Using Digital Health Technologies to Monitor Pain, Medication Adherence and Physical Activity in Young People with Juvenile Idiopathic Arthritis: A Feasibility Study
Abstract
1. Introduction
1.1. Utilising Digital Health Technology
1.2. InteractiveClinics—A Web-Based Platform for Digital Health Research
1.3. Definition
- (1.)
- Assessing if the intervention works as intended and is error-free;
- (2.)
- Demonstrating end-user acceptance [29].
2. Materials and Methods
2.1. Study Design
2.2. Sampling
2.3. Intervention
2.3.1. InteractiveClinics Setup
- Refurbished Apple iPhone (SE, 2016), loaded with pre-paid credit (AUD 30), selected because of the purchase price, a 4-inch screen, and the simplicity of the home button for children.
- Interactive Clinics app, free to download from the Apple or Google Play Store (already pre-set on the iPhone).
- Personal password, to access the secure, password-locked, web-based platform and to the summary of the data collected from the app.
- Training and support (approximately 10 min), which included demonstrations and written instructions. The contact details of SB were also placed within the phone’s address book, to gain further support if needed.
- Prepaid envelope, to return the watch and phone at the end of the study.
2.3.2. InteractiveClinics Modules
2.3.3. Pain Level Module
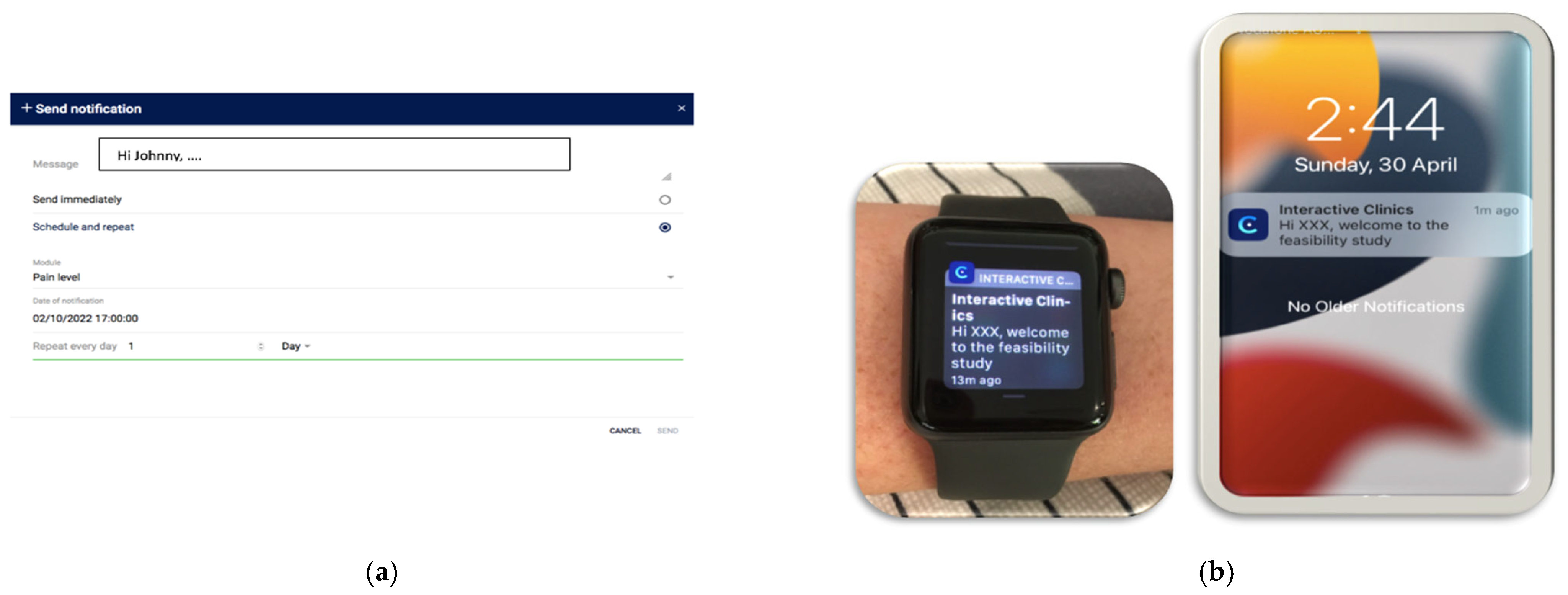
- Set up: Participants self-selected their pain notification time, and this was entered by the researchers (SB) into the web-based platform. A personal message to each participant was also created.
- During the study period: Personalised notifications were sent daily to the smartwatch and phone to remind participants to record their pain.
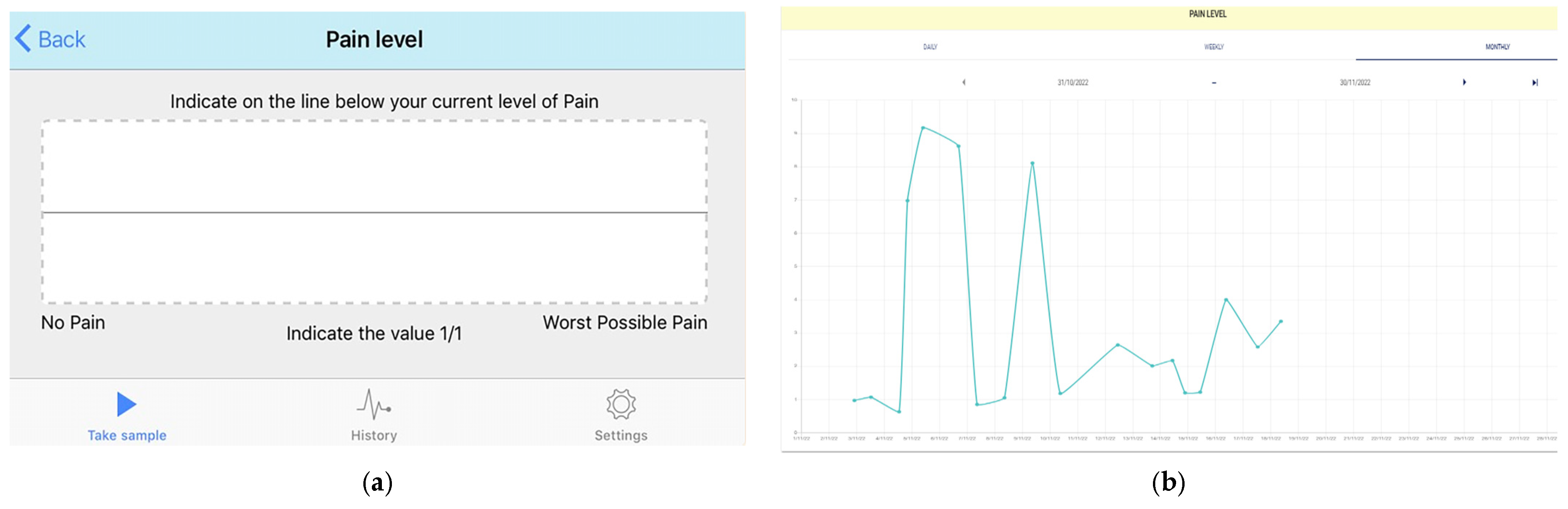
- User actions: Pain levels were recorded in the app using the validated electronic visual analogue scale (eVas) [32,33,34]. eVas utilises a simple horizontal line with defined pain limits. The left endpoint indicates no pain, and the right endpoint indicates the worst possible pain. This reporting scale has been found to be highly reliable and consistent with the original paper-based visual analogue scale (VAS) [32,34] (Figure 2).
- Data feedback: Visual feedback was then provided to the participants, displaying their daily pain level using the numerical score (0–10) and a monthly pain graph. The same information was available on the web-based platform, enabling the parent/carer and paediatric PR research teams to longitudinally monitor pain trends (Figure 2) (Also see Table 1 below).
2.3.4. Medication Adherence Module
- Setup: Each participant’s medication and administration times were entered into the web-based platform. This information could be viewed by the participants through the app.
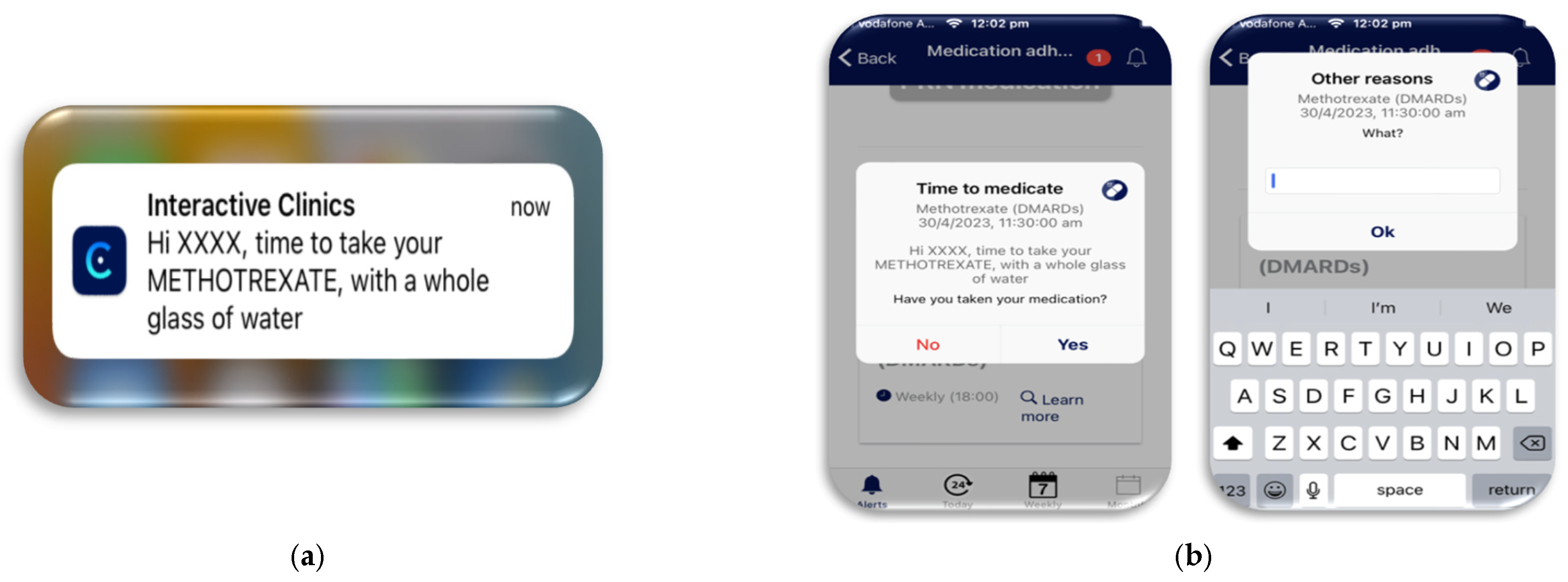
- During the study: Personalised notifications were sent to the watch and phone to remind the participants that their medications were due and how the medication should be taken. For example, methotrexate, with a whole glass of water (Figure 3).
- User actions: The participants were instructed to record their medication administration in the app. If the participants did not take their medication, they were then asked to provide a reason why, either from a pre-programmed list or a free text box to allow a unique answer (Figure 3). The medication module also allowed for additional pro-re-nata (PRN) medications to be recorded in case breakthrough pain medications were needed.
- Data feedback: Visual feedback was then provided to the participants, displaying their daily, weekly, and monthly medication usage in the app. Through the web-based platform, the parent/carer and PR research teams could also review the same information, allowing them to graphically track medication adherence (Figure 3) (Also see Table 1 below).
2.3.5. Physical Activity Level Module
- Setup: Each participant’s personal physical activity goals were set during the training session in accordance with their age and Australia’s Physical Activity and Sedentary Behaviour Guidelines [35]. Corresponding pamphlets were also provided to supply a variety of achievable exercise options, such as walking to school, and education on sedentary behaviour, such as extended sitting [35].
- User actions: Through participants simply wearing the watch each day, physical activity levels were automatically recorded by the biosensors in the watch measuring heart rate and the three-axis accelerometer measuring changes in velocity [36]. These data were then transferred from the phone’s commercial fitness app to the InteractiveClinics app and web-based platform. This synchronisation was dependent on a once-only authorisation on the phone and internet connectivity.
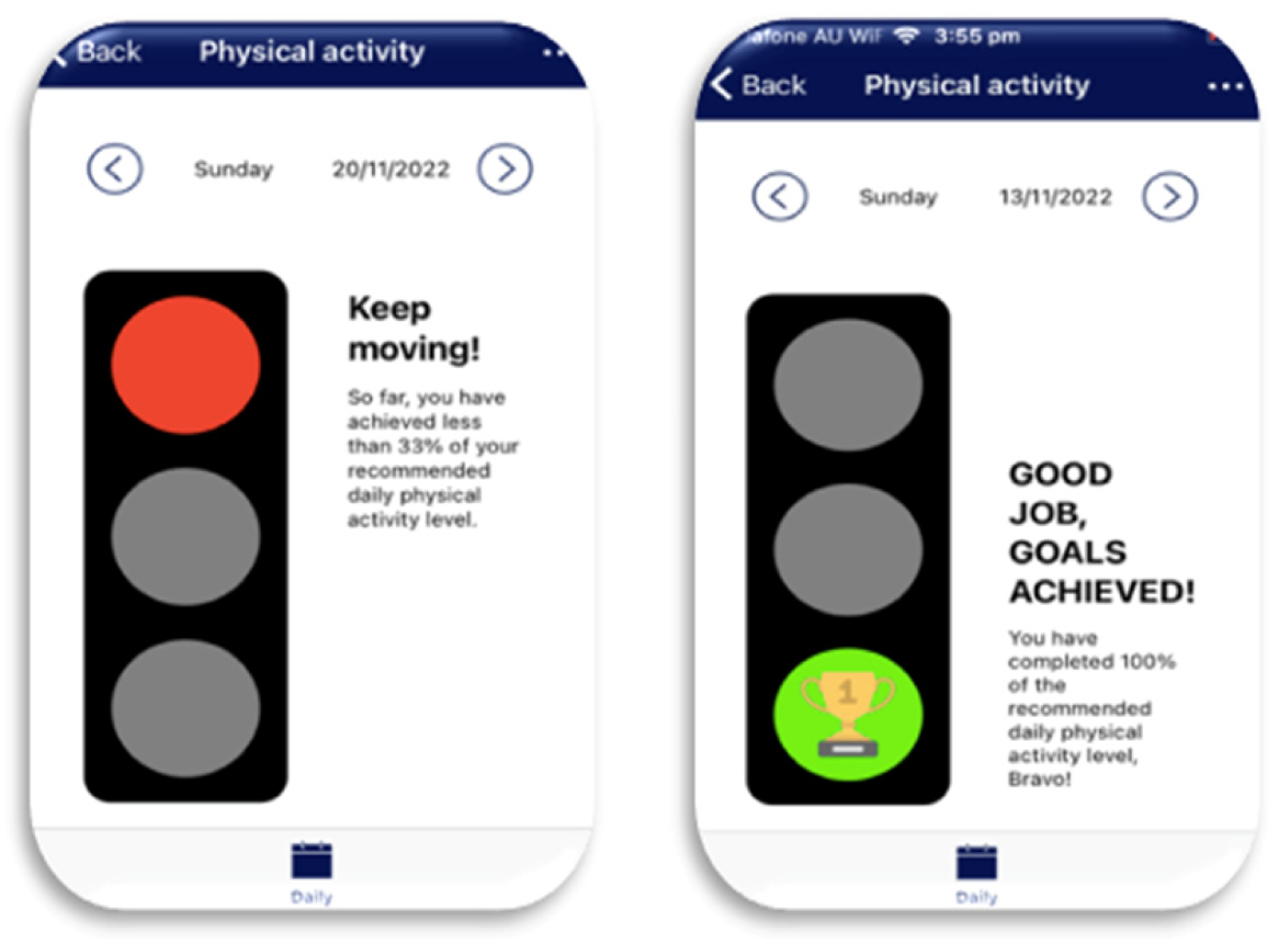
- Data feedback: Visual feedback was provided to participants in the app to motivate the participants to reach their daily goals (Figure 4). The web-based platform was more detailed by graphically displaying the participant’s daily and monthly physical activity levels, making these data available to the participant, parent/carer, and the research team (Also see Table 1 below).
| Module | Persuasive Influence | Data Collection Method |
|---|---|---|
| Pain level | Customised notifications to watch and phone | User action via app |
| Medication adherence | Customised notifications to watch and phone | User action via app |
| Physical activity levels | User action—wearing the watch | Automatic |
2.4. Participant Recruitment
2.5. Follow-Up of Participants
2.6. Ethical Considerations
2.7. Outcomes
2.7.1. Primary Feasibility Outcomes
- (1.)
- App usage: Measured by the percentage of patients who used InteractiveClinics daily. Only missed medication administration entries were recorded by the system.
- (2.)
- Confirmation of data integrity: Measured by the percentage of missing or incomplete data between the web-based platform and watch and phone’s Apple health app.
- (3.)
- Preliminary interpretation: By presenting the initial results of the study population’s data collected on InteractiveClinics web-based platform.
- For the pain module, using eVAS scores.
- For the medication module, the rate of medication administration.
- For the physical activity level module, daily kilojoules (light physical activity), moderate-to-vigorous physical activity, and stand hours.
- (4.)
- Watch wearing behaviour: Measured by the number of days that the watch was worn across the study period.
2.7.2. Secondary Outcomes
- (1.)
- Technical errors: measured by the percentage of participants’ needing technical support after intervention training.
2.8. Data Collection
2.9. Data Analysis
3. Results
3.1. Pain Level Module
3.2. Medication Adherence Module
Preliminary Interpretation
- Daily medication adherence: Overall, the web-based platform recorded 39% (71/182) of the expected medication entries.
- Twice-a-day medication adherence: One participant (8.3%, 1/12) required the same medication twice a day. This medication frequency could not be entered into the system due to a coding fault.
- Weekly medication adherence: Three medication entries of the expected eight (37.5%, 3/8) were recorded by three participants.
- Reasons for non-adherence: Only one participant utilised the text box to provide their reason for non-adherence, explaining they ‘arrived home late’ (Participant 6).
- PRN medication monitoring: Eight participants in this study often needed PRN medications for their pain or stiffness. Over 14 days, the web-based platform recorded four participants needing additional medication (mean: 3.5, SD: 2.4), for 2 to 6 days. When PRN medications were not needed, ten responses were also seen, for example, (there was) “no need” (Participant 7).
3.3. Physical Activity Module
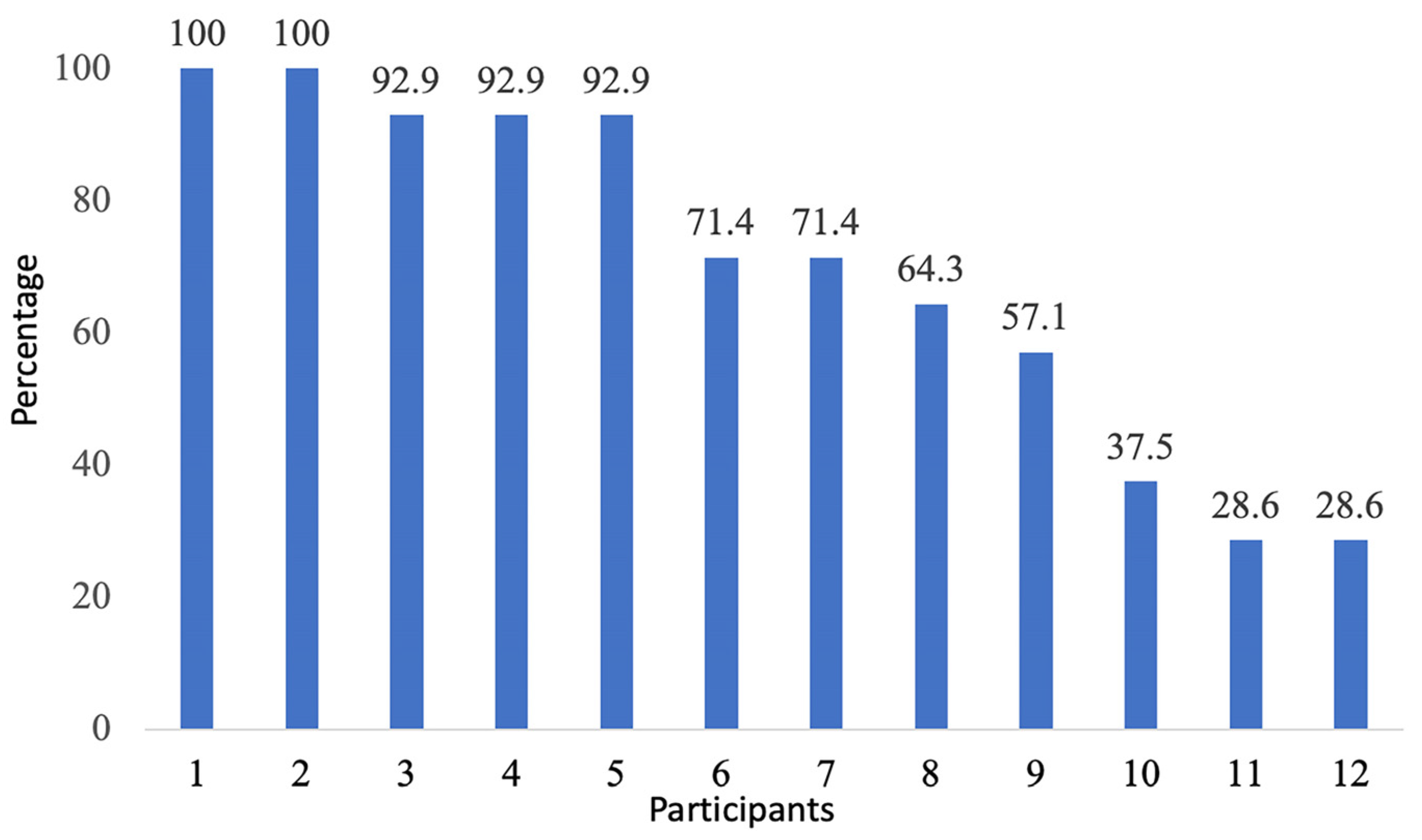
3.4. Smartwatch Wearing Behaviour
3.5. Ongoing Technical Support
4. Discussion
4.1. App Usage
4.2. Confirmation of Data Integrity
4.3. Preliminary Interpretation
4.3.1. Pain Level Module
4.3.2. Medication Adherence
4.3.3. Physical Activity Module
4.4. Watch Wearing Behaviour
4.5. Technical Issues
4.6. Limitations
4.6.1. Internal Limitations
4.6.2. External Limitations
4.7. Clinical Importance
4.8. Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parliament of the Commonwealth of Australia. Inquiry into Childhood Rheumatic Diseases: Interim Report; House of Representatives Standing Committee on Health, Aged Care and Sport: Canberra, Australia, 2022; pp. 1–73. Available online: https://parlinfo.aph.gov.au/parlInfo/download/committees/reportrep/024921/toc_pdf/InquiryintochildhoodrheumaticdiseasesInterimreport.pdf;fileType=application%2Fpdf (accessed on 3 November 2023).
- Petty, R.E.; Southwood, T.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. Available online: https://www.jrheum.org/content/31/2/390.long (accessed on 1 October 2023). [PubMed]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Giancane, G.; Consolaro, A.; Lanni, S.; Davì, S.; Schiappapietra, B.; Ravelli, A. Juvenile Idiopathic Arthritis: Diagnosis and Treatment. Rheumatol. Ther. 2016, 3, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Feger, D.M.; Longson, N.; Dodanwala, H.; Ostrov, B.E.; Olsen, N.J.; June, R.R. Comparison of Adults with Polyarticular Juvenile Idiopathic Arthritis to Adults With Rheumatoid Arthritis: A Cross-sectional Analysis of Clinical Features and Medication Use. J. Clin. Rheumatol. 2019, 25, 163–170. [Google Scholar] [CrossRef]
- Moorthy, L.N.; Peterson, M.G.; Hassett, A.L.; Lehman, T.J. Burden of childhood-onset arthritis. Pediatr. Rheumatol. 2010, 8, 20. [Google Scholar] [CrossRef]
- Porth, C.M.; Gaspard, K.J. Essentials of Pathophysiology [Electronic Resource]: Concepts of Altered Health States, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2015. [Google Scholar]
- Chomistek, K.; Johnson, N.; Stevenson, R.; Luca, N.; Miettunen, P.; Benseler, S.M.; Veeramreddy, D.; Schmeling, H. Patient-Reported Barriers at School for Children with Juvenile Idiopathic Arthritis. ACR Open Rheumatol. 2019, 1, 182–187. [Google Scholar] [CrossRef]
- Yildiz, D.; Fidanci, B.A. Holistic Approach of Children with JIA. Ann. Paediatr. Rheumatol. 2013, 2, 14–20. [Google Scholar] [CrossRef]
- McErlane, F.; Carrasco, R.; Kearsley-Fleet, L.; Baildam, E.M.; Wedderburn, L.R.; Foster, H.E.; Ioannou, Y.; Chieng, S.E.A.; Davidson, J.E.; Thomson, W.; et al. Growth patterns in early juvenile idiopathic arthritis: Results from the Childhood Arthritis Prospective Study (CAPS). Semin. Arthritis Rheum. 2018, 48, 53–60. [Google Scholar] [CrossRef]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Baildam, E.M.; Oldershaw, R.A. Juvenile idiopathic arthritis: From aetiopathogenesis to therapeutic approaches. Pediatr. Rheumatol. 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, L.; Kristensen, K.D.; Herlin, T. Juvenile idiopathic arthritis characteristics: Etiology and pathophysiology. Semin. Orthod. 2015, 21, 77–83. [Google Scholar] [CrossRef]
- Glerup, M.; Rypdal, V.; Arnstad, E.D.; Ekelund, M.; Peltoniemi, S.; Aalto, K.; Rygg, M.; Toftedal, P.; Nielsen, S.; Fasth, A.; et al. Long-Term Outcomes in Juvenile Idiopathic Arthritis: Eighteen Years of Follow-Up in the Population-Based Nordic Juvenile Idiopathic Arthritis Cohort. Arthritis Care Res. 2020, 72, 507–516. [Google Scholar] [CrossRef]
- Len, C.A.; Miotto e Silva, V.B.; Terreri, M.T. Importance of Adherence in the Outcome of Juvenile Idiopathic Arthritis. Curr. Rheumatol. Rep. 2014, 16, 410. [Google Scholar] [CrossRef]
- de Lalouvière, L.; Ioannou, Y.; Fitzgerald, M. Neural mechanisms underlying the pain of juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2014, 10, 205–211. [Google Scholar] [CrossRef]
- Bohr, A.H.; Nielsen, S.; Müller, K.; Karup Pedersen, F.; Andersen, L.B. Reduced physical activity in children and adolescents with Juvenile Idiopathic Arthritis despite satisfactory control of inflammation. Pediatr. Rheumatol. 2015, 13, 57. [Google Scholar] [CrossRef]
- Feldman, D.E.; De Civita, M.; Dobkin, P.L.; Malleson, P.N.; Meshefedjian, G.; Duffy, C.M. Effects of adherence to treatment on short-term outcomes in children with juvenile idiopathic arthritis. Arthritis Care Res. 2007, 57, 905–912. [Google Scholar] [CrossRef]
- Waite-Jones, J.M.; Majeed-Ariss, R.; Smith, J.; Stones, S.R.; Van Rooyen, V.; Swallow, V. Young People’s, Parents’, and Professionals’ Views on Required Components of Mobile Apps to Support Self-Management of Juvenile Arthritis: Qualitative Study. JMIR mHealth uHealth 2018, 6, e25. [Google Scholar] [CrossRef]
- Moses, J.C.; Adibi, S.; Shariful Islam, S.M.; Wickramasinghe, N.; Nguyen, L. Application of Smartphone Technologies in Disease Monitoring: A Systematic Review. Healthcare 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Razavi Termeh, V.; Sadeghi Niaraki, A. Design and Implementation of Ubiquitous Health System (U-Health) using Smart-Watches Sensors. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 607–612. Available online: https://isprs-archives.copernicus.org/articles/XL-1-W5/607/2015/isprsarchives-XL-1-W5-607-2015.pdf (accessed on 1 October 2023). [CrossRef]
- Reeder, B.; David, A. Health at Hand: A Systematic Review of Smart Watch Uses for Health and Wellness. J. Biomed. Inform. 2016, 63, 269–276. [Google Scholar] [CrossRef]
- Coda, A.; Sculley, D.; Santos, D. Harnessing interactive technologies to improve health outcomes in juvenile idiopathic arthritis. Pediatr. Rheumatol. 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, C.M.; Rocchio, R.A.; Roman, A.; King, C.E.; Sarrafzadeh, M. Wireless Sensor-Dependent Ecological Momentary Assessment for Pediatric Asthma mHealth Applications. In Proceedings of the 2017 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Philadelphia, PA, USA, 17–19 July 2017; pp. 137–146. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Topol, E.J. Moving from Digitalization to Digitization in Cardiovascular Care: Why is it Important and What Can it Mean for Patients and Providers? J. Am. Coll. Cardiol. 2015, 66, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.; Frontera, A. Smart-watches: A potential challenger to the implantable loop recorder? Europace 2016, 18, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S. Digital 2023 Deep-Dive: The Rise of Wearables. Datareportal 2023. Available online: https://datareportal.com/reports/digital-2023-deep-dive-the-rise-of-wearables (accessed on 15 October 2023).
- Telsyte. Telsyte Australian Smartphone & Wearable Devices Market Study 2022–2026, Android Share Growing in Australia. 2022. Available online: https://www.telsyte.com.au/announcements?month=09-2022 (accessed on 1 October 2023).
- Jat, A.S.; Grønli, T.M. Smart Watch for Smart Health Monitoring: A Literature Review. In Bioinformatics and Biomedical Engineering; Rojas, I., Valenzuela, O., Rojas, F., Herrera, L.J., Ortuño, F., Eds.; Lecture Notes in Computer Science; Springer International Publishing: New York, NY, USA, 2022; pp. 256–268. [Google Scholar] [CrossRef]
- World Health Organization. Monitoring and Evaluating Digital Health Interventions. In A Practical Guide to Conducting Research and Assessment; World Health Organization: Geneva, Switzerland, 2016; pp. 1–144. Available online: https://apps.who.int/iris/bitstream/handle/10665/252183/9789241511766-eng.pdf?sequence=1&isAllowed=y (accessed on 1 October 2023).
- Apple Inc. About Apple Watch Water-Resistance. 2023. Available online: https://support.apple.com/en-au/HT205000 (accessed on 1 October 2023).
- Takken, T.; Van Der Net, J.; Kuis, W.; Helders, P.J. Aquatic fitness training for children with juvenile idiopathic arthritis. Rheumatology 2003, 42, 1408–1414. [Google Scholar] [CrossRef]
- Maarj, M.; Pacey, V.; Tofts, L.; Clapham, M.; Gironès Garcia, X.; Coda, A. Validation of an Electronic Visual Analog Scale App for Pain Evaluation in Children and Adolescents With Symptomatic Hypermobility: Cross-sectional Study. JMIR Pediatr. Parent. 2022, 5, e41930. [Google Scholar] [CrossRef]
- Turnbull, A.; Sculley, D.; Escalona-Marfil, C.; Riu-Gispert, L.; Ruiz-Moreno, J.; Gironès, X.; Coda, A. Comparison of a Mobile Health Electronic Visual Analog Scale App with a Traditional Paper Visual Analog Scale for Pain Evaluation: Cross-Sectional Observational Study. J. Med. Internet Res. 2020, 22, e18284. [Google Scholar] [CrossRef]
- Escalona-Marfil, C.; Coda, A.; Ruiz-Moreno, J.; Riu-Gispert, L.M.; Gironès, X. Validation of an Electronic Visual Analog Scale mHealth Tool for Acute Pain Assessment: Prospective Cross-Sectional Study. J. Med. Internet Res. 2020, 22, e13468. [Google Scholar] [CrossRef]
- Department of Health and Aged Care. Collection of Physical Activity and Sedentary Behaviour Guidelines for All Ages, Commonwealth of Australia. 2019. Available online: https://www.health.gov.au/resources/collections/collection-of-physical-activity-and-sedentary-behaviour-guidelines-for-all-ages (accessed on 3 November 2023).
- Apple Developer. Getting Raw Accelerometer Events, Apple Inc. 2023. Available online: https://developer.apple.com/documentation/coremotion/getting_raw_accelerometer_events (accessed on 3 November 2023).
- Patton, M. Qualitative Research Methods and Evaluation: Integrating Theory into Practice, 4th ed.; Sage: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Bol, N.; Helberger, N.; Weert, J. Differences in mobile health app use: A source of new digital inequalities? Inf. Soc. 2018, 34, 183–193. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of Attrition and Dropout in App-Based Interventions for Chronic Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 229, e20283. [Google Scholar] [CrossRef]
- Floch, J.; Vilarinho, T.; Zettl, A.; Ibanez-Sanchez, G.; Calvo-Lerma, J.; Stav, E.; Haro, P.H.; Aalberg, A.L.; Fides-Valero, A.; Bayo Montón, J.L. Users’ Experiences of a Mobile Health Self-Management Approach for the Treatment of Cystic Fibrosis: Mixed Methods Study. JMIR mHealth uHealth 2020, 8, e15896. [Google Scholar] [CrossRef]
- Tabi, K.; Randhawa, A.S.; Choi, F.; Mithani, Z.; Albers, F.; Schnieder, M.; Nikoo, M.; Vigo, D.; Jang, K.; Demlova, R.; et al. Mobile Apps for Medication Management: Review and Analysis. JMIR mHealth uHealth 2019, 7, e13608. [Google Scholar] [CrossRef] [PubMed]
- Chédeville, G.; McGuire, K.; Cabral, D.A.; Shiff, N.J.; Rumsey, D.G.; Proulx-Gauthier, J.P.; Schmeling, H.; Berard, R.A.; Batthish, M.; Soon, G.; et al. Parent-Reported Medication Side Effects and Their Impact on Health-Related Quality of Life in Children With Juvenile Idiopathic Arthritis. Arthritis Care Res. 2022, 74, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Vandenberk, T.; Storms, V.; Lanssens, D.; De Cannière, H.; Smeets, C.J.; Thijs, I.M.; Batool, T.; Vanrompay, Y.; Vandervoort, P.M.; Grieten, L. A Vendor-Independent Mobile Health Monitoring Platform for Digital Health Studies: Development and Usability Study. JMIR mHealth uHealth 2019, 7, e12586. [Google Scholar] [CrossRef] [PubMed]
- Zens, M.; Grotejohann, B.; Tassoni, A.; Duttenhoefer, F.; Südkamp, N.P.; Niemeyer, P. Development of a Modular Research Platform to Create Medical Observational Studies for Mobile Devices. JMIR Res. Protoc. 2017, 6, e99. [Google Scholar] [CrossRef] [PubMed]
- Piwek, L.; Ellis, D.A.; Sally, A. Can Programming Frameworks Bring Smartphones into the Mainstream of Psychological Science? Front. Psychol. 2016, 7, 1252. [Google Scholar] [CrossRef] [PubMed]
- de Arriba-Pérez, F.; Caeiro-Rodríguez, M.; Santos-Gago, J.M. Collection and Processing of Data from Wrist Wearable Devices in Heterogeneous and Multiple-User Scenarios. Sensors 2016, 16, 1538. [Google Scholar] [CrossRef] [PubMed]
- Oughton, E.J.; Lehr, W.; Katsaros, K.; Selinis, I.; Bubley, D.; Kusuma, J. Revisiting Wireless Internet Connectivity: 5G vs. Wi-Fi 6. Telecommun. Policy 2021, 45, 102127. [Google Scholar] [CrossRef]
- Bos, G.; Lelieveld, O.T.; Armbrust, W.; Sauer, P.J.; Geertzen, J.H.; Dijkstra, P.U. Physical activity in children with Juvenile Idiopathic Arthritis compared to controls. Pediatr. Rheumatol. 2016, 14, 42. [Google Scholar] [CrossRef]
- Butler, S.; Sculley, D.; Santos, D.; Fellas, A.; Gironès, X.; Singh-Grewal, D.; Coda, A. Effectiveness of eHealth and mHealth Interventions Supporting Children and Young People Living With Juvenile Idiopathic Arthritis: Systematic Review and Meta-analysis. J. Med. Internet Res. 2022, 24, e30457. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, J.; Song, W.; Shen, Y. The Effectiveness of Wearable Devices as Physical Activity Interventions for Preventing and Treating Obesity in Children and Adolescents: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2022, 10, e32435. [Google Scholar] [CrossRef]
- Welch, V.; Wy, T.J.; Ligezka, A.; Hassett, L.; Croarkin, P.; Athreya, A.; Romanowicz, M. Use of Mobile and Wearable Artificial Intelligence in Child and Adolescent Psychiatry: Scoping Review. J. Med. Internet Res. 2022, 24, e33560. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, H.; Kim, R.; Lee, U.; Jeong, Y. Smartwatch Wearing Behavior Analysis: A Longitudinal Study. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–31. [Google Scholar] [CrossRef]
- Siepmann, C.; Kowalczuk, P. Understanding continued smartwatch usage: The role of emotional as well as health and fitness factors. Electron Mark. 2021, 31, 795–809. [Google Scholar] [CrossRef]
- Cibrian, F.L.; Monteiro, E.; Ankrah, E.; Beltran, J.A.; Tavakoulnia, A.; Schuck, S.E.B.; Hayes, G.R.; Lakes, K.D. Parents’ perspectives on a smartwatch intervention for children with ADHD: Rapid deployment and feasibility evaluation of a pilot intervention to support distance learning during COVID-19. PLoS ONE 2021, 16, e0258959. [Google Scholar] [CrossRef]
- Butler, S.; Sculley, D.; Santos, D.; Fellas, A.; Gironès, X.; Singh-Grewal, D.; Coda, A. Usability of eHealth and Mobile Health Interventions by Young People Living With Juvenile Idiopathic Arthritis: Systematic Review. JMIR Pediatr. Parent. 2020, 3, e15833. [Google Scholar] [CrossRef]
- Pew Internet Research Centre. Teens, Smartphones and Texting. 2012. Available online: https://www.pewresearch.org/internet/2012/03/19/teens-smartphones-texting/ (accessed on 1 November 2023).
- MacDougall, S.; Jerrott, S.; Clark, S.; Campbell, L.A.; Murphy, A.; Wozney, L. Text Message Interventions in Adolescent Mental Health and Addiction Services: Scoping Review. JMIR Mental Health 2021, 8, e16508. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.T.; Ponce, J.; Krönke, M.; Blower, S. Lack of ownership of mobile phones could hinder the rollout of mHealth interventions in Africa. eLife 2022, 11, e79615. [Google Scholar] [CrossRef] [PubMed]
- World Wide Web Foundation. Mobile Data Costs Fall but as Demand for Internet Services Surges, Progress Remains too Slow. 2021. Available online: https://webfoundation.org/2021/03/mobile-data-costs-fall-but-as-demand-for-internet-services-surges-progress-remains-too-slow/ (accessed on 1 November 2023).
- Bidargaddi, N.; Almirall, D.; Murphy, S.; Nahum-Shani, I.; Kovalcik, M.; Pituch, T.; Maaieh, H.; Strecher, V. To Prompt or Not to Prompt? A Microrandomized Trial of Time-Varying Push Notifications to Increase Proximal Engagement With a Mobile Health App. JMIR mHealth uHealth 2018, 6, e10123. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, N.; Benedetto, S. Driven by notifications—Exploring the effects of badge notifications on user experience. PLoS ONE 2022, 17, e0270888. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Ham, E.; Hilton, N.Z. Documentation of psychotropic pro re nata medication administration: An evaluation of electronic health records compared with paper charts and verbal reports. J. Clin. Nurs. 2018, 27, 3171–3178. [Google Scholar] [CrossRef]
- Mardani, A.; Paal, P.; Weck, C.; Jamshed, S.; Vaismoradi, M. Practical Considerations of PRN Medicines Management: An Integrative Systematic Review. Front. Pharmacol. 2022, 13, 759998. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.; Li, L.; Ouellette, C.; Hopkins, K.; McGillion, M.H.; Carter, N. Use of Synchronous Digital Health Technologies for the Care of Children With Special Health Care Needs and Their Families: Scoping Review. JMIR Pediatr. Parent. 2019, 2, e15106. [Google Scholar] [CrossRef] [PubMed]
- Mattison, G.; Canfell, O.J.; Forrester, D.; Dobbins, C.; Smith, D.; Reid, D.; Sullivan, C. A step in the right direction: The potential role of smartwatches in supporting chronic disease prevention in health care. Med. J. Aust. 2023, 218, 384–388. [Google Scholar] [CrossRef] [PubMed]
| Daily on the Web-Based Platform | When Support Required | |
|---|---|---|
| Pain | ||
| eVas [32,33,34] | ✓ a | |
| Medication adherence | ||
| Medication administration | ✓ | |
| Reasons for non-adherence | ✓ | |
| PRN medication administration | ✓ | |
| Physical activity levels | ||
| Kilojoules—light physical activity levels | ✓ | |
| Moderate-to-vigorous physical activity levels | ✓ | |
| Hourly stand goals | ✓ | |
| Watch wearing behaviour | ✓ | |
| Technical errors | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, S.; Sculley, D.; Santos, D.; Girones, X.; Singh-Grewal, D.; Coda, A. Using Digital Health Technologies to Monitor Pain, Medication Adherence and Physical Activity in Young People with Juvenile Idiopathic Arthritis: A Feasibility Study. Healthcare 2024, 12, 392. https://doi.org/10.3390/healthcare12030392
Butler S, Sculley D, Santos D, Girones X, Singh-Grewal D, Coda A. Using Digital Health Technologies to Monitor Pain, Medication Adherence and Physical Activity in Young People with Juvenile Idiopathic Arthritis: A Feasibility Study. Healthcare. 2024; 12(3):392. https://doi.org/10.3390/healthcare12030392
Chicago/Turabian StyleButler, Sonia, Dean Sculley, Derek Santos, Xavier Girones, Davinder Singh-Grewal, and Andrea Coda. 2024. "Using Digital Health Technologies to Monitor Pain, Medication Adherence and Physical Activity in Young People with Juvenile Idiopathic Arthritis: A Feasibility Study" Healthcare 12, no. 3: 392. https://doi.org/10.3390/healthcare12030392
APA StyleButler, S., Sculley, D., Santos, D., Girones, X., Singh-Grewal, D., & Coda, A. (2024). Using Digital Health Technologies to Monitor Pain, Medication Adherence and Physical Activity in Young People with Juvenile Idiopathic Arthritis: A Feasibility Study. Healthcare, 12(3), 392. https://doi.org/10.3390/healthcare12030392







