and treatment. J. Am. Geriatr. Soc. 2002, 50, 1431–1438. [Google Scholar] [CrossRef]
- Quigley, L.; Dozois, D.J.; Bagby, R.M.; Lobo, D.S.; Ravindran, L.; Quilty, L.C. Cognitive change in cognitive-behavioural therapy v. pharmacotherapy for adult depression: A longitudinal mediation analysis. Psychol. Med. 2019, 49, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef]
- Pampallona, S.; Bollini, P.; Tibaldi, G.; Kupelnick, B.; Munizza, C. Combined pharmacotherapy and psychological treatment for depression: A systematic review. Arch. Gen. Psychiatry 2004, 61, 714–719. [Google Scholar] [CrossRef]
- Johansson, R.; Andersson, G. Internet-based psychological treatments for depression. Expert. Rev. Neurother. 2012, 12, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Michalaak, E.E.; Swinson, R.P. Assessment Scales in Depression and Anxiety-CORPORATE: (Servier Edn); CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Mehdi, S.; Manohar, K.; Shariff, A.; Kinattingal, N.; Wani, S.U.D.; Alshehri, S.; Imam, M.T.; Shakeel, F.; Krishna, K.L. Omega-3 Fatty Acids Supplementation in the Treatment of Depression: An Observational Study. J. Pers. Med. 2023, 13, 224. [Google Scholar] [CrossRef]
- Guu, T.-W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R. International society for nutritional psychiatry research practice guidelines for omega-3 fatty acids in the treatment of major depressive disorder. Psychother. Psychosom. 2019, 88, 263–273. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Cheng, C.; Satyanarayanan, S.K.; Chiu, L.-T.; Chien, Y.-C.; Chuu, C.-P.; Lan, T.-H.; Su, K.-P. Omega-3 fatty acids and blood-based biomarkers in Alzheimer’s disease and mild cognitive impairment: A randomized placebo-controlled trial. Brain Behav. Immun. 2022, 99, 289–298. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190, Erratum in Transl. Psychiatry 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, K. The role of polyunsaturated fatty acids in neuronal signaling in depression and cognitive processes. Arch. Biochem. Biophys. 2023, 737, 109555. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, M.-Q.; Xue, Y.; Yang, R.; Tang, M.-M. Dietary of n-3 polyunsaturated fatty acids influence neurotransmitter systems of rats exposed to unpredictable chronic mild stress. Behav. Brain Res. 2019, 376, 112172. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016, 4, Cd009002. [Google Scholar] [CrossRef]
- Wei, B.Z.; Li, L.; Dong, C.W.; Tan, C.C.; Xu, W. The Relationship of Omega-3 Fatty Acids with Dementia and Cognitive Decline: Evidence from Prospective Cohort Studies of Supplementation, Dietary Intake, and Blood Markers. Am. J. Clin. Nutr. 2023, 117, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Basun, H.; Cederholm, T.; Faxén-Irving, G.; Garlind, A.; Grut, M.; Vedin, I.; Palmblad, J.; Wahlund, L.O.; Eriksdotter-Jönhagen, M. Omega-3 supplementation in mild to moderate Alzheimer’s disease: Effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry 2008, 23, 161–169. [Google Scholar] [CrossRef]
- Freund-Levi, Y.; Vedin, I.; Hjorth, E.; Basun, H.; Faxén Irving, G.; Schultzberg, M.; Eriksdotter, M.; Palmblad, J.; Vessby, B.; Wahlund, L.O.; et al. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer’s disease: The OmegAD study. J. Alzheimers Dis. 2014, 42, 823–831. [Google Scholar] [CrossRef]
- Giudici, K.V.; de Souto Barreto, P.; Beard, J.; Cantet, C.; Araujo de Carvalho, I.; Rolland, Y.; Vellas, B. Effect of long-term omega-3 supplementation and a lifestyle multidomain intervention on intrinsic capacity among community-dwelling older adults: Secondary analysis of a randomized, placebo-controlled trial (MAPT study). Maturitas 2020, 141, 39–45. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kato, S.; Tanabe, Y.; Katakura, M.; Mamun, A.A.; Ohno, M.; Hossain, S.; Onoda, K.; Yamaguchi, S.; Shido, O. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes. Geriatr. Gerontol. Int. 2017, 17, 330–337. [Google Scholar] [CrossRef]
- Maltais, M.; de Souto Barreto, P.; Pothier, K.; Cantet, C.; Andrieu, S.; Rolland, Y.; Vellas, B. Lifestyle multidomain intervention, omega-3 supplementation, or both for reducing the risk of developing clinically relevant depressive symptoms in older adults with memory complaints? Secondary analysis from the MAPT trial. Exp. Gerontol. 2019, 120, 28–34. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Mozzoni, M.; Antoniello, N.; Cazzola, R.; Savarè, R.; Cerutti, R.; Grossi, E.; Cestaro, B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012, 15, 46–54. [Google Scholar] [CrossRef]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef]
- Van de Rest, O.; Geleijnse, J.M.; Kok, F.J.; van Staveren, W.A.; Olderikkert, M.G.; Beekman, A.T.; de Groot, L.C. Effect of fish oil supplementation on quality of life in a general population of older Dutch subjects: A randomized, double-blind, placebo-controlled trial. J. Am. Geriatr. Soc. 2009, 57, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.; Higgins, J.; Altman, D.; Thomas, J.; Chandler, J. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.4. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 1 August 2023).
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (Updated August 2022). Available online: https://training.cochrane.org/handbook/current/chapter-06 (accessed on 13 August 2021).
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.; Salanti, G. Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 285–320. [Google Scholar]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. Nutr. 2017, 57, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Cloutier, S.; Mellah, S.; Willis, S.; Vellas, B.; Andrieu, S.; Coley, N.; Ngandu, T. Is more always better? Dose effect in a multidomain intervention in older adults at risk of dementia. Alzheimers Dement. 2022, 18, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Grenyer, B.F.; Crowe, T.; Owen, A.J.; Grigonis-Deane, E.M.; Howe, P.R. Improvement of major depression is associated with increased erythrocyte DHA. Lipids 2013, 48, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Loria, G.; Salvati, S.; Di Biase, A.; Conte, G.; Villella, C.; Righino, E.; Ciciarelli, C.; Bria, P.; La Torre, G. DHA effects in Parkinson disease depression. Basal Ganglia 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Wani, A.L.; Bhat, S.A.; Ara, A. Omega-3 fatty acids and the treatment of depression: A review of scientific evidence. Integr. Med. Res. 2015, 4, 132–141. [Google Scholar] [CrossRef]
- Luo, X.D.; Feng, J.S.; Yang, Z.; Huang, Q.T.; Lin, J.D.; Yang, B.; Su, K.P.; Pan, J.Y. High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: A network meta-analysis. BMC Psychiatry 2020, 20, 248. [Google Scholar] [CrossRef]
- Trebatická, J.; Dukát, A.; Ďuračková, Z.; Muchová, J. Cardiovascular diseases, depression disorders and potential effects of omega-3 fatty acids. Physiol. Res. 2017, 66, 363. [Google Scholar] [CrossRef]
- Gu, M.; Li, X.; Yan, L.; Zhang, Y.; Yang, L.; Li, S.; Song, C. Endogenous ω-3 fatty acids in Fat-1 mice attenuated depression-like behaviors, spatial memory impairment and relevant changes induced by olfactory bulbectomy. Prostaglandins Leukot. Essent. Fat. Acids 2021, 171, 102313. [Google Scholar] [CrossRef]
- Wu, K.; Gao, X.; Shi, B.; Chen, S.; Zhou, X.; Li, Z.; Gan, Y.; Cui, L.; Kang, J.X.; Li, W.; et al. Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer’s disease. Neuroscience 2016, 333, 345–355. [Google Scholar] [CrossRef]
- Song, C.; Shieh, C.-H.; Wu, Y.-S.; Kalueff, A.; Gaikwad, S.; Su, K.-P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef]
- Charles, C.N.; Msagati, T.; Swai, H.; Chacha, M. Microalgae: An alternative natural source of bioavailable omega-3 DHA for promotion of mental health in East Africa. Sci. Afr. 2019, 6, e00187. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Samieri, C.; Cunnane, S.C. Long-chain omega3 polyunsaturated fatty acids and cognition in older people: Interaction with APOE genotype. OCL 2016, 23, D111. [Google Scholar] [CrossRef][Green Version]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA is More Effective than DHA to Improve Depression-Like Behavior, Glia Cell Dysfunction and Hippcampal Apoptosis Signaling in a Chronic Stress-Induced Rat Model of Depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T. Nutrition and Feeding of Fish; Springer: Berlin/Heidelberg, Germany, 1989; Volume 260. [Google Scholar]
- Parada, J.; Aguilera, J. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Cristina, N.M.; Lucia, D.A. Nutrition and Healthy Aging: Prevention and Treatment of Gastrointestinal Diseases. Nutrients 2021, 13, 4337. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.-A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858. [Google Scholar] [CrossRef]
- Löhr, J.M.; Panic, N.; Vujasinovic, M.; Verbeke, C. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.A.; Steinberger, J.; Sinaiko, A.R.; Moran, A.; Jacobs, D.R., Jr.; Kelly, A.S.; Dengel, D.R. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity 2015, 23, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Maraki, M.I.; Sidossis, L.S. The latest on the effect of prior exercise on postprandial lipaemia. Sports Med. 2013, 43, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Van der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E., Jr.; Marangell, L.B.; Richardson, A.J.; Lake, J.; et al. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef]
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention, and interactions with statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Authors and Year | Country | Dose and Method | Sample | Treatment Duration | Results | Outcome | Type/Genres |
|---|---|---|---|---|---|---|---|
| Freund-Levi et al., 2008 [29] | Sweden | Dose: 1.7 g DHA and 0.6 g EPA daily. Method: Randomized, double-blind, placebo-controlled. Patients received either omega-3 supplements or placebo. | Size: 204 patients enrolled, 174 completed the study. Characteristics: Patients with Alzheimer’s disease, living in their own homes, stable dose of acetylcholine esterase inhibitors. Mean age: 74 years. Gender distribution: 51% female. | 12 months (6 months randomized to omega-3 or placebo, followed by 6 months of omega-3 for all). | No significant overall treatment effects on neuropsychiatric symptoms, daily living activities, or caregiver’s burden. Some significant effects observed in specific domains (NPI agitation domain in APOEv4 carriers, MADRS scores in non-APOEv4 carriers). | MADRS | |
| Freund-Levi et al., 2014 [30] * | Sweden | Dose: 430 mg DHA and 150 mg EPA per capsule, total of four capsules daily. Additional Components: 4 mg vitamin E per capsule. Placebo: Corn oil with 0.6 g linoleic acid per capsule. Method: Randomized, double-blind, placebo-controlled. Patients received either omega-3 supplements or placebo oil. | Size: 41 patients enrolled, 37 completed the study. 17 omega-3 fatty acid (ω-3 FA) group. 20 placebo group. Characteristics: Patients diagnosed with Alzheimer’s disease according to the DSM-IV criteria, having an MMSE score between 15 and 30. Mean age: 70 years. Gender distribution: 40.5% female. | 6 months. | No significant difference in treatment effect between supplemented and non-supplemented patients. | - | |
| Giudici et al., 2020 [31] * | Netherlands | Dose: 400 mg DHA and ≤112.5 mg EPA per capsule, two capsules daily. Method: Randomized assignment into one of four groups: 1. Multidomain intervention plus omega-3 supplementation; 2. Multidomain intervention plus placebo; 3. Omega-3 supplementation only; 4. Placebo. Multidomain intervention components: Cognitive stimulation, physical activity, and nutritional counseling. | Size: 1445 participants. Characteristics: Participants must have self-reported memory loss and inability to carry out an instrumental activity of daily living. Mean age: 75.3 years. Gender distribution: 64.2% female. | 36 months. | No significant between-group differences were observed in the decrease in intrinsic capacity (IC) Z-score among all groups after 3 years when comparing each intervention group with participants taking placebo. Additionally, no long-term effects were noted in the omega-3 supplementation groups compared to placebo. | - | |
| Hashimoto et al., 2017 [32] | Japan | Dose: 860 mg DHA and 203 mg EPA daily for the intervention group; 53 mg DHA and 15 mg EPA daily for the control group. Method: Double-blind study. Participants received daily cooked meals including fish sausages, with differing DHA and EPA contents. Participants were blinded to the food products. | Size: 75 participants enrolled, 66 completed the study. Characteristics: Patients with Alzheimer’s disease, elderly individuals from care facilities and a nursing home in Shimane prefecture, Japan, including 10 men and 65 women. Mean age: 88.5 years. Gender distribution: 86.67% female. | 12 months. | Significant increases in the levels of EPA and DHA, as well as in the DHA/AA and EPA/AA ratios, were observed in the active group compared to the control group after 12 months. Furthermore, certain aspects of cognitive functions, measured by MMSE and HDS-R scores, demonstrated significant improvements, suggesting benefits against age-related cognitive decline. | SDS | |
| Lin et al., 2022 [21] | Taiwan | Dose: Pure EPA group: 1.6 gm daily; Pure DHA group: 0.7 gm daily; Combination group: 0.8 gm EPA and 0.35 gm DHA daily. Method: Participants were divided into three groups, each receiving two 0.5 gm capsules twice a day. The placebo group received soybean oil capsules. | Size: 163 participants. Characteristics: Condition distribution: 93 with MCI, 70 with mild to moderate Alzheimer’s disease. Source: Mainly elderly individuals from veteran retirement centers. Mean age: 77.9 years. Gender distribution: 33.75% female. | 24 months. | No significant differences were observed among treatment groups and placebo regarding cognitive, functional ability, and mood status outcomes after 24 months. This suggests that n-3 PUFA supplementation might not have significant effects on the disease progression rate in MCI and AD patients. | GDS | |
| Maltais et al., 2019 [33] * | France | Dose: Two soft capsules daily, each containing 400 mg DHA and up to 112.5 mg EPA. Method: Participants were assigned to one of four groups for a duration of 3 years: 1. Multidomain intervention plus omega-3; 2. Omega-3 only; 3. Multidomain intervention only; 4. Placebo. Multidomain intervention components: Nutritional and physical activity counseling, and cognitive training. | Size: 1680 participants. Characteristics: Participants must have self-reported memory loss, and slow gait speed. Demographics: Community-dwelling men and women aged 70 years or older. Mean age: 75.2 years. Gender distribution: 64.73% female. | 36 months. | No significant effect of any intervention (multidomain, omega-3, or their combination) was found on the progression of depressive symptoms. The incidences of developing minimum clinically meaningful, moderate, and severe depressive symptoms were not significantly different across the four groups. | - | |
| Rondanelli et al., 2012 [34] | Italy | Dose: Two capsules daily, each containing DHA 720 mg, EPA 286 mg, vitamin E 16 mg, soy phospholipids 160 mg, tryptophan 95 mg, and melatonin 5 mg, taken 1 h before bedtime. Method: Randomized, double-blind, placebo-controlled. Participants received either the described capsules or placebo capsules containing non-fish oils without omega-3 or omega-6 fatty acids. | Size: 25 participants: 11 supplement group; 14 placebo group. Characteristics: Participants with MCI. Mean age: 86 years. Gender distribution: 80.20% female. | 12 weeks. | Not statistically significant, the trend of improvement in depressive symptoms as evaluated with GDS was observed in the supplement group after 12 weeks of treatment with the dietary supplement. | GDS | |
| Sinn et al., 2012 [35] | Australia | Dose: EPA-rich fish oil group: 1.67 g EPA and 0.16 g DHA; DHA-rich fish oil group: 1.55 g DHA and 0.40 g EPA; Control group: Safflower oil with 2.72 g linoleic acid (LA). Method: Randomized allocation into one of three groups. | Size: 50 volunteers recruited, 40 completed assessments. Characteristics: Participants must have self-reported memory loss. Mean age: 74.03 years. Gender distribution: 33% female. | 6 months. | Significant improvement in depression (GDS) scores was observed in the EPA and DHA groups compared with the control group after 6 months. However, no significant treatment effects were found on the physical or mental quality of life outcomes. In the DHA treatment group, initial letter fluency scores significantly improved compared with the control group. | GDS | |
| van de Rest et al., 2009 [36] * | Netherlands | Dose: High dose group: 1800 mg per day of EPA-DHA; Low dose group: 400 mg per day of EPA-DHA; Placebo group. Method: Randomized, double-blind, placebo-controlled. Participants received either 1800 mg, 400 mg of EPA-DHA, or a placebo. | Size: 302 participants. Characteristics: Participants were aged 65 years and older. A score of more than 21 on the MMSE. Mean age: 69.8 years. Gender distribution: 55% male. | 26 weeks. | No significant differences were observed in the quality of life scores among the three groups after 13 and 26 weeks of intervention. | - | |
| Subgroup | k | Effect Size (SMD) | 95% Confidence Interval | p |
|---|---|---|---|---|
| Intervention type | ||||
| EPA | 4 | −0.169 | −0.454 to 0.116 | 0.246 |
| DHA | 6 | −0.247 | −0.482 to −0.013 | 0.039 |
| DHA+EPA | 5 | −0.061 | −0.228 to 0.105 | 0.470 |
| DHA+OS | 1 | −0.815 | −1.636 to 0.007 | 0.052 |
| Length | ||||
| 3 m | 1 | −0.815 | −1.636 to 0.007 | 0.052 |
| 6 m | 7 | −0.177 | −0.415 to 0.062 | 0.146 |
| 12 m | 5 | −0.147 | −0.334 to 0.039 | 0.120 |
| ≥24 m | 3 | −0.138 | −0.417 to 0.140 | 0.331 |
| Cognition | ||||
| MCI | 3 | −0.934 | −1.412 to −0.456 | < 0.001 |
| Moderate | 11 | −0.073 | −0.200 to 0.055 | 0.265 |
| Severe | 2 | −0.295 | −0.620 to 0.031 | 0.076 |
| DHA/Dosage (Day) | ||||
| High | 5 | −0.226 | −0.484 to 0.031 | 0.085 |
| Moderate | 4 | −0.172 | −0.436 to 0.093 | 0.203 |
| Low | 4 | −0.156 | −0.432 to 0.119 | 0.267 |
| EPA/Dosage (Day) | ||||
| High | 4 | −0.169 | −0.454 to 0.116 | 0.246 |
| Moderate | 5 | −0.061 | −0.228 to 0.105 | 0.470 |
| Low | 2 | −0.953 | −1.534 to −0.373 | 0.001 |
| Rating scale | ||||
| GDS | 12 | −0.172 | −0.329 to −0.016 | 0.031 |
| non-GDS | 4 | −0.126 | −0.302 to 0.051 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Ting, B.; Chen, D.T.-L.; Hsu, W.-T.; Lin, S.-C.; Kuo, C.-Y.; Wang, M.-F. Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 536. https://doi.org/10.3390/healthcare12050536
Chang Y-Y, Ting B, Chen DT-L, Hsu W-T, Lin S-C, Kuo C-Y, Wang M-F. Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis. Healthcare. 2024; 12(5):536. https://doi.org/10.3390/healthcare12050536
Chicago/Turabian StyleChang, Yen-Yun, Berne Ting, Daniel Tzu-Li Chen, Wei-Ti Hsu, Song-Chow Lin, Chun-Yen Kuo, and Ming-Fu Wang. 2024. "Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis" Healthcare 12, no. 5: 536. https://doi.org/10.3390/healthcare12050536
APA StyleChang, Y.-Y., Ting, B., Chen, D. T.-L., Hsu, W.-T., Lin, S.-C., Kuo, C.-Y., & Wang, M.-F. (2024). Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis. Healthcare, 12(5), 536. https://doi.org/10.3390/healthcare12050536









