Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses
Abstract
1. Introduction
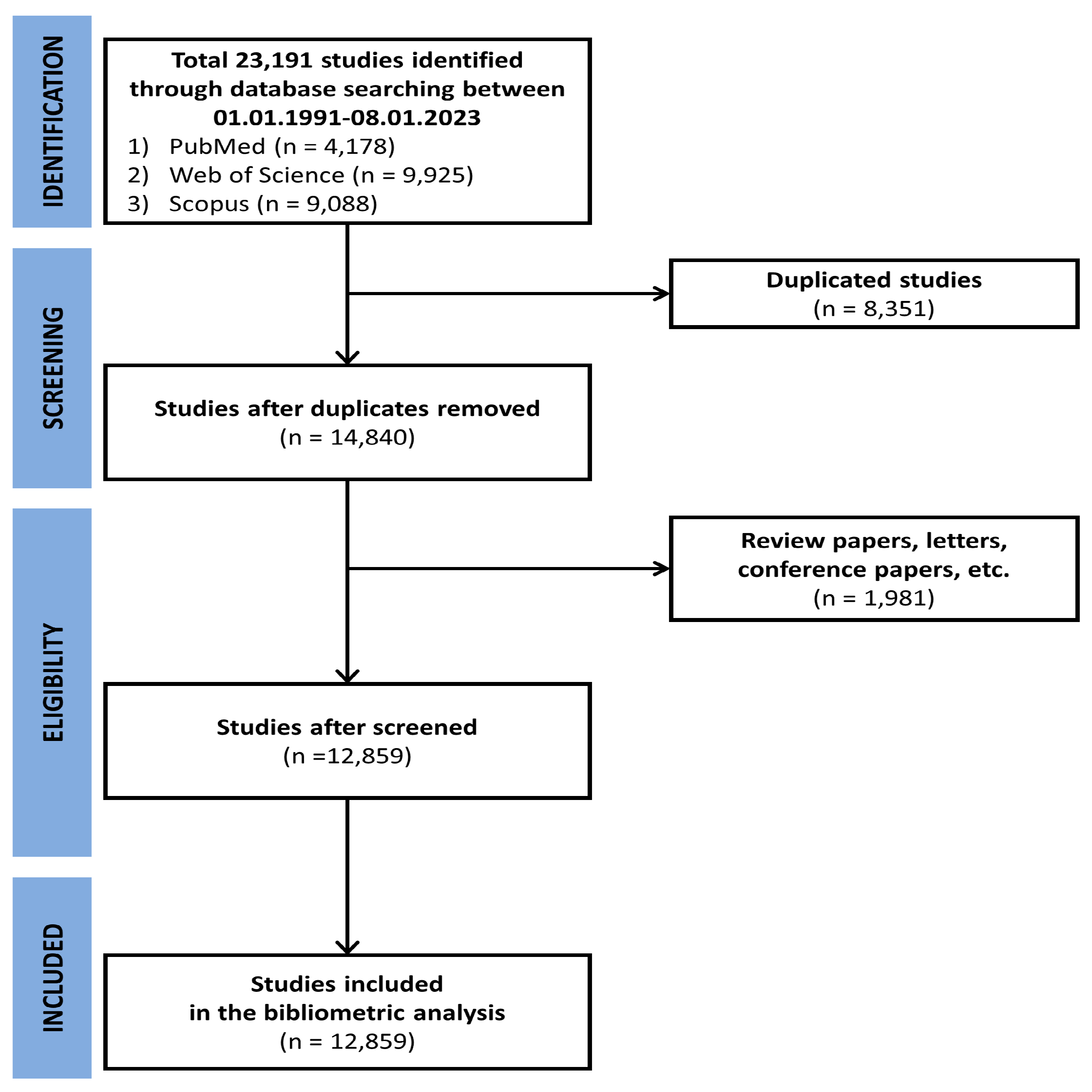
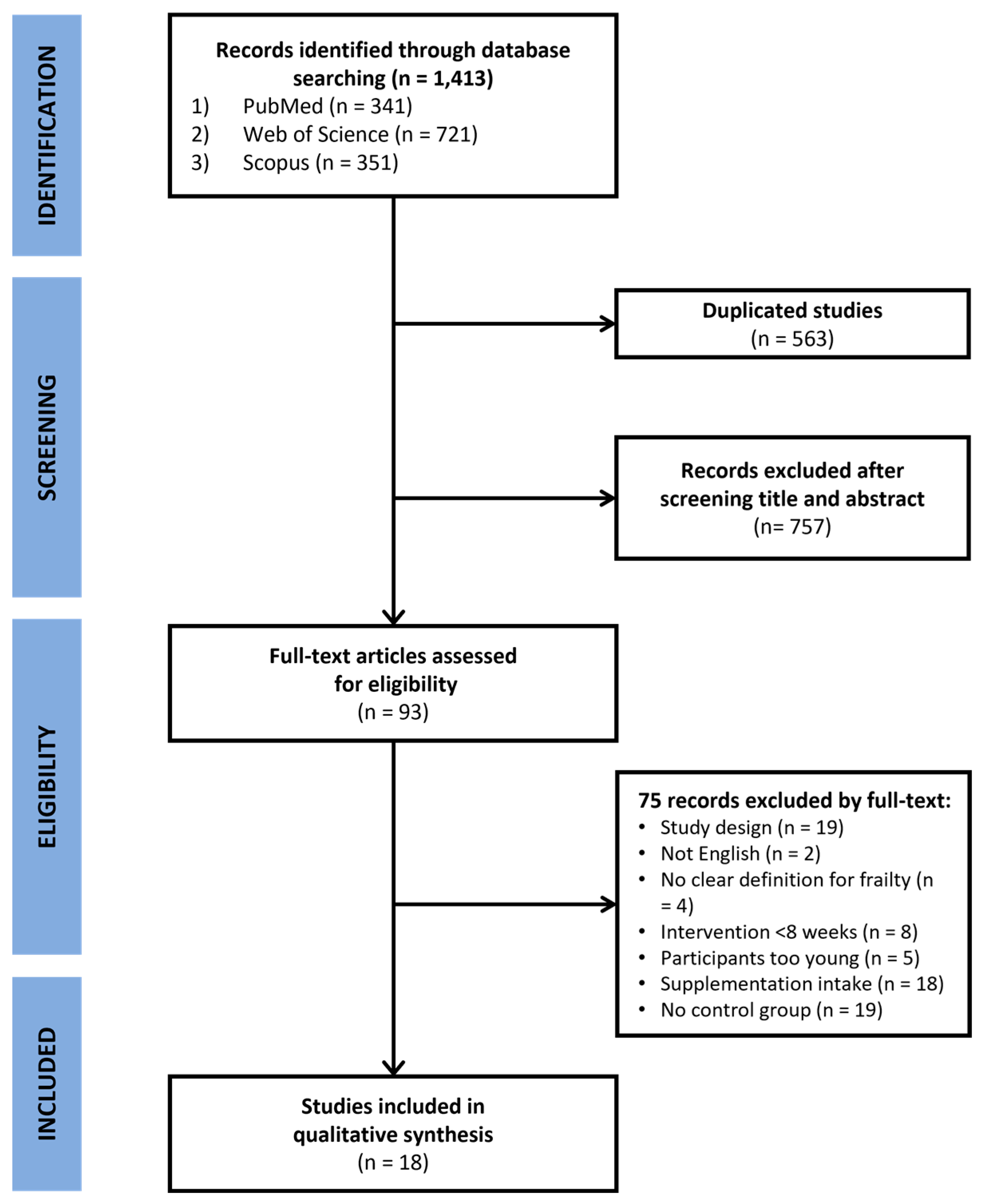
2. Materials and Methods
2.1. Bibliometric Analysis Method
2.2. Meta-Analysis Method
2.2.1. Data Extraction
2.2.2. Statistical Analysis
2.2.3. Quality Assessment
3. Results
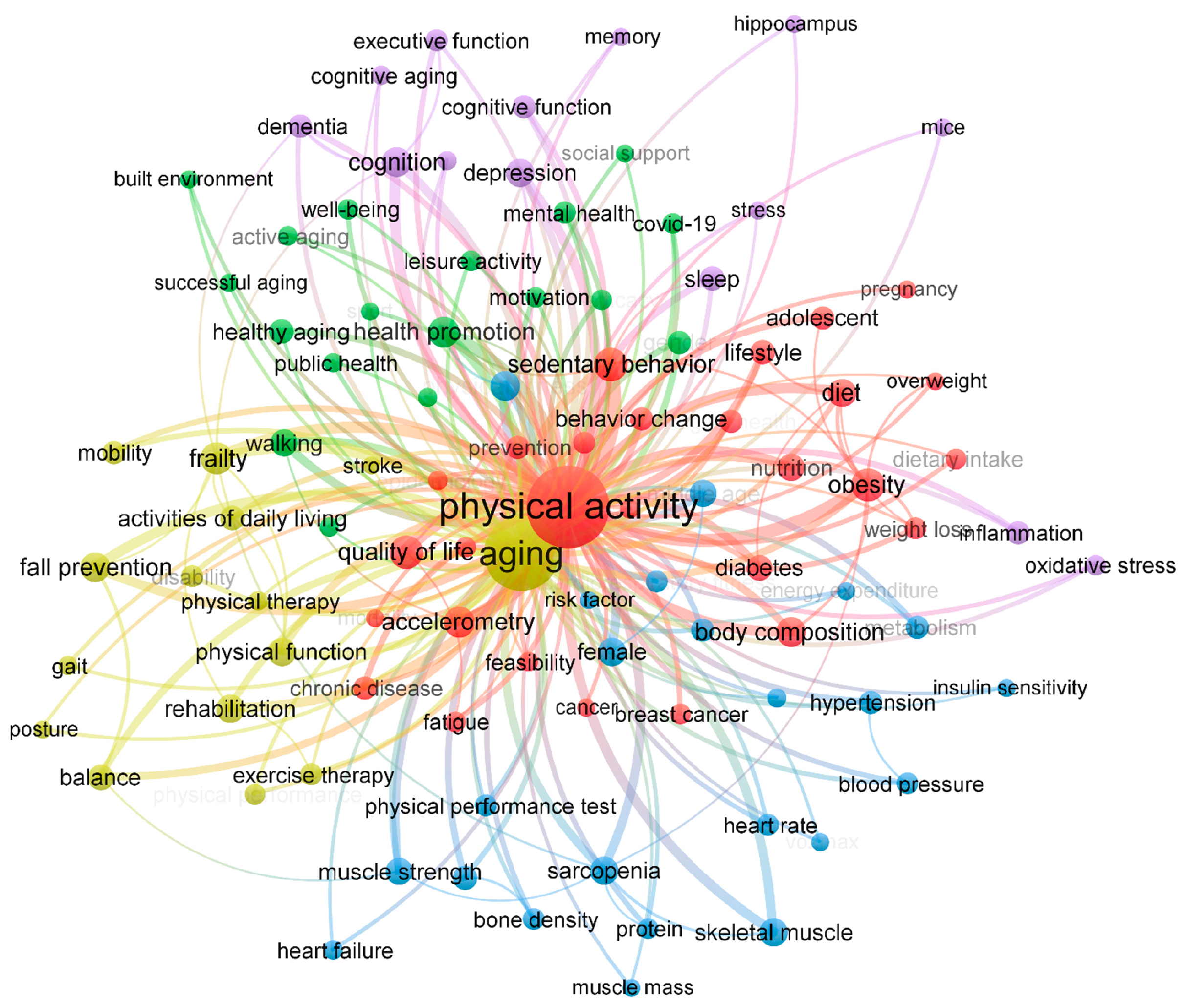
3.1. Bibliometric Analysis
3.1.1. Trend of Publication Year-Wise
3.1.2. Keyword Analysis
3.1.3. Trends in Keywords by Time
3.2. Meta-Analysis
3.2.1. Quality Check
3.2.2. Study Characteristics
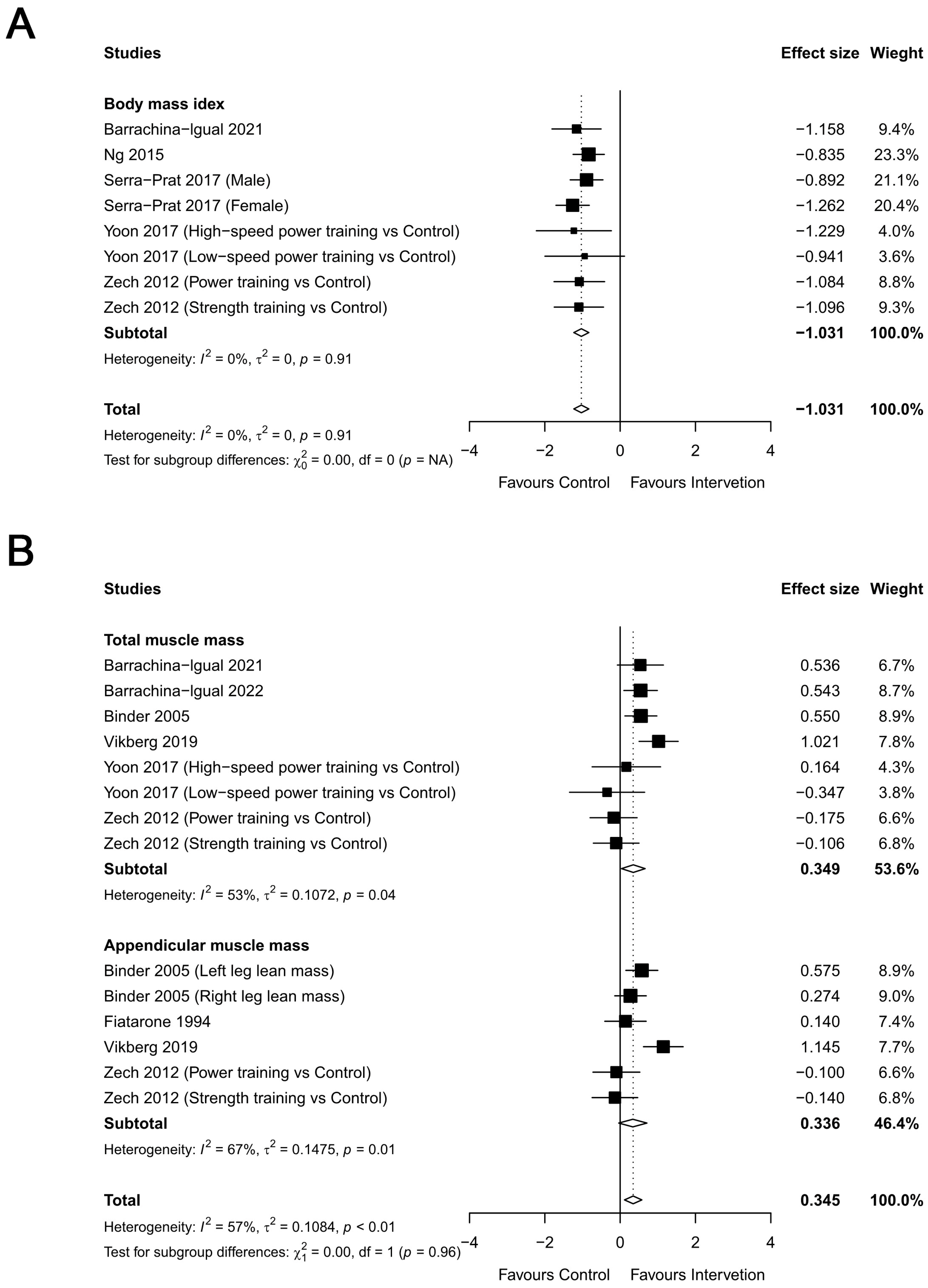
3.2.3. Body Composition
3.2.4. Muscular Strength
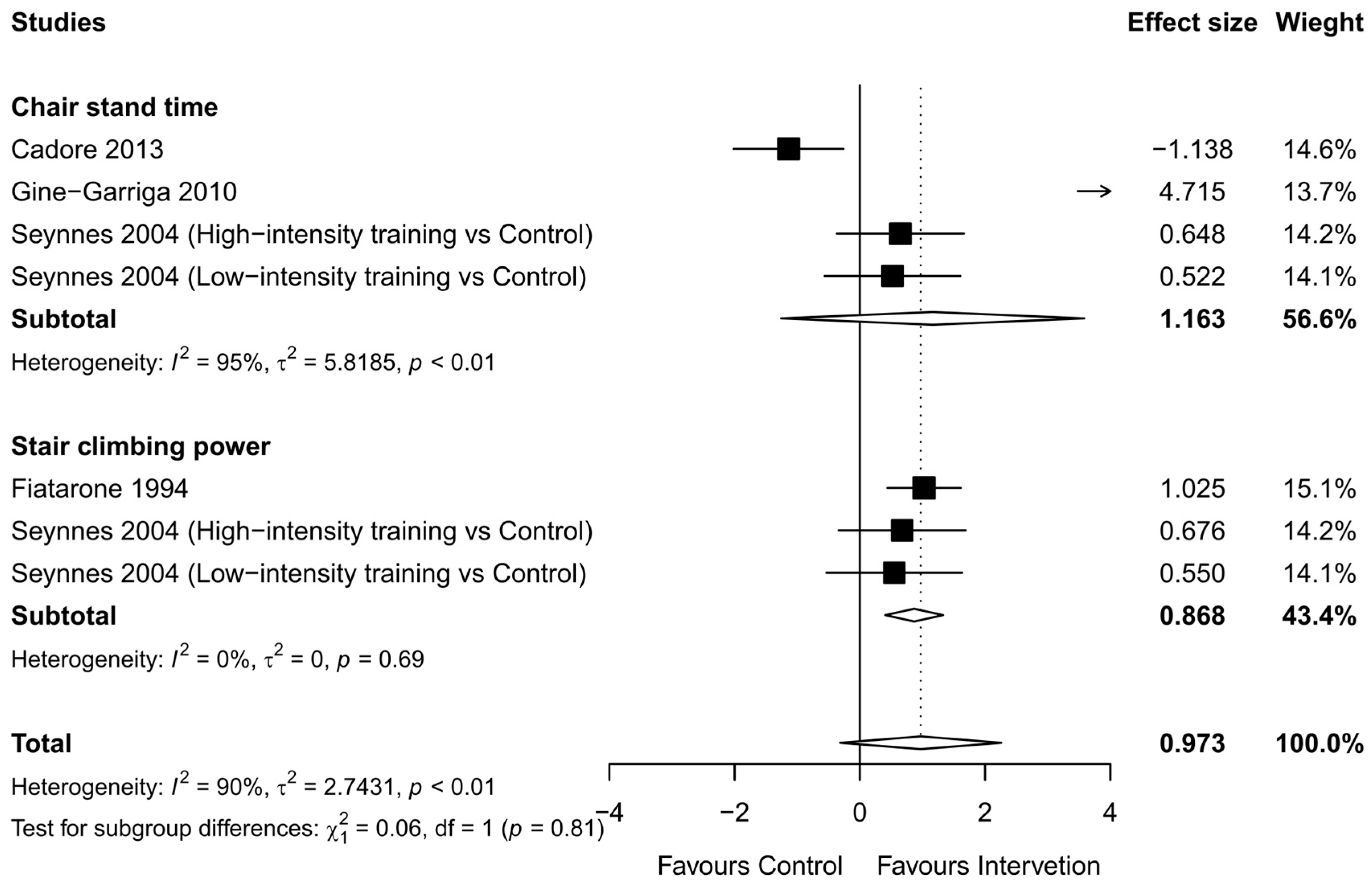
3.2.5. Physical Function
3.2.6. Functional Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Betancourt, L.; Sun, Y. Molecular Endocrinology and Physiology of the Aging Central Nervous System. Endocr. Rev. 2005, 26, 203–250. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- Seene, T.; Kaasik, P. Muscle weakness in the elderly: Role of sarcopenia, dynapenia, and possibilities for rehabilitation. Eur. Rev. Aging Phys. Act. 2012, 9, 109–117. [Google Scholar] [CrossRef]
- Duchowny, K.A.; Clarke, P.J.; Peterson, M.D. Muscle weakness and physical disability in older americans: Longitudinal findings from the U.S. health and retirement study. J. Nutr. Health Aging 2018, 22, 501–507. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Dixon, A. The united nations decade of healthy ageing requires concerted global action. Nat. Aging 2021, 1, 2. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in older persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Kuzuya, M. Process of physical disability among older adults—Contribution of frailty in the super-aged society. Nagoya J. Med. Sci. 2012, 74, 31–37. [Google Scholar]
- Garcia-Garcia, F.J.; Gutierrez Avila, G.; Alfaro-Acha, A.; Amor Andres, M.S.; De Los Angeles de la Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo study for healthy aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015.
- Jadczak, A.D.; Makwana, N.; Luscombe-Marsh, N.; Visvanathan, R.; Schultz, T.J. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: An umbrella review of systematic reviews. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 752–775. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Morley, J.E.; Izquierdo, M. Exercise, aging and frailty: Guidelines for increasing function. J. Nutr. Health Aging 2021, 25, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Di Lorito, C.; Long, A.; Byrne, A.; Harwood, R.H.; Gladman, J.R.F.; Schneider, S.; Logan, P.; Bosco, A.; van der Wardt, V. Exercise interventions for older adults: A systematic review of meta-analyses. J. Sport Health Sci. 2021, 10, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Levin, O.; Netz, Y.; Zivcorresponding, G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: A systematic review. Eur. Rev. Aging Phys. Act. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Rebelo-Marques, A.; De Sousa Lages, A.; Andrade, R.; Ribeiro, C.F.; Mota-Pinto, A.; Carrilho, F.; Espregueira-Mendes, J. Aging hallmarks: The benefits of physical exercise. Front. Endocrinol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Graham, Z.A.; Lavin, K.M.; O’Bryan, S.M.; Thalacker-Mercer, A.E.; Buford, T.W.; Ford, K.M.; Broderick, T.J.; Bamman, M.M. Mechanisms of exercise as a preventative measure to muscle wasting. Am. J. Physiol. Cell Physiol. 2021, 321, C40–C57. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Szychowska, A.; Drygas, W. Physical activity as a determinant of successful aging: A narrative review article. Aging Clin. Exp. Res. 2022, 34, 1209–1214. [Google Scholar] [CrossRef]
- Ellegaard, O.; Wallin, J.A. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef]
- Li, L.-L.; Ding, G.; Feng, N.; Wang, M.-H.; Ho, Y.-S. Global stem cell research trend: Bibliometric analysis as a tool for mapping of trends from 1991 to 2006. Scientometrics 2009, 80, 39–58. [Google Scholar] [CrossRef]
- Zitt, M.; Ramanana-Rahary, S.; Bassecoulard, E. Relativity of citation performance and excellence measures: From cross-field to cross-scale effects of field-normalisation. Scientometrics 2005, 63, 373–401. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual Version 1.6.18; Univeristeit Leiden: Leiden, The Netherlands, 2022; pp. 1–54. [Google Scholar]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Müller, S.; Scharhag, J. The intensity and effects of strength training in the elderly. Dtsch. Ärzteblatt Int. 2011, 108, 359. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef]
- Chandler, J.M.; Duncan, P.W.; Kochersberger, G.; Studenski, S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch. Phys. Med. Rehabil. 1998, 79, 24–30. [Google Scholar] [CrossRef]
- Binder, E.F.; Schechtman, K.B.; Ehsani, A.A.; Steger-May, K.; Brown, M.; Sinacore, D.R.; Yarasheski, K.E.; Holloszy, J.O. Effects of exercise training on frailty in community-dwelling older adults: Results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2002, 50, 1921–1928. [Google Scholar] [CrossRef]
- Seynnes, O.; Fiatarone Singh, M.A.; Hue, O.; Pras, P.; Legros, P.; Bernard, P.L. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J. Gerontol. Ser. A 2004, 59, M503–M509. [Google Scholar] [CrossRef]
- Binder, E.F.; Yarasheski, K.E.; Steger-May, K.; Sinacore, D.R.; Brown, M.; Schechtman, K.B.; Holloszy, J.O. Effects of progressive resistance training on body composition in frail older adults: Results of a randomized, controlled trial. J. Gerontol. Ser. A 2005, 60, 1425–1431. [Google Scholar] [CrossRef]
- Boshuizen, H.C.; Stemmerik, L.; Westhoff, M.H.; Hopman-Rock, M. The effects of physical therapists’ guidance on improvement in a strength-training program for the frail elderly. J. Aging Phys. Act. 2005, 13, 5–22. [Google Scholar] [CrossRef]
- Giné-Garriga, M.; Guerra, M.; Pagès, E.; Manini, T.M.; Jiménez, R.; Unnithan, V.B. The effect of functional circuit training on physical frailty in frail older adults: A randomized controlled trial. J. Aging Phys. Act. 2010, 18, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Zech, A.; Drey, M.; Freiberger, E.; Hentschke, C.; Bauer, J.M.; Sieber, C.C.; Pfeifer, K. Residual effects of muscle strength and muscle power training and detraining on physical function in community-dwelling prefrail older adults: A randomized controlled trial. BMC Geriatr. 2012, 12, 68. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Feng, L.; Nyunt, M.S.Z.; Feng, L.; Niti, M.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; Yap, P.; et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A Randomized Controlled Trial. Am. J. Med. 2015, 128, 1225–1236.e1221. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Sist, X.; Domenich, R.; Jurado, L.; Saiz, A.; Roces, A.; Palomera, E.; Tarradelles, M.; Papiol, M. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: A randomised controlled trial. Age Ageing 2017, 46, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Kang, D.; Kim, H.-j.; Kim, J.-S.; Song, H.S.; Song, W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr. Gerontol. Int. 2017, 17, 765–772. [Google Scholar] [CrossRef]
- Sahin, U.K.; Kirdi, N.; Bozoglu, E.; Meric, A.; Buyukturan, G.; Ozturk, A.; Doruk, H. Effect of low-intensity versus high-intensity resistance training on the functioning of the institutionalized frail elderly. Int. J. Rehabil. Res. 2018, 41, 211–217. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Chen, R.; Wu, Q.; Wang, D.; Li, Z.; Liu, H.; Liu, G.; Cui, Y.; Song, L. Effects of elastic band exercise on the frailty states in pre-frail elderly people. Physiother. Theory Pract. 2020, 36, 1000–1008. [Google Scholar] [CrossRef]
- Barrachina-Igual, J.; Martínez-Arnau, F.M.; Pérez-Ros, P.; Flor-Rufino, C.; Sanz-Requena, R.; Pablos, A. Effectiveness of the PROMUFRA program in pre-frail, community-dwelling older people: A randomized controlled trial. Geriatr. Nurs. 2021, 42, 582–591. [Google Scholar] [CrossRef]
- Barrachina-Igual, J.; Pablos, A.; Pérez-Ros, P.; Flor-Rufino, C.; Martínez-Arnau, F.M. Frailty status improvement after 5-month multicomponent program PROMUFRA in community-dwelling older people: A randomized controlled trial. J. Clin. Med. 2022, 11, 4077. [Google Scholar] [CrossRef] [PubMed]
- Swales, B.; Whittaker, A.; Ryde, G. P04-11 A physical activity intervention designed to improve multi-dimensional health and functional capacity of frail older adults in residential care: A randomised controlled feasibility study. Eur. J. Public Health 2022, 32 (Suppl. 2), ckac095-065. [Google Scholar] [CrossRef]
- Hedges, L.; Olkin, I. Statistical Methods in Meta-Analysis. J. Educ. Stat. 1985, 13, 75–78. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Çoğaltay, N.; Karadağ, E. Introduction to Meta-Analysis. In Leadership and Organizational Outcomes—Meta-Analysis of Empirical Studies; Springer International: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Waltman, L.; van Eck, N.J.; Noyons, E.C.M. A unified approach to mapping and clustering of bibliometric networks. J. Informetr. 2010, 4, 629–635. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Leeson, G.W. The growth, ageing and urbanisation of our world. J. Popul. Ageing 2018, 11, 107–115. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Dent, E. Trajectories, transitions, and trends in frailty among older adults: A review. Ann. Geriatr. Med. Res. 2022, 26, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O.; Godin, J.; Mayo, A.; Cahill, L.; Rockwood, K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Kirkland, J.L. Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 2016, 86, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Meeuwsen, S.; Horgan, G.W.; Elia, M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin. Nutr. 2010, 29, 560–566. [Google Scholar] [CrossRef]
- Monteil, D.; Walrand, S.; Vannier-Nitenberg, C.; Oost, B.V.; Bonnefoy, M. The relationship between frailty, obesity and social deprivation in non-institutionalized elderly people. J. Nutr. Health Aging 2020, 24, 821–826. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Koopman, R.; van Loon, L.J.C. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef]
- Dibble, L.E.; Hale, T.F.; Marcus, R.L.; Droge, J.; Gerber, J.P.; LaStayo, P.C. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov. Disord. 2006, 21, 1444–1452. [Google Scholar] [CrossRef]
- Denys, K.; Cankurtaran, M.; Janssens, W.; Petrovic, M. Metabolic syndrome in the elderly: An overview of the evidence. Acta Clin. Belg. 2009, 64, 23–34. [Google Scholar] [CrossRef]
- Bårdstu, H.B.; Andersen, V.; Fimland, M.S.; Raastad, T.; Saeterbakken, A.H. Muscle strength is associated with physical function in community-dwelling older adults receiving home care: A cross-sectional study. Front. Public Health 2022, 10, 856632. [Google Scholar] [CrossRef] [PubMed]
- Wickramarachchi, B.; Torabi, M.R.; Perera, B. Effects of physical activity on physical fitness and functional ability in older adults. Gerontol. Geriatr. Med. 2023, 9, 23337214231158476. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip strength and health in aging adults. Sports Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.F.; Leveille, S.G.; Kiely, D.K.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? J. Gerontol. Ser. A 2003, 58, M728–M733. [Google Scholar] [CrossRef]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving mobility in older adults with physical frailty and sarcopenia: Opportunities, challenges, and recommendations for physical activity interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Skelton, D.A.; Beyer, N. Exercise and injury prevention in older people. Scand. J. Med. Sci. Sports 2003, 13, 77–85. [Google Scholar] [CrossRef]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar]
- Thrane, G.; Joakimsen, R.M.; Thornquist, E. The association between timed up and go test and history of falls: The Tromsø study. BMC Geriatr. 2007, 7, 1. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 171–176. [Google Scholar] [CrossRef]
- Lemos, E.C.W.M.; Guadagnin, E.C.; Mota, C.B. Influence of strength training and multicomponent training on the functionality of older adults: Systematic review and meta-analysis. Rev. Bras. Cineantropometria Desempenho Hum. 2020, 22, e60707. [Google Scholar] [CrossRef]
- Henwood, T.R.; Taaffe, D.R. Short-term resistance training and the older adult: The effect of varied programmes for the enhancement of muscle strength and functional performance. Clin. Physiol. Funct. Imaging 2006, 26, 305–313. [Google Scholar] [CrossRef] [PubMed]

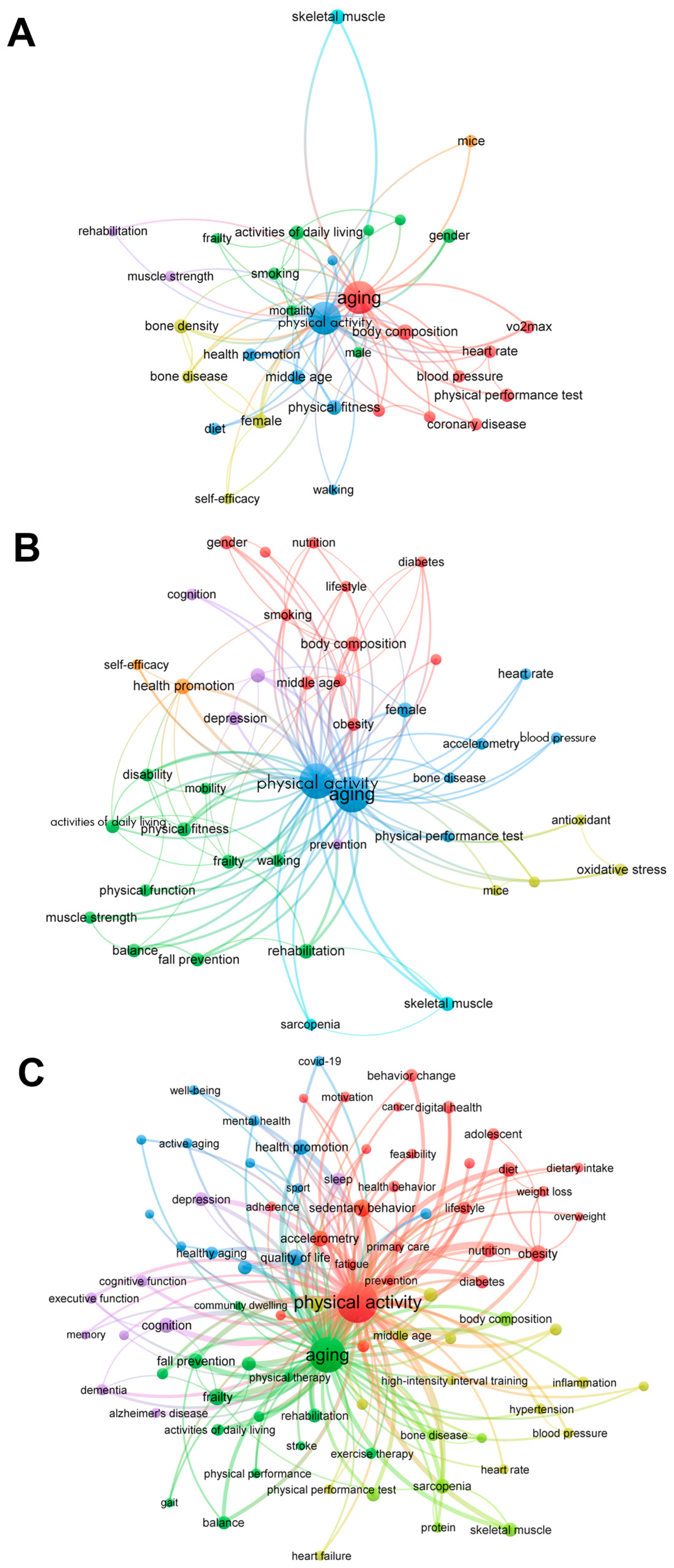


| 1991–2000 | 2001–2010 | 2011–August 2023 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Keyword | Occurrences | Total Link Strength | Keyword | Occurrences | Total Link Strength | Keyword | Occurrences | Total Link Strength | |
| 1 | body composition | 29 | 77 | body composition | 50 | 145 | sedentary behavior | 485 | 1290 |
| 2 | female | 25 | 68 | health promotion | 58 | 136 | obesity | 447 | 1225 |
| 3 | physical fitness | 24 | 68 | rehabilitation | 48 | 116 | quality of life | 443 | 1108 |
| 4 | middle age | 23 | 57 | quality of life | 41 | 115 | frailty | 424 | 1126 |
| 5 | activities of daily living | 19 | 56 | female | 46 | 113 | accelerometry | 356 | 951 |
| 6 | bone density | 19 | 51 | frailty | 43 | 103 | cognition | 329 | 899 |
| 7 | gender | 19 | 47 | physical fitness | 42 | 98 | health promotion | 326 | 902 |
| 8 | skeletal muscle | 23 | 45 | depression | 33 | 96 | fall prevention | 311 | 820 |
| 9 | health promotion | 15 | 45 | obesity | 31 | 92 | physical fitness | 283 | 760 |
| 10 | bone disease | 15 | 43 | skeletal muscle | 40 | 91 | diet | 279 | 807 |
| 11 | smoking | 13 | 43 | gender | 38 | 90 | body composition | 265 | 668 |
| 12 | heart rate | 15 | 38 | fall prevention | 37 | 90 | sarcopenia | 260 | 719 |
| 13 | VO2 max | 15 | 35 | balance | 33 | 83 | physical function | 256 | 688 |
| 14 | cardiovascular disease | 11 | 35 | disability | 29 | 82 | depression | 253 | 653 |
| 15 | risk factor | 10 | 35 | diet | 26 | 81 | rehabilitation | 252 | 619 |
| 16 | diet | 13 | 31 | smoking | 23 | 75 | skeletal muscle | 245 | 602 |
| 17 | blood pressure | 11 | 31 | middle age | 33 | 70 | nutrition | 228 | 666 |
| 18 | coronary disease | 11 | 28 | mobility | 20 | 68 | walking | 227 | 607 |
| 19 | longitudinal study | 8 | 27 | activities of daily living | 33 | 67 | female | 223 | 520 |
| 20 | mortality | 8 | 27 | walking | 27 | 64 | diabetes | 212 | 523 |
| 21 | male | 8 | 24 | muscle strength | 26 | 62 | muscle strength | 203 | 524 |
| 22 | physical performance test | 16 | 23 | physical function | 26 | 59 | balance | 192 | 500 |
| 23 | mice | 12 | 23 | nutrition | 19 | 58 | middle age | 182 | 360 |
| 24 | muscle strength | 8 | 23 | oxidative stress | 23 | 51 | healthy aging | 178 | 400 |
| 25 | leisure activity | 8 | 21 | cognition | 20 | 50 | lifestyle | 176 | 546 |
| 26 | self-efficacy | 8 | 17 | lifestyle | 18 | 50 | sleep | 166 | 445 |
| 27 | frailty | 7 | 17 | sarcopenia | 21 | 47 | digital health | 157 | 471 |
| 28 | walking | 7 | 16 | self-efficacy | 17 | 47 | behavior change | 156 | 412 |
| 29 | questionnaire | 7 | 16 | diabetes | 17 | 46 | adolescent | 152 | 369 |
| 30 | rehabilitation | 7 | 13 | prevention | 16 | 43 | activities of daily living | 143 | 392 |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | PEDro Score (0–10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiatarone et al., 1994 [27] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Chandler et al., 1998 [28] | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Binder et al., 2002 [29] | Y | Y | N | Y | N | N | Y | N | N | Y | Y | 5 |
| Seynnes et al., 2004 [30] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Binder et al., 2005 [31] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Boshuizen et al., 2005 [32] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Giné-Garriga et al., 2010 [33] | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | 6 |
| Zech et al., 2012 [34] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Cadore et al., 2014 [35] | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | 6 |
| Ng et al., 2015 [36] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Serra-Prat et al., 2017 [37] | Y | Y | Y | Y | N | N | N | N | N | Y | Y | 5 |
| Yoon et al., 2017 [38] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Sahin et al., 2018 [39] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Vikberg et al., 2019 [40] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Chen et al., 2020 [41] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Barrachina-Igual et al., 2021 [42] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Barrachina-Igual et al., 2022 [43] | Y | Y | N | Y | N | N | Y | N | N | Y | Y | 5 |
| Swales et al., 2022 [44] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Study (Year, Country) | N (IG/CG) | Age (Years) | Gender (M/F) | Duration (Frequency) | Intervention | CG |
|---|---|---|---|---|---|---|
| Fiatarone et al. 1994 (USA) [27] | 51 (25/26) | IG 86.2 ± 5.0 CG 89.2 ± 4.0 | 21/30 | 10 (3×/week) | Progressive lower extremity RT
| - |
| Chandler et al. 1998 (USA) [28] | 100 (50/50) | 77.6 ± 7.6 | 50/50 | 10 (3×/week) | Progressive lower extremity RT
| No RT was allowed, but aerobic or flexibility exercises were permitted. |
| Binder et al. 2002 (USA) [29] | 115 (66/49) | IG 83.0 ± 4.0 CG 83.0 ± 4.0 | 55/60 | 36 (2–3×/week) | Three approximately 3-month-long phases of ET
| Home-based exercise program focused primarily on flexibility (60 min, 2–3×/week) was performed. |
| Seynnes et al. 2004 (France) [30] | 22 (HI 8/LI 6/CG 8) | 81.5 | N/A | 10 (3×/week) | Progressive lower extremity RT
| - |
| Binder et al. 2005 (USA) [31] | 91 (53/38) | 83.0 ± 4.0 | 42/49 | 36 (3×/week) | Multi-component exercise program
| Home-based exercise program focused primarily on flexibility (60 min, 2–3×/week) was performed. |
| Boshuizen et al. 2005 (Netherlands) [32] | 72 (HG 24/MG 26/CG 22) | HG 80.0 ± 6.7 MG 79.3 ± 7.0 CG 77.2 ± 6.5 | 4/68 | 10 (3×/week) | Lower extremity RT
| - |
| Giné-Garriga et al. 2010 (Spain) [33] | 51 (26/25) | 84.0 ± 2.9 | 20/31 | 12 (2×/week) | Progressive lower extremity RT + balance training
| - |
| Zech et al. 2012 (Germany) [34] | 69 (ST 23/PT 24/CG 22) | ST 77.8 ± 6.1 PT 77.4 ± 6.2 CG 75.9 ± 7.8 | N/A | 12 (2×/week) | Progressive RT + balance training
| - |
| Cadore et al. 2014 (Spain) [35] | 24 (11/13) | IG 93.4 ± 3.2 CG 90.1 ± 1.1 | 7/14 | 12 (2×/week) | Multi-component exercise program
| Mobility exercises were performed 30 min per day (4×/week). |
| Ng et al. 2015 (Singapore) [36] | 98 (48/50) | 70.0 ± 4.7 | 43/55 | 24 (2×/week) | Progressive RT + functional tasks + balance training
| - |
| Serra-Prat et al. 2017 (Spain) [37] | 172 (80/92) | IG 77.9 ± 5.0 CG 78.8 ± 4.9 | 75/97 | 48 (4×/week) | Multi-component exercise program
| - |
| Yoon et al. 2017 (Korea) [38] | 58 (HSPT 19/LSST 19/CG 20) | HSPT 75.0 ± 0.9 LSST 76.0 ± 1.3 CG 78.0 ± 1.0 | 0/58 | 12 (2×/week) | Progressive RT with elastic bands
| Static and dynamic stretching exercises (60 min, 1×/week) were performed. |
| Sahin et al. 2018 (Turkey) [39] | 48 (HI 16/LI 16/CG 16) | HI 84.2 ± 6.9 LI 84.5± 4.8 CG 85.4 ± 4.7 | N/A | 8 (3×/week) | Whole body RT
| - |
| Vikberg et al. 2019 (Sweden) [40] | 70 (36/34) | 70.9 ± 0.03 | 32/38 | 10 (3×/week) | Whole body RT, with a focus on strengthening of the lower-extremity
| - |
| Chen et al. 2020 (China) [41] | 70 (35/35) | IG 77.0 ± 5.2 CG 75.3 ± 6.0 | 23/43 | 8 (3×/week) | Whole body RT with elastic bands
| - |
| Barrachina-Igual et al. 2021 (Spain) [42] | 50 (27/23) | 75.0 ± 7.0 | 13/37 | 12 (2×/week) | Multi-component exercise program
| - |
| Barrachina-Igual et al. 2022 (Spain) [43] | 81 (39/42) | 77.6 ± 7.5 | 13/68 | 20 (2×/week) | Multi-component exercise program
| Continue their routine daily activities. |
| Swales et al. 2022 (UK) [44] | 11 (6/5) | 86.1 ± 7.2 | 4/7 | 6 (3×/week) | RT
| - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kim, D.; Kim, S.K. Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses. Healthcare 2024, 12, 197. https://doi.org/10.3390/healthcare12020197
Choi Y, Kim D, Kim SK. Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses. Healthcare. 2024; 12(2):197. https://doi.org/10.3390/healthcare12020197
Chicago/Turabian StyleChoi, Yerim, Daekyoo Kim, and Seung Kyum Kim. 2024. "Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses" Healthcare 12, no. 2: 197. https://doi.org/10.3390/healthcare12020197
APA StyleChoi, Y., Kim, D., & Kim, S. K. (2024). Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses. Healthcare, 12(2), 197. https://doi.org/10.3390/healthcare12020197





