Degree of Uncertainty in Reporting Imaging Findings for Necrotizing Enterocolitis: A Secondary Analysis from a Pilot Randomized Diagnostic Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Determination of Uncertainty
2.4. Outcomes and Variables of Interest
2.5. Statistical Analysis
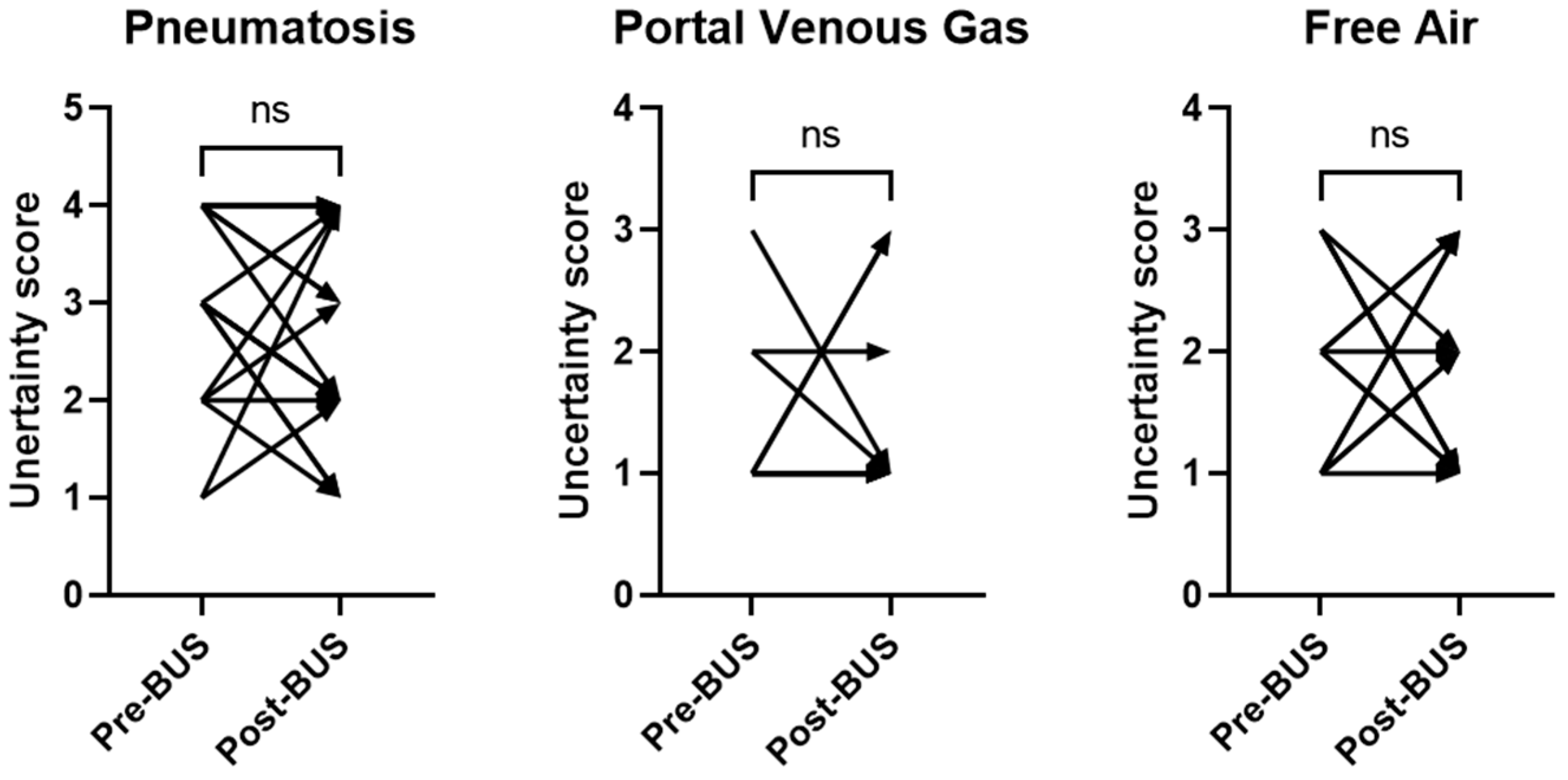
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Alganabi, M.; Lee, C.; Bindi, E.; Li, B.; Pierro, A. Recent advances in understanding necrotizing enterocolitis. F1000Research 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Zani, A.; Eaton, S.; Puri, P.; Rintala, R.; Lukac, M.; Bagolan, P.; Kuebler, J.F.; Hoellwarth, M.E.; Wijnen, R.; Tovar, J.; et al. EUPSA Network, International survey on the management of necrotizing enterocolitis. Eur. J. Pediatr. Surg. 2015, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ahle, M.; Ringertz, H.G.; Rubesova, E. The role of imaging in the management of necrotising enterocolitis: A multispecialist survey and a review of the literature. Eur. Radiol. 2018, 28, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Valpacos, M.; Arni, D.; Keir, A.; Aspirot, A.; Wilde, J.C.H.; Beasley, S.; De Luca, D.; Pfister, R.E.; Karam, O. Diagnosis and Management of Necrotizing Enterocolitis: An International Survey of Neonatologists and Pediatric Surgeons. Neonatology 2018, 113, 170–176. [Google Scholar] [CrossRef] [PubMed]
- van Druten, J.; Khashu, M.; Chan, S.S.; Sharif, S.; Abdalla, H. Abdominal ultrasound should become part of standard care for early diagnosis and management of necrotising enterocolitis: A narrative review. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F551–F559. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Gordon, S.; Yang, M.; Weekes, J.; Dance, L. Abdominal Ultrasound Assists the Diagnosis and Management of Necrotizing Enterocolitis. Adv. Neonatal Care 2021, 21, 365–370. [Google Scholar] [CrossRef]
- Kim, J.H. Role of Abdominal US in Diagnosis of NEC. Clin. Perinatol. 2019, 46, 119–127. [Google Scholar] [CrossRef]
- Alexander, K.M.; Chan, S.S.; Opfer, E.; Cuna, A.; Fraser, J.D.; Sharif, S.; Khashu, M. Implementation of bowel ultrasound practice for the diagnosis and management of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 96–103. [Google Scholar] [CrossRef]
- Epelman, M.; Daneman, A.; Navarro, O.M.; Morag, I.; Moore, A.M.; Kim, J.H.; Faingold, R.; Taylor, G.; Gerstle, J.T. Necrotizing enterocolitis: Review of state-of-the-art imaging findings with pathologic correlation. Radiographics 2007, 27, 285–305. [Google Scholar] [CrossRef]
- Brady, A.; Laoide, R.Ó.; McCarthy, P.; McDermott, R. Discrepancy and error in radiology: Concepts, causes and consequences. Ulster Med. J. 2012, 81, 3–9. [Google Scholar] [PubMed]
- Wattamwar, K.; Garg, T.; Wheeler, C.A.; Burns, J. Diagnostic Uncertainty in Radiology: A Perspective for Trainees and Training Programs. Radiographics 2022, 42, E193–E196. [Google Scholar] [CrossRef]
- Bruno, M.A.; Petscavage-Thomas, J.; Abujudeh, H.H. Communicating Uncertainty in the Radiology Report. AJR Am. J. Roentgenol. 2017, 209, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Reiner, B. Uncovering and improving upon the inherent deficiencies of radiology reporting through data mining. J. Digit. Imaging 2010, 23, 109–118. [Google Scholar] [CrossRef]
- Mata, A.G.; Rosengart, R.M. Interobserver variability in the radiographic diagnosis of necrotizing enterocolitis. Pediatrics 1980, 66, 68–71. [Google Scholar] [CrossRef]
- Rehan, V.K.; Seshia, M.M.; Johnston, B.; Reed, M.; Wilmot, D.; Cook, V. Observer variability in interpretation of abdominal radiographs of infants with suspected necrotizing enterocolitis. Clin. Pediatr. 1999, 38, 637–643. [Google Scholar] [CrossRef]
- El-Kady, S.; Petel, D.; Baird, R. Inter-rater agreement in the evaluation of abdominal radiographs for necrotizing enterocolitis. J. Pediatr. Surg. 2014, 49, 733–735. [Google Scholar] [CrossRef]
- Di Napoli, A.; Di Lallo, D.; Perucci, C.A.; Schifano, P.; Orzalesi, M.; Franco, F.; De Carolis, M.P. Inter-observer reliability of radiological signs of necrotising enterocolitis in a population of high-risk newborns. Paediatr. Perinat. Epidemiol. 2004, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Chan, S.; Jones, J.; Sien, M.; Robinson, A.; Rao, K.; Opfer, E. Feasibility and acceptability of a diagnostic randomized clinical trial of bowel ultrasound in infants with suspected necrotizing enterocolitis. Eur. J. Pediatr. 2022, 181, 3211–3215. [Google Scholar] [CrossRef]
- Reiner, B.I. Quantifying Analysis of Uncertainty in Medical Reporting: Creation of User and Context-Specific Uncertainty Profiles. J. Digit. Imaging 2018, 31, 379–382. [Google Scholar] [CrossRef]
- Merritt, C.R.; Goldsmith, J.P.; Sharp, M.J. Sonographic detection of portal venous gas in infants with necrotizing enterocolitis. AJR Am. J. Roentgenol. 1984, 143, 1059–1062. [Google Scholar] [CrossRef]
- Lindley, S.; Mollitt, D.L.; Seibert, J.J.; Golladay, E.S. Portal vein ultrasonography in the early diagnosis of necrotizing enterocolitis. J. Pediatr. Surg. 1986, 21, 530–532. [Google Scholar] [CrossRef]
- Robberecht, E.A.; Afschrift, M.; De Bel, C.E.; Van Haesebrouck, P.J.; Van Bever, H.P.; De Wit, M.; Leroy, J.G. Sonographic demonstration of portal venous gas in necrotizing enterocolitis. Eur. J. Pediatr. 1988, 147, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Faingold, R. Technical aspects of abdominal ultrasound and color Doppler assessment of bowel viability in necrotizing enterocolitis. Pediatr. Radiol. 2018, 48, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Anupindi, S.; Keener, H.; Rompel, O. Ultrasound of the bowel in children: How we do it. Pediatr. Radiol. 2010, 40, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Faingold, R.; Daneman, A.; Tomlinson, G.; Babyn, P.S.; Manson, D.E.; Mohanta, A.; Moore, A.M.; Hellmann, J.; Smith, C.; Gerstle, T.; et al. Necrotizing enterocolitis: Assessment of bowel viability with color doppler US. Radiology 2005, 235, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.G.; Rajderkar, D.A.; Sistrom, C.L.; Slater, R.M.; Mancuso, A.A. Bubbles in the belly: How well do radiology trainees recognize pneumatosis in pediatric patients on plain film? Br. J. Radiol. 2022, 95, 20211101. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, C.L.; Rice, H.E. The Duke Abdominal Assessment Scale: Initial experience. Expert. Rev. Gastroenterol. Hepatol. 2010, 4, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Coursey, C.A.; Hollingsworth, C.L.; Gaca, A.M.; Maxfield, C.; Delong, D.; Bisset, G. Radiologists’ agreement when using a 10-point scale to report abdominal radiographic findings of necrotizing enterocolitis in neonates and infants. AJR Am. J. Roentgenol. 2008, 191, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Markiet, K.; Szymanska-Dubowik, A.; Janczewska, I.; Domazalska-Popadiuk, I.; Zawadzka-Kepczynska, A.; Bianek-Bodzak, A. Agreement and reproducibility of radiological signs in NEC using The Duke Abdominal Assessment Scale (DAAS). Pediatr. Surg. Int. 2017, 33, 335–340. [Google Scholar] [CrossRef]
- Callen, A.L.; Dupont, S.M.; Price, A.; Laguna, B.; McCoy, D.; Do, B.; Talbott, J.; Kohli, M.; Narvid, J. Between Always and Never: Evaluating Uncertainty in Radiology Reports Using Natural Language Processing. J. Digit. Imaging 2020, 33, 1194–1201. [Google Scholar] [CrossRef]
- Crombé, A.; Seux, M.; Bratan, F.; Bergerot, J.-F.; Banaste, N.; Thomson, V.; Lecomte, J.-C.; Gorincour, G. What Influences the Way Radiologists Express Themselves in Their Reports? A Quantitative Assessment Using Natural Language Processing. J. Digit. Imaging 2022, 35, 993–1007. [Google Scholar] [CrossRef]
- Panicek, D.M.; Hricak, H. How Sure Are You, Doctor? A Standardized Lexicon to Describe the Radiologist’s Level of Certainty. AJR Am. J. Roentgenol. 2016, 207, 2–3. [Google Scholar] [CrossRef]
- Glazer, D.I.; Budiawan, E.; Burk, K.S.; Shinagare, A.B.; Lacson, R.; Boland, G.W.; Khorasani, R. Adoption of a diagnostic certainty scale in abdominal imaging: 2-year experience at an academic institution. Abdom. Radiol. 2022, 47, 1187–1195. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Alper, D.P.; Hashemi, S.R.; Chai, J.L.; Hammer, M.M.; Boland, G.W.; Khorasani, R. Early Adoption of a Certainty Scale to Improve Diagnostic Certainty Communication. J. Am. Coll. Radiol. 2020, 17, 1276–1284. [Google Scholar] [CrossRef]
- Das, J.P.; Panicek, D.M. Added Value of a Diagnostic Certainty Lexicon to the Radiology Report. Radiographics 2021, 41, E64–E65. [Google Scholar] [CrossRef]
- Godwin, B.D.; Drake, F.T.; Simianu, V.V.; Shriki, J.E.; Hippe, D.S.; Dighe, M.; Bastawrous, S.; Cuevas, C.; Flum, D.; Bhargava, P. A novel reporting system to improve accuracy in appendicitis imaging. AJR Am. J. Roentgenol. 2015, 204, 1212–1219. [Google Scholar] [CrossRef]
- Wibmer, A.; Vargas, H.A.; Sosa, R.; Zheng, J.; Moskowitz, C.; Hricak, H. Value of a standardized lexicon for reporting levels of diagnostic certainty in prostate MRI. AJR Am. J. Roentgenol. 2014, 203, W651–W657. [Google Scholar] [CrossRef] [PubMed]
- Maizlin, N.N.; Somers, S. The Role of Clinical History Collected by Diagnostic Imaging Staff in Interpreting of Imaging Examinations. J. Med. Imaging Radiat. Sci. 2019, 50, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lacson, R.; Laroya, R.; Wang, A.; Kapoor, N.; Glazer, D.I.; Shinagare, A.; Ip, I.K.; Malhotra, S.; Hentel, K.; Khorasani, R. Integrity of clinical information in computerized order requisitions for diagnostic imaging. J. Am. Med. Inform. Assoc. 2018, 25, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Babcook, C.J.; Norman, G.R.; Coblentz, C.L. Effect of clinical history on the interpretation of chest radiographs in childhood bronchiolitis. Investig. Radiol. 1993, 28, 214–217. [Google Scholar] [CrossRef]
- Berbaum, K.S.; Franken, E.A.; Dorfman, D.D.; Lueben, K.R. Influence of clinical history on perception of abnormalities in pediatric radiographs. Acad. Radiol. 1994, 1, 217–223. [Google Scholar] [CrossRef]
- Castillo, C.; Steffens, T.; Sim, L.; Caffery, L. The effect of clinical information on radiology reporting: A systematic review. J. Med. Radiat. Sci. 2021, 68, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Binkovitz, L.A.; Unsdorfer, K.M.L.; Thapa, P.; Kolbe, A.B.; Hull, N.C.; Zingula, S.N.; Thomas, K.B.; Homme, J.L. Pediatric appendiceal ultrasound: Accuracy, determinacy and clinical outcomes. Pediatr. Radiol. 2015, 45, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Unsdorfer, K.M.L.; An, J.Y.; Binkovitz, L.A. Pediatric appendiceal ultrasound: Maintaining accuracy, increasing determinacy and improving clinical outcomes following the introduction of a standardized reporting template. Pediatr. Radiol. 2021, 51, 265–272. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Template |
|---|---|
| AXR | There are no findings to suggest bowel obstruction, free intraperitoneal gas, or pneumatosis. |
| There is no portal venous gas. | |
| BUS | There is no bowel wall thickening (>2.7 mm). |
| There is no bowel wall thinning (<1.0 mm). | |
| There is no bowel wall hyperechogenicity. | |
| There is no pneumatosis intestinalis. | |
| There is no portal venous gas. | |
| There is no pneumoperitoneum. | |
| There are no focal fluid collections with complex echoes. | |
| There is no free fluid. | |
| There is normal bowel wall perfusion. | |
| Peristalsis is present. |
| Score | Degree of Uncertainty | Example |
|---|---|---|
| 1 | Lowest level of uncertainty. No equivocation. | No pneumatosis. |
| No portal venous gas. | ||
| No pneumoperitoneum. | ||
| 2 | Low level of uncertainty. Minimal equivocation. | No definite pneumatosis. |
| No supine evidence of pneumoperitoneum. | ||
| No obvious free air. | ||
| 3 | Intermediate level of uncertainty. Intermediate degree of equivocation. | No findings to suggest pneumatosis, portal venous gas, or free air. |
| Small area of possible linear lucency along the bowel wall. | ||
| Suggestion of interim mural air within the bowel wall. | ||
| 4 | Highest level of uncertainty. High degree of equivocation. | Cannot exclude pneumatosis. |
| No obvious pneumatosis although evaluation is limited. | ||
| Mild mottled lucencies which may represent pneumatosis or stool. |
| Baseline Characteristics | AXR Group (n = 8) | AXR Plus BUS Group (n = 8) |
|---|---|---|
| Gestational age, weeks | 26.9 ± 2.5 | 27.4 ± 2.1 |
| Birth weight, grams | 1056 ± 399 | 1022 ± 381 |
| Male sex, no. (%) | 5 (63) | 2 (25) |
| White race, no. (%) | 4 (50) | 6 (75) |
| Small for gestational age, no. (%) | 0 (0) | 1 (13) |
| Maternal age, years a | 25 ± 7 | 31 ± 7 |
| Caesarian delivery, no. (%) | 3 (38) | 5 (63) |
| Apgar score < 5 at 1 min, no. (%) b | 4 (50) | 7 (88) |
| Apgar score < 5 at 5 min, no. (%) b | 3 (38) | 2 (25) |
| Antenatal corticosteroids, no. (%) | 8 (100) | 7 (88) |
| Surfactant, no. (%) | 8 (100) | 6 (75) |
| No. of NEC concern episodes | 1 (1–3) | 1 (1–3) |
| No. of imaging studies | ||
| AXR | 49 | 64 |
| BUS | 0 | 24 |
| BUS n = 24 | AXR n = 113 | p | |
|---|---|---|---|
| Complete report, n (%) | 23 (96) | 59 (52) | 0.0001 |
| Pneumatosis | 24 (100) | 111 (98) | 1.0 |
| Portal venous gas | 24 (100) | 67 (59) | 0.0001 |
| Free air | 23 (96) | 95 (84) | 0.19 |
| Use of standardized template, n (%) | 21 (88) | 18 (16) | 0.0001 |
| AXR + BUS Arm n = 64 | AXR Arm n = 49 | p | |
|---|---|---|---|
| Complete report, n (%) | 35 (55) | 24 (49) | 0.55 |
| Pneumatosis | 63 (98) | 48 (98) | 1.0 |
| Portal venous gas | 42 (66) | 25 (51) | 0.12 |
| Free Air | 54 (84) | 41 (84) | 0.92 |
| Use of standardized template, n (%) | 9 (14) | 9 (18) | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuna, A.; Rathore, D.; Bourret, K.; Opfer, E.; Chan, S. Degree of Uncertainty in Reporting Imaging Findings for Necrotizing Enterocolitis: A Secondary Analysis from a Pilot Randomized Diagnostic Trial. Healthcare 2024, 12, 511. https://doi.org/10.3390/healthcare12050511
Cuna A, Rathore D, Bourret K, Opfer E, Chan S. Degree of Uncertainty in Reporting Imaging Findings for Necrotizing Enterocolitis: A Secondary Analysis from a Pilot Randomized Diagnostic Trial. Healthcare. 2024; 12(5):511. https://doi.org/10.3390/healthcare12050511
Chicago/Turabian StyleCuna, Alain, Disa Rathore, Kira Bourret, Erin Opfer, and Sherwin Chan. 2024. "Degree of Uncertainty in Reporting Imaging Findings for Necrotizing Enterocolitis: A Secondary Analysis from a Pilot Randomized Diagnostic Trial" Healthcare 12, no. 5: 511. https://doi.org/10.3390/healthcare12050511
APA StyleCuna, A., Rathore, D., Bourret, K., Opfer, E., & Chan, S. (2024). Degree of Uncertainty in Reporting Imaging Findings for Necrotizing Enterocolitis: A Secondary Analysis from a Pilot Randomized Diagnostic Trial. Healthcare, 12(5), 511. https://doi.org/10.3390/healthcare12050511






