Delivery of Allied Health Interventions Using Telehealth Modalities: A Rapid Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
- How effective are allied health interventions delivered using telehealth modalities compared with interventions delivered face-to-face of a comparable dose?
- What are the telehealth modalities and interventions used by allied health clinicians?
- What outcomes are influenced (impairment, activity limitation, participation)?
- Are there differences in adherence and safety between telehealth and face-to-face delivery?
2. Method
2.1. Identification and Selection of Trials
| Design: |
|
| Participants: |
|
Intervention: allied health intervention delivered via telehealth
|
|
Comparison: face-to-face allied health intervention
|
|
| Outcome measures: |
|
|
| Exclusion criteria: |
|
|
|
2.2. Assessment of Quality of Trials
2.3. Data Extraction and Analysis
3. Results
3.1. The Flow of Trials through the Review
3.2. Characteristics of Included Trials
3.3. Technologies Used
3.4. Risk of Bias
3.5. Design and Effects of Neuropsychology Interventions
3.6. Design and Effects of Occupational Therapy Interventions
3.6.1. Cognition
3.6.2. Burden of Care
3.6.3. Motor Outcomes
3.7. Design and Effects of Physiotherapy Interventions
3.7.1. Joint Range of Motion
3.7.2. Strength
3.7.3. Pain
3.7.4. Gait and Physical Activity
3.7.5. Balance
Exercise Capacity
3.7.6. Function and Disability
3.7.7. Anxiety and Depression
3.7.8. Health-Related Quality of Life
3.8. Design and Effects of Psychology Interventions
3.8.1. Depression
3.8.2. Anxiety
3.8.3. PTSD
3.8.4. Pain
3.8.5. Binge Eating and Purging
3.8.6. Health-Related Quality of Life
3.9. Design and Effects of Speech Pathology Interventions
3.9.1. Cognition–Communication
3.9.2. Communication
3.9.3. Health-Related Quality of Life
3.10. Design and Effects of Podiatry Interventions
3.11. Allied Health: Adverse Events, Adherence, and Satisfaction
3.11.1. Study-Related and Possibly Study-Related Adverse Events
3.11.2. Adherence
3.11.3. Satisfaction
3.12. Feasibility of Telehealth in Allied Health
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| HRQoL | Health-Related Quality of Life |
| CBT | Cognitive Behavioral Therapy |
| PTSD | Post-Traumatic Stress Disorder |
| CPT | Cognitive Processing Therapy |
| PET | Prolonged Exposure Therapy |
References
- Ryu, S. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth 2009 (Global Observatory for eHealth Series, Volume 2). Healthc. Inform. Res. 2012, 18, 153–155. [Google Scholar] [CrossRef]
- Kruse, C.; Fohn, J.; Wilson, N.; Nunez Patlan, E.; Zipp, S.; Mileski, M. Utilization Barriers and Medical Outcomes Commensurate with the Use of Telehealth Among Older Adults: Systematic Review. JMIR Med. Inform. 2020, 8, e20359. [Google Scholar] [CrossRef] [PubMed]
- Hickey, S.; Gomez, J.; Meller, B.; Schneider, J.C.; Cheney, M.; Nejad, S.; Schulz, J.; Goverman, J. Interactive home telehealth and burns: A pilot study. Burns 2017, 43, 1318–1321. [Google Scholar] [CrossRef]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and Facilitators That Influence Telemedicine-Based, Real-Time, Online Consultation at Patients’ Homes: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef]
- Tabachnick, N.; Klugman, D.J. No Name-A Study of Anonymous Suicidal Telephone Calls. Psychiatry 1965, 28, 79–88. [Google Scholar] [CrossRef]
- Antonioni, D.T. A Field Study Comparison of Counselor Empathy, Concreteness and Client Self-Exploration in Face-to-Face and Telephone Counseling during First and Second Interviews. Ph.D. Thesis, The University of Wisconsin-Madison, Ann Arbor, MI, USA, 1973. [Google Scholar]
- Miller, W.B. The telephone in outpatient psychotherapy. Am. J. Psychother. 1973, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.M.; Russell, T.G.; Gray, L.C. Feasibility of using an in-home video conferencing system in geriatric rehabilitation. J. Rehabil. Med. 2011, 43, 364–366. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Gregersen, M.; Pallesen, S.H.; Damsgaard, E.M.; Damsgaard, E.M. A group-based real-time videoconferencing telerehabilitation programme in recently discharged geriatric patients: A feasibility study. Eur. Geriatr. Med. 2021, 12, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Appleby, E.; Gill, S.T.; Hayes, L.K.; Walker, T.L.; Walsh, M.; Kumar, S. Effectiveness of telerehabilitation in the management of adults with stroke: A systematic review. PLoS ONE 2019, 14, e0225150. [Google Scholar] [CrossRef]
- Grona, S.L.; Bath, B.; Busch, A.; Rotter, T.; Trask, C.; Harrison, E. Use of videoconferencing for physical therapy in people with musculoskeletal conditions: A systematic review. J. Telemed. Telecare 2018, 24, 341–355. [Google Scholar] [CrossRef]
- Axelsson, E.; Hedman-Lagerlof, E. Cognitive behavior therapy for health anxiety: Systematic review and meta-analysis of clinical efficacy and health economic outcomes. Expert Rev. Pharmacoeconomics Outcomes Res. 2019, 19, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Adey-Wakeling, Z.; Crotty, M.; Lannin, N.A.; George, S.; Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020, 1, CD010255. [Google Scholar] [CrossRef] [PubMed]
- Coughtrey, A.E.; Pistrang, N. The effectiveness of telephone-delivered psychological therapies for depression and anxiety: A systematic review. J. Telemed. Telecare 2018, 24, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ownsworth, T.; Arnautovska, U.; Beadle, E.; Shum, D.H.K.; Moyle, W. Efficacy of Telerehabilitation for Adults with Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2018, 33, E33–E46. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.; Lowman, J. Telepractice for Adult Speech-Language Pathology Services: A Systematic Review. Perspect. ASHA Spec. Interest Groups 2020, 5, 326–338. [Google Scholar] [CrossRef]
- Caristia, S.; Ferranti, M.; Skrami, E.; Raffetti, E.; Pierannunzio, D.; Palladino, R.; Carle, F.; Saracci, R.; Badaloni, C.; Barone-Adesi, F.; et al. Effect of national and local lockdowns on the control of COVID-19 pandemic: A rapid review. Epidemiol. Prev. 2020, 44, 60–68. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, C.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2019. [Google Scholar]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the International Health Informatics Symposium, Miami, FL, USA, 28–30 January 2012. [Google Scholar]
- Giummarra, M.J.; Lau, G.; Gabbe, B.J. Evaluation of text mining to reduce screening workload for injury-focused systematic reviews. Inj. Prev. 2020, 26, 55–60. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health. 2021. Available online: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health (accessed on 14 October 2020).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, rev ed.; Lindner, R., Ed.; Routledge: New York, NY, USA, 1988; Volume 67, p. 1007. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Moher, D.L.; Tetzlaff., J.A.; Altman, D.G. The PRISMA Group, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Chen, J.; Jin, W.; Dong, W.S.; Jin, Y.; Qiao, F.L.; Zhou, Y.F.; Ren, C.C. Effects of Home-based Telesupervising Rehabilitation on Physical Function for Stroke Survivors with Hemiplegia: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 152–160. [Google Scholar] [CrossRef]
- Jelcic, N.; Agostini, M.; Meneghello, F.; Bussè, C.; Parise, S.; Galano, A.; Tonin, P.; Dam, M.; Cagnin, A. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: A pilot study. Clin. Interv. Aging 2014, 9, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Chen, C.-H.; Chen, Y.-Y.; Huang, W.-T.; Lai, J.-S.; Yu, S.-M.; Chang, Y.-J. Bidirectional and multi-user telerehabilitation system: Clinical effect on balance, functional activity, and satisfaction in patients with chronic stroke living in long-term care facilities. Sensors 2014, 14, 12451–12466. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Ho, J.; Duffecy, J.; Reifler, D.; Sokol, L.; Burns, M.N.; Jin, L.; Siddique, J. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: A randomized trial. Jama 2012, 307, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, M.; Marco, E.; Coll, M.; Escalada, F.; Ballester, A.; Cinca, C.; Belmonte, R.; Muniesa, J.M. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthroplasty: A randomized controlled trial. J. Rehabil. Med. 2013, 45, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Lum, P.S.; Brennan, D.M.; Barman, J.; Bowman, M.H.; Taylor, A.; Mckay, S.; Sloman, S.B.; Morris, D.M.; et al. Tele-rehabilitation of upper-extremity hemiparesis after stroke: Proof-of-concept randomized controlled trial of in-home constraint-induced movement therapy. Restor. Neurol. Neurosci. 2021, 39, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Topfer, N.F.; Wrede, N.; Theurer, C.; Wilz, G. Face-to-face versus telephone-based cognitive-behavioral therapy for family caregivers of people with dementia. J. Clin. Psychol. 2023, 79, 2270–2287. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.; Liu, E.; Clemson, L.; Davies, O.; Gray, L.; Gitlin, L.N.; Crotty, M. Does Telehealth Delivery of a Dyadic Dementia Care Program Provide a Noninferior Alternative to Face-To-Face Delivery of the Same Program? A Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry 2020, 28, 673–682. [Google Scholar] [CrossRef]
- Sanford, J.A.; Griffiths, P.C.; Richardson, P.; Hargraves, K.; Butterfield, T.; Hoenig, H. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: A randomized clinical trial. J. Am. Geriatr. Soc. 2006, 54, 1641–1648. [Google Scholar] [CrossRef]
- Torpil, B.; Pekcetin, E.; Pekcetin, S. The effectiveness of cognitive rehabilitation intervention with the telerehabilitation method for amnestic mild cognitive impairment: A feasibility randomized controlled trial. J. Telemed. Telecare 2023. [Google Scholar] [CrossRef]
- Aily, J.B.; de Noronha, M.; Approbato Selistre, L.F.; Ferrari, R.J.; White, D.K.; Mattiello, S.M. Face-to-face and telerehabilitation delivery of circuit training have similar benefits and acceptability in patients with knee osteoarthritis: A randomised trial. J. Physiother. 2023. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Dosbaba, F.; Hartman, M.; Batalikova, K.; Spinar, J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: A randomized controlled trial. Medicine 2020, 99, e19556. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A.; Mahajan, J. Clinical outcomes of remote asynchronous telerehabilitation are equivalent to traditional therapy following total knee arthroplasty: A randomized control study. J. Telemed. Telecare 2017, 23, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.S.; McDonald, C.F.; Mahal, A.; Alison, J.A.; Wootton, R.; Hill, C.J.; Zanaboni, P.; O’Halloran, P.; Bondarenko, J.; Macdonald, H.; et al. Telerehabilitation for chronic respiratory disease: A randomised controlled equivalence trial. Thorax 2022, 77, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Dadarkhah, A.; Rezaimoghadam, F.; Najafi, S.; Mohebi, B.; Azarakhsh, A.; Rezasoltani, Z. Remote Versus in-Person Exercise Instruction for Chronic Nonspecific Low Back Pain Lasting 12 Weeks or Longer: A Randomized Clinical Trial. J. Natl. Med. Assoc. 2021, 113, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Dias Correia, F.; Nogueira, A.; Magalhães, I.; Guimarães, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Pires, J.; Seabra, R.; et al. Digital Versus Conventional Rehabilitation After Total Hip Arthroplasty: A Single-Center, Parallel-Group Pilot Study. JMIR Rehabil. Assist. Technol. 2019, 6, e14523. [Google Scholar] [CrossRef] [PubMed]
- Dias Correia, F.; Molinos, M.; Luis, S.; Carvalho, D.; Carvalho, C.; Costa, P.; Seabra, R.; Francisco, G.; Bento, V.; Lains, J. Digitally Assisted Versus Conventional Home-Based Rehabilitation After Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2022, 101, 237–249. [Google Scholar] [CrossRef]
- Doiron-Cadrin, P.; Kairy, D.; Vendittoli, P.A.; Lowry, V.; Poitras, S.; Desmeules, F. Feasibility and preliminary effects of a tele-prehabilitation program and an in-person prehablitation program compared to usual care for total hip or knee arthroplasty candidates: A pilot randomized controlled trial. Disabil. Rehabil. 2020, 42, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Preston, E.; Dennis, S.; Canning, C.G.; Allen, N.E. Home-based exercise monitored with telehealth is feasible and acceptable compared to centre-based exercise in Parkinson’s disease: A randomised pilot study. Clin. Rehabil. 2021, 35, 728–739. [Google Scholar] [CrossRef]
- Godtfredsen, N.; Frolich, A.; Bieler, T.; Beyer, N.; Kallemose, T.; Wilcke, T.; Ostergaard, L.; Andreassen, H.F.; Martinez, G.; Lavesen, M.; et al. 12-months follow-up of pulmonary tele-rehabilitation versus standard pulmonary rehabilitation: A multicentre randomised clinical trial in patients with severe COPD. Respir. Med. 2020, 172, 106129. [Google Scholar] [CrossRef]
- Hansen, H.; Bieler, T.; Beyer, N.; Kallemose, T.; Wilcke, J.T.; Ostergaard, L.M.; Frost Andeassen, H.; Martinez, G.; Lavesen, M.; Frolich, A.; et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: A randomised multicentre trial. Thorax 2020, 75, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Mahal, A.; Hill, C.J.; Lee, A.L.; Burge, A.T.; Cox, N.S.; Moore, R.; Nicolson, C.; O’Halloran, P.; Lahham, A.; et al. Home-based rehabilitation for COPD using minimal resources: A randomised, controlled equivalence trial. Thorax 2017, 72, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Bruning, J.; Morris, N.R.; Mandrusiak, A.; Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: A randomised trial. J. Physiother. 2017, 63, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kraal, J.J.; Peek, N.; Van den Akker-Van Marle, M.E.; Kemps, H.M. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: Short-term results of the FIT@Home study. Eur. J. Prev. Cardiol. 2014, 21, 26–31. [Google Scholar] [CrossRef]
- Kraal, J.J.; Van den Akker-Van Marle, M.E.; Abu-Hanna, A.; Stut, W.; Peek, N.; Kemps, H.M. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur. J. Prev. Cardiol. 2017, 24, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, usability, and cost-benefit of a virtual reality-based telerehabilitation program for balance recovery after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e412. [Google Scholar] [CrossRef] [PubMed]
- Moffet, H.; Tousignant, M.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Belzile, É.L.; Ranger, P.; et al. Patient Satisfaction with In-Home Telerehabilitation After Total Knee Arthroplasty: Results from a Randomized Controlled Trial. Telemed. J. e-Health 2017, 23, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Moffet, H.; Tousignant, M.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Ranger, P.; Belzile, É.L.; et al. In-Home Telerehabilitation Compared with Face-to-Face Rehabilitation After Total Knee Arthroplasty: A Noninferiority Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2015, 97, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, D.; Pazzinatto, M.F.; Crossley, K.M.; Azevedo, F.M.; Barton, C.J. Novel Stepped Care Approach to Provide Education and Exercise Therapy for Patellofemoral Pain: Feasibility Study. J. Med. Internet Res. 2020, 22, e18584. [Google Scholar] [CrossRef]
- Odole, A.; Ojo, O. Is Telephysiotherapy an Option for Improved Quality of Life in Patients with Osteoarthritis of the Knee? Int. J. Telemed. Appl. 2014, 2014, 903816. [Google Scholar] [CrossRef]
- Odole, A.C.; Ojo, O.D. A Telephone-based Physiotherapy Intervention for Patients with Osteoarthritis of the Knee. Int. J. Telerehabilitation 2013, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Onan, D.; Ulger, O.; Martelletti, P. Effects of spinal stabilization exercises delivered using telerehabilitation on outcomes in patients with chronic neck pain: A randomized controlled trial. Expert Rev. Neurother. 2023, 23, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Paratz, J.; Cottrell, M. A six-week physical therapy exercise program delivered via home-based telerehabilitation is comparable to in-person programs for patients with burn injuries: A randomized, controlled, non-inferiority clinical pilot trial. Burn. J. Int. Soc. Burn Inj. 2023, 49, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Tarakci, E.; Tarakci, D.; Hajebrahimi, F.; Budak, M. Supervised exercises versus telerehabilitation. Benefits for persons with multiple sclerosis. Acta Neurol. Scand. 2021, 144, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Low Choy, N.L.; Brauer, S.G. Center-Based Group and Home-Based Individual Exercise Programs Have Similar Impacts on Gait and Balance in People with Multiple Sclerosis: A Randomized Trial. PM&R 2020, 13, 9–18. [Google Scholar]
- Chavooshi, B.; Mohammadkhani, P.; Dolatshahee, B. Telemedicine vs. in-person delivery of intensive short-term dynamic psychotherapy for patients with medically unexplained pain: A 12-month randomized, controlled trial. J. Telemed. Telecare 2017, 23, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Bombardier, C.H.; Vannoy, S.; Dyer, J.; Ludman, E.; Dikmen, S.; Marshall, K.; Barber, J.; Temkin, N. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: A randomized controlled trial. J. Neurotrauma 2015, 32, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Germain, V.; Marchand, A.; Bouchard, S.; Drouin, M.S.; Guay, S. Effectiveness of Cognitive Behavioural Therapy Administered by Videoconference for Posttraumatic Stress Disorder. Cogn. Behav. Ther. 2009, 38, 42–53. [Google Scholar] [CrossRef]
- Herbert, M.S.; Afari, N.; Liu, L.; Heppner, P.; Rutledge, T.; Williams, K.; Eraly, S.; VanBuskirk, K.; Nguyen, C.; Bondi, M.; et al. Telehealth Versus In-Person Acceptance and Commitment Therapy for Chronic Pain: A Randomized Noninferiority Trial. J. Pain 2017, 18, 200–211. [Google Scholar] [CrossRef]
- Himelhoch, S.; Medoff, D.; Maxfield, J.; Dihmes, S.; Dixon, L.; Robinson, C.; Potts, W.; Mohr, D.C. Telephone based cognitive behavioral therapy targeting major depression among urban dwelling, low income people living with HIV/AIDS: Results of a randomized controlled trial. Aids Behav. 2013, 17, 2756–2764. [Google Scholar] [CrossRef]
- Karagiozi, K.; Margaritidou, P.; Tsatali, M.; Marina, M.; Dimitriou, T.; Apostolidis, H.; Tsiatsos, T.; Tsolaki, M. Comparison of on Site versus Online Psycho Education Groups and Reducing Caregiver Burden. Clin. Gerontol. 2022, 45, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Thorp, S.R.; Moreno, L.; Wells, S.Y.; Glassman, L.H.; Busch, A.C.; Zamora, T.; Rodgers, C.S.; Allard, C.B.; Morland, L.A.; et al. Videoconferencing psychotherapy for veterans with PTSD: Results from a randomized controlled non-inferiority trial. J. Telemed. Telecare 2020, 26, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Lleras de Frutos, M.; Medina, J.C.; Vives, J.; Casellas-Grau, A.; Marzo, J.L.; Borras, J.M.; Ochoa-Arnedo, C. Video conference vs face-to-face group psychotherapy for distressed cancer survivors: A randomized controlled trial. Psycho-Oncol. 2020, 29, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Luxton, D.D.; Pruitt, L.D.; Wagner, A.; Smolenski, D.J.; Jenkins-Guarnieri, M.A.; Gahm, G. Home-based telebehavioral health for U.S. military personnel and veterans with depression: A randomized controlled trial. J. Consult. Clin. Psychol. 2016, 84, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Maieritsch, K.P.; Smith, T.L.; Hessinger, J.D.; Ahearn, E.P.; Eickhoff, J.C.; Zhao, Q. Randomized controlled equivalence trial comparing videoconference and in person delivery of cognitive processing therapy for PTSD. J. Telemed. Telecare 2015, 22, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Beaulieu-Prévost, D.; Guay, S.; Bouchard, S.; Drouin, M.S.; Germain, V. Relative Efficacy of Cognitive-Behavioral Therapy Administered by Videoconference for Posttraumatic Stress Disorder: A Six-Month Follow-Up. J. Aggress. Maltreatment Trauma 2011, 20, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Marrone, S.; Mitchell, J.E.; Crosby, R.; Wonderlich, S.; Jollie-Trottier, T. Predictors of response to cognitive behavioral treatment for bulimia nervosa delivered via telemedicine versus face-to-face. Int. J. Eat. Disord. 2009, 42, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.E.; Crosby, R.D.; Wonderlich, S.A.; Crow, S.; Lancaster, K.; Simonich, H.; Swan-Kremeier, L.; Lysne, C.; Cook Myers, T. A randomized trial comparing the efficacy of cognitive–behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav. Res. Ther. 2008, 46, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Morland, L.A.; Pierce, K.; Wong, M.Y. Telemedicine and coping skills groups for Pacific Island veterans with post-traumatic stress disorder: A pilot study. J. Telemed. Telecare 2004, 10, 286–289. [Google Scholar] [CrossRef]
- Morland, L.; Greene, C.; Rosen, C.; Foy, D.; Reilly, P.; Shore, J.; He, Q.; Frueh, B. Telemedicine for Anger Management Therapy in a Rural Population of Combat Veterans with Posttraumatic Stress Disorder: A Randomized Noninferiority Trial. J. Clin. Psychiatry 2010, 71, 855–863. [Google Scholar] [CrossRef]
- Morland, L.A.; Mackintosh, M.A.; Rosen, C.S.; Willis, E.; Resick, P.; Chard, K.; Frueh, B.C. Telemedicine versus in-person delivery of cognitive processing therapy for women with posttraumatic stress disorder: A randomized noninferiority trial. Depress Anxiety 2015, 32, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; White, C.; Lynch, A.; Mohammed, K. Telephone-delivered individual cognitive behavioural therapy for cancer patients: An equivalence randomised trial. Psycho-Oncol. 2017, 26, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; O’Brian, S.; Onslow, M.; Block, S.; Jones, M.; Packman, A. Randomized controlled non-inferiority trial of a telehealth treatment for chronic stuttering: The Camperdown Program. Int. J. Lang. Commun. Disord. 2010, 45, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, J.; Baird, A.; Steele, R.; Harvey, S. Computer-based treatment of poststroke language disorders: A non-inferiority study of telerehabilitation compared to in-person service delivery. Aphasiology 2018, 32, 290–311. [Google Scholar] [CrossRef]
- Rietdijk, R.; Power, E.; Attard, M.; Heard, R.; Togher, L. Improved Conversation Outcomes After Social Communication Skills Training for People with Traumatic Brain Injury and Their Communication Partners: A Clinical Trial Investigating In-Person and Telehealth Delivery. J. Speech Lang. Hear. Res. 2020, 63, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, R.; Power, E.; Attard, M.; Heard, R.; Togher, L. A Clinical Trial Investigating Telehealth and In-Person Social Communication Skills Training for People with Traumatic Brain Injury: Participant-Reported Communication Outcomes. J. Head Trauma Rehabil. 2020, 35, 241–253. [Google Scholar] [CrossRef]
- Rietdijk, R.; Power, E.; Attard, M.; Togher, L. Acceptability of telehealth-delivered rehabilitation: Experiences and perspectives of people with traumatic brain injury and their carers. J. Telemed. Telecare 2022, 28, 122–134. [Google Scholar] [CrossRef]
- Theodoros, D.G.; Hill, A.J.; Russell, T.G. Clinical and Quality of Life Outcomes of Speech Treatment for Parkinson’s Disease Delivered to the Home Via Telerehabilitation: A Noninferiority Randomized Controlled Trial. Am. J. Speech Lang. Pathol. 2016, 25, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.; Caute, A.; Haigh, Z.; Galliers, J.; Wilson, S.; Kessie, A.; Hirani, S.; Hegarty, B.; Marshall, J. A comparison of remote therapy, face to face therapy and an attention control intervention for people with aphasia: A quasi-randomised controlled feasibility study. Clin. Rehabil. 2016, 30, 359–373. [Google Scholar] [CrossRef]
- Rasmussen, O.W.; Lauszus, F.F.; Loekke, M. Telemedicine compared with standard care in type 2 diabetes mellitus: A randomized trial in an outpatient clinic. J. Telemed. Telecare 2016, 22, 363–368. [Google Scholar] [CrossRef]
- Wilbright, W.A.; Birke, J.A.; Patout, C.A.; Varnado, M.; Horswell, R. The Use of Telemedicine in the Management of Diabetes-Related Foot Ulceration: A Pilot Study. Adv. Ski. Wound Care 2004, 17, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, H.; Hui, E.; Woo, J. The acceptability of telemedicine for podiatric intervention in a residential home for the elderly. J. Telemed. Telecare 2003, 9, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; Chen, J.; Chunara, R.; Testa, P.A.; Nov, O. COVID-19 transforms health care through telemedicine: Evidence from the field. J. Am. Med. Inf. Assoc. 2020, 27, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Wosik, J.; Fudim, M.; Cameron, B.; Gellad, Z.F.; Cho, A.; Phinney, D.; Curtis, S.; Roman, M.; Poon, E.G.; Ferranti, J.; et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inf. Assoc. 2020, 27, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.; Berghofer, K.; Long, J.; Prescott, A.; Caboral-Stevens, M. Satisfaction with the use of telehealth during COVID-19: An integrative review. Int. J. Nurs. Stud. Adv. 2020, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.; Yu, M.; Drangsholt, S.; Ng, E.; Culligan, P.J.; Schlegel, P.N.; Hu, J.C. Patient Satisfaction with Telemedicine During the COVID-19 Pandemic: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e20786. [Google Scholar] [CrossRef] [PubMed]
- Giummarra, M.J.; Arnold, C.A.; Beck, B.B. Evaluation of the Relationship Between Geographic Proximity and Treatment for People Referred to a Metropolitan Multidisciplinary Pain Clinic. Pain Med. 2021, 22, 1993–2006. [Google Scholar] [CrossRef]
- Smith, M.A.; Tomita, M.R. Combined effects of telehealth and modified constraint-induced movement therapy for individuals with chronic hemiparesis. Int. J. Telerehabilitation 2020, 12, 51–62. [Google Scholar] [CrossRef]










| Study | Participant Characteristics Number of Participants Mean Age, Years (SD) Sex (F/M) Diagnosis/Population | Intervention and Comparator | Outcome Measures |
|---|---|---|---|
| Neuropsychology | |||
| Jelcic 2014 [26] | n = 17 Age = 84.1 (5.7) Sex = 12 F, 5 M Early-stage Alzheimer’s disease | Lexical-semantic stimulation to enhance semantic verbal processing FTF = Day center-based group therapy (3–4 participants), 60 min daily Telehealth = Day center-based group VC (3–4 participants) via Skype® (Microsoft Corporation, Redmond, WA, USA) and computer program with exercises, trained operator present to facilitate interaction with therapist, 60 min daily Duration = 3 months | Attention and executive functions (DCT and TMT) Feasibility Global cognitive performance (MMSE) Lexical-semantic abilities (VNT and phonemic and semantic fluency) Memory (FDST, ROCF, Brief Story Recall, RAVLT) Satisfaction Visual-spatial abilities (ROCF Copy Test) |
| Occupational Therapy | |||
| Chen 2017 [25] | n = 54 Age = 61.2 (12.1) Sex = 21 F, 33 M Stroke with hemiplegia | Rehabilitation exercise (Bobath and PNF) and electromyography-triggered neuromuscular stimulation FTF = Outpatient setting, exercises 1 h × per weekday and ETNS 20 min × 2 per weekday Telehealth = Home-based VC, training logs, same program as FTF Duration = 12 weeks (60 sessions total) | Adverse events Balance (BBS) Caregiver Strain (CSI) Disability (MRS) Function (MBI) |
| Laver 2020 [33] | n = 63 Age = 70.3 (14.5) (carers) 80 (6.8) (adults with dementia) Sex = 25 F, 38 M Dementia, carers of adults with dementia | Carer program to problem solve, educate, and build skills FTF = Home visits, approximately 60 min Telehealth = 2 home visits followed by home-based VC via h264 videoconference codecs (Tandberg 550 MXP; Cisco Systems, San Jose, CA, USA) on laptop, tablet, or smartphone, ≤6 sessions Duration = 16 weeks, ≤8 sessions | Adverse events Caregiver mastery (CMI) Function (CAFU) Perceived change (PCS) |
| Torpil 2023 [35] | n = 68 Age = 70.0 (2.7) Sex = 10 F, 24 M Amnesic mild cognitive impairment | Cognitive rehabilitation FTF = Center-based, 2 × 45 min sessions per week Telehealth = Home-based VC via Skype® (Microsoft Corporation, Redmond, WA, USA), Zoom (Zoom Video Communications, San Jose, CA, USA), or WhatsApp Messenger (Meta Platforms, Mountain View, CA, USA) according to participants’ preferences and existing technology, 2 × 45 min sessions per week Duration = 12 weeks | Cognitive skills (LOTCA-G) Feasibility |
| Occupational Therapy and Physiotherapy | |||
| Cramer 2019 [32] | n = 124 Age = 61.0 (14.0) Sex = 38 F, 90 M Stroke—within 3 months of stroke onset | Arm motor therapy plus stroke education FTF = Outpatient setting with 18 supervised and 18 unsupervised 70 min sessions, use of standard exercise hardware for functional tasks Telehealth = Home-based VC as per FTF, content matched with FTF; use of 12 input devices (e.g., PlayStation® Move controller (Sony, Japan) or trackpad) for functional tasks Duration = 30 days | Adverse events Motor skills (FM) |
| Sanford 2006 [34] | n = 32 Age 61.4 (12.9) Sex = 5 F, 27 M Community-dwelling older adults with new mobility devices | Mobility and transfer tasks FTF = Home visits, 1 × 60 min session per week of PT/OT Telehealth = Home-based VC operated by a research assistant in the home 1 × 60 min session per week with remote PT/OT Duration = 4 weeks, 4 sessions | Mobility self-efficacy (FES) Self-reported physical functioning |
| Uswatte 2021 [30] | n = 24 Age = 59.6 (13.2) Sex = 10 F, 14 M Chronic stroke | Upper Limb Constraint-Induced Movement Therapy FTF = In lab, 1:1 sessions 3.5 h per day for 10 consecutive weekdays Telehealth = VC 1:1 sessions at home using Tele-AutoCITE (developed in C#.NET 2007 (Microsoft, Redmond, WA, USA), as per FTF Duration = 2 weeks, 10 sessions | Arm Use (MAL) Motor capacity (WMFT) Satisfaction |
| Physiotherapy | |||
| Aily 2023 [36] | n = 100 Age = 55.0 (8.0) Sex = 60 F, 40 M Knee osteoarthritis | Circuit training of upper and lower limb, trunk, and global exercises FTF = Center-based group exercise, 3 sessions per week Telehealth = Home-based asynchronous exercise 3 sessions per week via video recordings (DVD), with 20 min telephone calls every 1–3 weeks (total of 7) Duration = 14 weeks | Adherence, acceptability Body composition (fat, lean mass, muscle architecture) Gait speed (40 m fast-paced walk test) Pain (VAS and Pain Catastrophizing Syndrome) Pain, stiffness, and functional capacity (WOMAC, stair climb test, 30-second chair stand test) Strength (isometric peak torque) |
| Batalik 2020 [37] | n = 56 Age = 57.1 (7.2) Sex = 14F, 42M Cardiovascular disease and myocardial revascularization | Cardiac rehabilitation including exercise and educational booklet FTF = Center-based group exercise and education, 3 × 60 min sessions per week Telehealth = Home-based asynchronous exercise, 3 × 60 min sessions per week, weekly telephone feedback as recommendations, advice, and motivation Duration = 12 weeks, 36 sessions | Adherence Adverse events Health-Related Quality of Life (SF-36) Physical fitness (maximal CPET text) |
| Bini 2017 [38] | n = 29 Age = 63.3 (NR) Sex = 13 F, 15 M Total knee arthroplasty | Exercise protocol (customized type/number and frequency of exercises) FTF = Outpatient setting Telehealth = Home-based asynchronous video application (CaptureProof, San Francisco, CA, USA) on iPod touch (Apple, Cupertino, CA, USA) and web-based interface; participants videoed themselves performing exercises which were reviewed with feedback and progression by physiotherapist. Outpatient physiotherapy was available Duration = Minimum 3 months, sessions as required | Function (KOOS-PS) Health-Related Quality of Life (VR-12) Pain (VAS) Range |
| Cox 2022 [39] | n = 142 Age = 67.5 (9.0) Sex = 76 F, 66 M Chronic respiratory disease | Pulmonary rehabilitation including exercise physiologists and nurses, at least 30 min of lower limb aerobic training at 60% Peak VO2 plus progressive resistance training. Home exercises 3 sessions per week (unsupervised) with home diary, self-management education (book and brochure for online resources) FTF = Center-based, group sessions (8–12 participants) Telehealth = Home-based VC using iPad (Apple, Cupertino, CA, USA) and Zoom (Zoom Video Communications, San Jose, CA, USA) with initial home visit by physiotherapist, followed by group sessions (4–6 participants), stationary exercise bicycle, pulse oximeter Duration = 8 weeks, 16 sessions total | Adverse events Anxiety and depression (HADS) Change in dyspnoea (Modified Medical Research Council scale) Functional exercise capacity (6MWT) Health-Related Quality of Life (CRQ) Hospitalization Psychological well-being Self-efficacy Symptoms |
| Dadarkhah 2021 [40] | n = 56 Age = 49.5 (8.9) Sex = 32 F, 24 M Chronic and non-specific low back pain | Core stability, flexibility, and strengthening program with logbook FTF = Clinic-based exercise, 3 sessions per week Telehealth = Home-based asynchronous exercise 2 sessions per day for 4 weeks, 3 × 10 min telephone calls per week Duration = 4 weeks, 12 sessions | Disability (ODQ) Pain (VAS) |
| Dias Correia 2019 [41] | n = 66 Age = 64.4 (9.2) Sex = 31 F, 35 M Arthroscopic rotator cuff repair | Shoulder strengthening and range of movement exercises FTF = Home visits for supervised exercise 3 × 30–60 min sessions per week plus ≥ 2 sessions unsupervised per week (30 sessions) Telehealth = Home-based asynchronous exercise via app, 5 × 15–30 min per week, digitally monitored via inertial motion trackers (chest, upper arm, and wrist) and progressed via PT through web portal. Plus, 13 × home visits by PT Duration = 8 weeks | Adherence Adverse events Shoulder function (QuickDASH) |
| Dias Correira 2022 [42] | n = 50 Age = 61.7 (6.9) Sex = 39 F, 11 M Total hip arthroplasty | Hip strengthening and range of movement exercises FTF = Home visits for supervised exercise 3 × 60 min sessions per week plus ≥ 2 sessions unsupervised per week Telehealth = Home-based exercise via app 5–7 ×x ≥ 30 min sessions per week, asynchronous monitoring by physiotherapist. Telephone call weeks 2 and 6, with FTF visit week 4, additional home visits as required Duration = 8 weeks | Adherence Adverse events Hip function (HOOS) Hip range Mobility (TUG) Patient satisfaction |
| Doiron-Cadrin 2020 [43] | n = 23 Age = 65.6 (9.5) Sex = 17 F, 6 M Awaiting total hip or knee replacement | Lower limb strengthening, proprioception, cardiovascular warm-up and education, with exercise logbook FTF = Outpatient setting, 2 sessions per week, with independent exercise program 3 sessions per week Telehealth = Home-based supervised exercise 1:1 VC through web platform REACTS LiteVR® (Technologies innovatrices d’imagerie, Montreal, QC, Canada) on iPad (Apple, Cupertino, CA, USA) 2 × week plus independent exercise program as per FTF. Backup VC options of Skype® (Microsoft Corporation, Redmond, WA, USA) and FaceTime® (Apple, Cupertino, CA, USA) Duration = 12 weeks | Feasibility Function (Lower Extremity Functional Scale, WOMAC, TUG, GRC, Stair test) Gait speed (self-paced walk) Satisfaction |
| Flynn 2020 [44] | n = 40 Age = 72 (6.9) Sex = 10 F, 30 M Parkinson’s Disease | Exercises in 2 phase study: first phase center-based, second phase split into FTF and telehealth groups. FTF = Center-based group exercises 3 × 60 min sessions per week Telehealth = Home-based with prescribed exercises from PhysioTherapy eXercises website (https://www.physiotherapyexercises.com), 3 × 45–60 min sessions per week (unsupervised), telephone calls to monitor and progress Duration = 5 weeks | Adherence Acceptability Feasibility |
| Hansen 2020 [45,46] | n = 134 Age = 68.3 (9.0) Sex = 74 F, 60 M Severe COPD | Pulmonary rehabilitation including nurses, Structured exercise and education with activity monitor (activePAL) (PAL Technologies Ltd., Glasgow, UK) FTF = Outpatient group program, 2 × 60 min sessions per week Telehealth = Home-based group VC on single touch screen device, 3 × 35 min sessions per week Duration = 10–12 weeks | Activity levels (steps per day) Anxiety and depression (HADS) Function (30 second-STS) Functional exercise capacity (6MWT) Health-Related Quality of Life (EQ-5D) |
| Holland 2017 [47] | n = 166 Age = 69 (11.5) Sex = 67 F, 99 M COPD | Pulmonary rehabilitation (aerobic and resistance exercise and self-management education) FTF = Center-based group program, 2 sessions per week Telehealth = Home-based: first week goal setting and supervision of exercise, ≥30 min of unsupervised aerobic training on most days of week. Weekly telephone call by physiotherapist including motivational interviewing. Education component, pedometer, and exercise diary Duration = 8 weeks | Adverse events Anxiety and depression (HADS) Attendance Dyspnea (modified Medical Research Council dyspnoea scale) Functional exercise capacity (6MWT) Health-Related Quality of Life (CRQ) Self-efficacy (PRAISE) |
| Hwang 2017 [48] | n = 53 Age = 67.5 (12.3) Sex = 13 F, 40 M Chronic heart failure | Cardiac rehabilitation including exercise and multidisciplinary education FTF = Hospital-based (outpatient) group program Telehealth = Home-based group VC (Adobe Connect 9.2) (Adobe, Systems Inc., San Jose, CA, USA) on laptop with chat function. Pre-recorded education as slides with audio. Telephone technical support was available. Self-monitored vital signs (equipment supplied) Duration = 12 weeks, 2 sessions per week | Attendance Adverse events Balance (BOOMER) Functional exercise capacity (6MWT) Functional outcomes (TUGT) Health-Related Quality of Life (EQ-5D) Strength (grip, quadriceps) Patient satisfaction |
| Kraal 2014, 2017 [49,50] | n = 90 Age = 59.2 (8.5) Sex = 10 F, 80 M Cardiac disease—low-moderate risk after myocardial infarction, unstable angina or revascularization procedure | Cardiac rehabilitation including exercise specialists FTF = Center-based group training at 70–85% maximal heart rate, 2–3 × 45–60 min sessions per week Telehealth = Home/personal gym-based training at 70–85% maximal heart rate, ≥3 × 45–60 min sessions per week. First 3 sessions were outpatient supervised. Weekly telephone calls by physiotherapist or exercise specialist for goal setting, feedback/progression, and motivational interviewing. Web application (Garmin Connect) and heart rate monitor (Garmin Fore-runner 70) (Garmin™, Kansas, MO, USA) Duration = 12 weeks | Adherence Adverse events Exercise capacity (Peak VO2) Health-Related Quality of Life (SF-36) Physical activity level (PAEE) |
| Lin 2014 [27] | n = 17 Age = 75.1 (2.9) Sex = 7 F, 10 M Stroke with proximal active movement in upper extremity in hemiparetic side, living in long-term care facilities | Standing balance training including volunteers FTF = Care-facility-based group physiotherapy (2 people), 3 × 50 min sessions per week Telehealth = Group VC (2 people) with 3D animation exercise videos and interactive games (National Taiwan University and Lunghwa University of Science and Technology, Taiwan) with touch screen capabilities exercises matched as per FTF, 3 × 50 min sessions per week, vital signs monitored with bi-directional sensor devices. Volunteer assisting on participant-end guided by remote physiotherapist Duration = 4 weeks, 12 sessions | Balance (BBS) Function (Barthel Index) Satisfaction |
| Llorens 2015 [51] | n = 30 Age = 55.5 (8.4) Sex = 13 F, 17 M Stroke with residual hemiparesis (stroke onset >6 months) | Virtual-Reality balance and complementary exercises with motion-sensing device (Microsoft Kinect) (Microsoft Corporation, Redmond, WA, USA) FTF = Clinic-based VR 3 × 45 min sessions per week plus 2 sessions per week conventional physiotherapy Telehealth = Home-based VR (laptop and television), 3 × 45 min sessions per week plus clinic-based conventional physiotherapy 2 sessions per week, weekly interview to progress Duration = 7 weeks, 20 sessions | Balance (BBS) Cost Gait (POMA-G) Mobility (POMA-B) Motivation (IMI) Usability (SUS) |
| Moffet 2015, 2017 {Moffet, 2017 #20647;Moffet, 2015 #20646} | n = 205 Age = 66.0 (8.0) Sex = 105 F, 100 M Total knee arthroplasty | Progressive exercise program of mobility, strength, function, and balance, with home exercise program and education FTF = Home visits, 2 × 45–60 min sessions per week Telehealth = Home-based VC sessions on h264 videoconference codecs (Tandberg 550 MXP; Cisco Systems, San Jose, CA, USA), 2 × 45–60 min sessions per week Duration = 2 months, 16 sessions | Adverse events Function, symptoms, and Quality of Life (KOOS) Functional exercise capacity (6MWT) Knee Range Knee Strength Pain, stiffness, physical function (WOMAC) Satisfaction |
| Odole 2013, 2014 {Odole, 2014 #16766;Odole, 2013 #16765} | n = 50 Age = 55.5 (7.6) Sex = 24 F, 26 M Osteoarthritis of the knee | Structured Exercise (not specified) FTF = Clinic-based exercise 3 sessions per week Telehealth = Home-based asynchronous exercise 3 sessions per week (as per FTF), with structured telephone monitoring and coaching 3 sessions per week, logbook Duration = 6 weeks, 18 sessions | Disability Function (IKHOAM) Health-Related Quality of Life (WHOQoL-Bref) Pain (VAS) |
| de Oliveira Silva 2020 [54] | n = 35 Age = 31.5 (5.9) Sex = 27 F, 8 M Patellofemoral pain, 6 weeks after self-management exercise program | Education and exercise, content as per ‘My Knee Cap’ website (mykneecap.trekeducation.org) FTF = Private practice clinic Telehealth = VC via Skype® (Microsoft Corporation, Redmond, WA, USA) Duration = 12 weeks, maximum of 8 sessions | Adverse Events Disability (AKPS) Feasibility Knee self-efficacy (K-SES) Pain (VAS and PCS) Quality of Life (Knee Injury Quality of Life subscale) Recovery (GROC) |
| Onan 2023 [57] | n = 31 Age = 38.5 (10.7) Sex = 22 F, 9 M Neck pain | Spinal stabilization exercises FTF = Clinic-based 3 sessions per week Telehealth = Home-based VC and video exercises, 3 sessions per week Duration = 8 weeks, 24 sessions | Disability (NDI) Neck function (Neck functional capacity evaluation test, Neck Awareness Questionnaire) Neck muscle size Pain (VAS) Satisfaction |
| Piqueras 2013 [29] | n = 181 Age = 73.3 (6.5) Sex = 131 F, 50 M Total knee arthroplasty | TKA Rehabilitation Clinical Protocol FTF = Outpatient setting—60 min sessions each weekday for 10 days Telehealth = Home-based 60 min sessions each weekday for 10 days, with interactive virtual software–hardware platform; in total, 5 sessions supervised by physiotherapist, 5 sessions at home with remote monitoring and progression, telephone contact if necessary. Wireless sensor WAGYRO (Wireless accelerometer and gyroscope) (Shimmer Research, Dublin, Ireland) to monitor knee range and movement Duration = 2 weeks, 10 sessions | Knee range Muscle strength (kg) Mobility (TUG) Pain (VAS) Pain, stiffness, and functional capacity (WOMAC) |
| Plaza 2023 [58] | n = 45 Age = 46.8 (15.0) Sex = 14 F, 31 M Burns ≤ 25% Total Body Surface Area | Individualized including range of motion and strengthening exercises, education, and home exercise program FTF = Outpatient-based, 30–60 min 1:1 sessions Telehealth = Home-based VC using eHAB® (NeoRehab, Brisbane, Australia) with patient’s existing technologies, 30–60 min 1:1 sessions, as per FTF Duration = 6 weeks, range 3–12 sessions (minimum 1 per fortnight—maximum 2 per week) | Adherence Burn-scar impact (BBSIP) Grip and quadriceps strength Feasibility Health-Related Quality of Life (AQoL-4D) Joint range (degrees) Pain (VAS) Patient and therapist satisfaction Self-efficacy for exercise Technical disruptions |
| Tarakci 2021 [59] | n = 30 Age = 40.3 (10.7) Sex = 23 F, 7 M Relapsing-Remitting Multiple Sclerosis | Structured exercise program of progressive resistance exercise with PNF, stretching, balance, coordination, and ambulation FTF = Center-based exercise, 3 × 60 min supervised sessions per week (non-consecutive days) Telehealth = Home-based exercise, 3 × 60 min sessions per week, with VC calls to monitor adherence and progression, plus center-based session 1 per month to check/revise exercises Duration = 12 weeks, 36 sessions | Fatigue (FSS) Function (FIM) Health-Related Quality of Life (NHP, QoLS) |
| Williams 2020 [60] | n = 50 Age = 52 (10.5) Sex = 38 F, 12 M Multiple Sclerosis | Progressive functional and balance training FTF = Center-based group sessions, 2 × 60 min sessions per week, at least 2 days apart Telehealth = Home-based exercise 2 × 60 min sessions per week (independent), with activity diary and telephone support by a physiotherapist each fortnight Duration = 8 weeks, 16 sessions | Adherence Adverse events Balance (BBS) Functional walking capacity (6MWT) Gait speed (10MWT) |
| Psychology | |||
| Chavooshi 2017 [61] | n = 81 Age = 32 (6.2) Sex = 29 F, 52 M Medically unexplained pain | Intensive short-term dynamic psychotherapy, based on CPT FTF = Clinic-based, 1 × 60 min session per week Telehealth = Home-based VC via Skype® (Microsoft Corporation, Redmond, WA, USA), 1 × 60 min session per week Duration = 16 weeks, 16 sessions | Anxiety and depression (DASS-21) Emotion regulation (ERQ) Pain intensity (NPRS) Health-Related Quality of Life (QOLI) Stress (MAAS) |
| Fann 2015 [62] | n = 58 Age = 45.4 (14.1) Sex = 24 F, 34 M Major depression after Traumatic Brain Injury | CBT with workbook and homework, encouraged to attend with support persons FTF = Center-based, 1 × 30–60 min session per week, personalized mailed follow-up letter with mutually agreed-upon exercises, workbook, and homework Telehealth = Home-based telephone calls, as per FTF Duration = 12 weeks, 12 sessions | Adverse events Depression (HAMD-17, SCL-20) Satisfaction |
| Germain 2009 [63,71] | n = 68 Age = 42.1 (12.1) Sex = 44 F, 24 M PTSD | CBT FTF = Center-based, 1 x 60 min session per week Telehealth = Center-based VC and fax (to send/receive written material during therapy), 1 × 60 min session per week Duration = 16–25 weeks | Anxiety (BAI) Depression (BDI-II) Function (ACF) Health-Related Quality of Life (SF-12) PTSD (MPSS) |
| Herbert 2017 [64] | n = 128 Age = 52 (13.3) Sex = 23 F, 105 M Chronic pain in veterans | Acceptance and Commitment Therapy FTF = Medical center/outpatient clinic, 1 × 60 min session per week Telehealth = VC (individual) at clinic of their choice–1 × 60 min session per week Duration = 8 weeks, 8 sessions | Activity level/disability/function (MPI) Anxiety—pain-related (PASS-20) Depression Health-Related Quality of Life (SF-12) Pain (BPI, CPAQ) Satisfaction (CSQ) Sleep quality (PSQI) |
| Himelhoch 2013 [65] | n = 31 Age = 45.1 (8.3) Sex = 25 F, 6 M Population = People living with HIV/AIDS with major depression | CBT FTF = Clinic-based–10 × 60 min sessions Telehealth = Telephone CBT —10 × 60 min sessions Duration = 14 weeks | Adverse events Depression (HAM-D) Satisfaction (SIMH) |
| Karagiozi 2022 [66] | n = 82 Age = 44.5 (10.6) Sex = not stated Caregivers of people living with dementia | Psychoeducational Program FTF = Day Center Group Sessions, 1 × 60 min session per week Telehealth = Home-based Group VC, 1 × 60 min session per week Duration = 4 months, 16 sessions | Anxiety (BAI) Carer Burden (ZBI) Depression (BDI) |
| Liu 2020 [67] | n = 207 Age = 48.4 (14.1) Sex = 45 F, 154 M PTSD in veterans | CPT: Cognitive, including social workers and counsellors FTF = VA Hospital-based, 1 × 60 min 1:1 session per week Telehealth = Veterans Affairs outpatient setting VC, 1 × 60 min 1:1 session per week Duration = 12 weeks, 12 sessions | Depression (PHQ-9) PTSD (CAPS, PCL-S) |
| Lleras de Frutos 2020 [68] | n = 269 Age = 49.9 (8.6) Sex = 269 F, 0 M Cancer in women | Positive Psychotherapy for Cancer FTF = Center-based group therapy (8–12 patients) 1 × 90–120 min session per week Telehealth = Home-based group VC (5–6 patients) via ViTAM (Video Teleassistance and Monitoring, University of Girona, Girona, Spain); 1 × 90–120 min session per week and 1 × FTF session (final) Duration = 12 weeks, 12 sessions | Adherence Anxiety and Depression (HADS) PTSD (PCL, PTGI) |
| Luxton 2016 [69] | n = 121 Age = 45% aged 19–29 Sex = 22 F, 99 M Depression in veterans | Behavioral Activation Treatment for Depression (a component of CBT) FTF = Clinic-based, 1 session per week Telehealth = Home-based VC, 1 session per week Duration = 8 weeks, 8 sessions | Adverse events Anxiety (BAI) Depression (BDI-II) Hopelessness (BHS) PTSD (PCL) Satisfaction (CSQ-8) |
| Maieritsch 2016 [70] | n = 90 Age = 30.9 (6.1) Sex = 7 F, 84 M PTSD | CPT including social workers FTF = Hospital setting, 1–2 × 50 min 1:1 sessions per week Telehealth = VA Hospital, VC with clinician at a distant VA hospital, 1–2 × 50 min 1:1 sessions per week Duration = 18 weeks | Depression severity PTSD (CAPS) |
| Mitchell 2008, Marrone [72,73] | n = 128 Age = 29.0 (10.6) Sex = 126 F, 2 M Bulimia nervosa | CBT FTF = Center-based, 60 min sessions Telehealth = VC at a distal site, 60 min sessions Duration = 16 weeks, 20 sessions | Absence of binge eating and purging Depression (HAM-D, BDI) Health-Related Quality of Life (SF-36) |
| Mohr 2012 [28] | n = 325 Age = 47.7 (13.0) Sex = 252 F, 73 M Depression | CBT with workbook FTF = Clinic-based sessions, 45 min sessions (2 in first 2 weeks, then weekly for 12 weeks, and 2 sessions over 4 weeks) Telehealth = Home-based telephone calls, same as FTF Duration = 18 weeks, 18 sessions | Adverse events Attendance Depression (HAM-D, PHQ-9) |
| Morland 2004 [74] | n = 18 Age = Not stated, inclusion criteria range 18–60 Sex = M PTSD in veterans | Psychoeducation for coping skills FTF = Veterans Center, group sessions Telehealth = Veterans Center, VC group sessions, with backup clinician on-site and technician available for technical problems Duration = 8 weeks, 8 sessions | Information retention PTSD (PCL) Satisfaction—patient and clinician |
| Morland 2010 [75] | n = 125 Age = 54.7 (9.6) Sex = 125 M PTSD in combat veterans | Anger management therapy (CBT) FTF = VA clinical site, Group sessions, 2 sessions per week Telehealth = VA clinical site group VC sessions with remote therapist, 2 sessions per week Duration = 6 weeks, 12 sessions | Adherence Adverse events Anger (NAS, STAXI-2) PTSD (CAPS, PCL) Satisfaction (CPOSS-VA) |
| Morland 2015 [76] | n = 126 Age = 46.4 (11.9) Sex = 126 F PTSD in women | CPT FTF = VA clinical site, 1–2 × 90 min 1:1 sessions per week Telehealth = VA clinical site VC with remote psychologist, 1–2 × 90 min 1:1 sessions per week Duration = 6 weeks, 12 sessions | Adherence PTSD (CAPS) Satisfaction (CPOSS-VA, TSAS) Treatment engagement (TEQ) |
| Topfer [31] | n = 188 Age = 63.5 (11.4) Sex = 150 F, 38 M Caregivers of people living with dementia | CBT with extended Tele.TAnDem FTF = Home visits, 50 min Telehealth = Home-based telephone sessions, 50 min Duration = 6 months, 12 sessions | Caregiver burden (BEHAVE-AD) Depression (CES–D) Emotional wellbeing (VAS) Health complaints Health-Related Quality of Life (WHOQoL-BREF) Satisfaction (CSQ-8) |
| Watson 2017 [77] | n = 118 Age = 50.4 (13.3) Sex = 85 F, 33 M Cancer | CBT with workbook and CD FTF = Center-based Telehealth = Home-based via telephone Duration = 12 weeks, up to 8 sessions | Anxiety and depression (HADS) Helpless/hopelessness (MAC) Patient-reported outcomes (PRO) Satisfaction |
| Speech Pathology | |||
| Carey 2010 [78] | n = 40 Age = 65% 18–30 years old Sex = 7 F, 33 M Chronic stuttering | ‘Camperdown Program’ FTF = Center-based ‘Camperdown Program’—individual teaching, group practice, individual problem solving, and maintenance Telehealth = Home-based via telephone, adapted ‘Camperdown Program’ (content as per FTF), mailed audiotapes, voicemail for speech samples, feedback by SP as needed, and home practice Duration = Not specified | Fluency (% syllables stuttered) Patient satisfaction |
| Meltzer 2017 [79] | n = 44 Age = 64.2 (10.8) Sex = 17 F, 27 M Communication disorders with chronic stroke | Supported conversation techniques to partners FTF = Center-based, 1 × 60 min session per week Telehealth = Home-based VC via WebEx® (Cisco, San Jose, CA, USA) or VSee (VSee, San Jose, CA, USA), 1 × 60 min session per week at home or telehealth site, supported with iPad (Apple, Cupertino, CA, USA) with TalkPath Software (Lingraphica Inc., Princeton, NJ, USA; Steele et al., 2014) for exercises/home program Duration = 10 weeks, 10 sessions | Communication (confidence and effectiveness) (WAB-R, CCRSA, CETI) Cognition (CLQT) |
| Rietdijk 2020 [80,81,82] | n = 36 Age = 54 (range 20–68), 42 (range 19–66) Sex = 31 F, 5 M Moderate to severe Traumatic Brain Injury with social communication skills deficits | TBIconneCT training with participant and communication partner, with manual and email summary of session content FTF = Home visits, TBIconneCT training, 1 × 90 min 1:1 session per week Telehealth = Home-based TBIconneCT training delivered via VC using Skype® (Microsoft Corporation, Redmond, USA) on their own home computer, 1 × 90 min 1:1 session per week Duration = 10 weeks, 10 sessions | Adherence Attendance Conversation Function (FAVRES) |
| Theodoros 2016 [83] | n = 31 Age = 71.0 (8.8) Sex = 10 F, 21 M Parkinson’s Disease | Lee Silverman Voice Treatment FTF = Clinic-based, 4 × 60 min sessions per week Telehealth = Home-based VC using eHAB® (V2.0, NeoRehab, Brisbane, Australia) with acoustic software and microphone, treatment as per FTF Duration = 4 weeks, 16 sessions | Acoustic Measures Health-Related Quality of Life (Dysarthria Impact Profile, PDQ-39) Perceptual measures |
| Woolf 2016 [84] | n = 20 Age = 59.7 (12.1) Sex = 6 F, 14 M Chronic post-stroke aphasia following left hemisphere stroke | Semantic verification, picture naming, and self-administered practice FTF = University lab, 2 × 60 min sessions per week plus computer-based homework Telehealth = Home-based VC via FaceTime® (Apple, Cupertino, CA, USA) using iPad (Apple, Cupertino, CA, USA), workbook, 2 × 60 min sessions per week computer-based homework. Therapist was either university-based or at a clinical site Duration = 4 weeks, 8 sessions | Compliance and satisfaction Feasibility Word retrieval |
| Outcome | Studies | Sample Size Telehealth | Sample Size Face-to-Face | Outcome Measure | Mean Difference between Groups (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Cognition | Torpil 2023 [35] | 34 | 34 | LOTCA-G Orientation Visual perception Spatial perception Motor praxis Visuomotor Thinking operation Memory Attention/concentration Total test | 0.18 (−0.09, 0.45) 0.83 (0.55, 1.11) 0.67 (0.34, 1.00) 0.83 (0.49, 1.17) 0.20 (−0.53, 0.93) 0.15 (−0.22, 0.52) −0.02 (−0.42, 0.38) −0.09 (−0.33, 0.15) 2.73 (0.98, 4.48) | 0.20 <0.001 * <0.001 * <0.001 * 0.59 0.43 0.92 0.45 0.002 * |
| Burden of Care | Laver 2020 [33] | 25 | 27 | Caregiving Mastery Index | 0.09 (−1.26, 1.45) | 0.891 |
| Laver 2020 [33] | 26 | 27 | Perceived Change Scale | 0.07 (−1.31, 1.16) | 0.905 | |
| Chen 2017 [25] | 27 | 27 | Caregiver Strain Index | 0.41 (−0.66, 1.49) | 0.666 | |
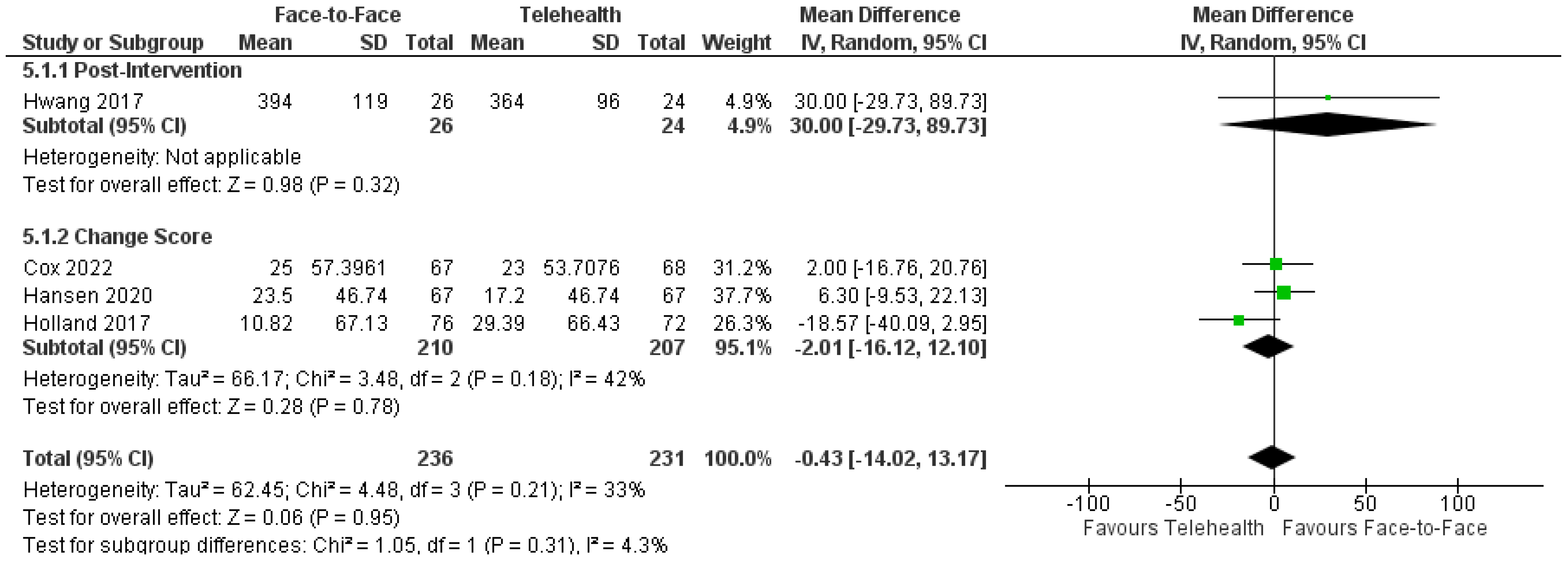
| Motor outcomes | Chen 2017 [25] | 27 | 27 | Function—mBI | 2.08 (−5.17, 9.34) | 0.897 |
| Sanford 2006 | 16 | 16 | Mobility—Falls Efficacy Scale | Effect estimates not reported | NR | |
| Chen 2017 [25] | 27 | 27 | Berg Balance Scale | 0.92 (−1.27, 3.10) | 0.912 | |
| Chen 2017 [25] | 27 | 27 | Upper limb activity—mRS | 2.33 (−8.86, 13.52) | 0.901 | |
| Cramer 2019 [32] | 62 | 62 | Upper limb activity—Fugl-Meyer assessment | 0.06 (−2.14, 2.26) | NR | |
| Cramer 2019 [32] | 62 | 62 | Box and Block | 0.95 (−1.90, 3.80) | 0.512 | |
| Cramer 2019 [32] | 62 | 62 | Stroke Impact Scale—hand motor domain | −5.19 (−11.80, 1.42) | 0.124 | |
| Chen 2017 [25] | 27 | 27 | Lower limb activity—RMS | 5.08 (−11.11, 21.28) | 0.583 | |
| Uswatte 2021 [30] | 12 | 12 | Motor Activity Log—Arm Use Scale | 0.20 (−0.78, 1.18) | 0.69 | |
| Uswatte 2021 [30] | 12 | 12 | Wolf Motor Function Test (repetitions per minute) | −0.30 (−4.72, 4.12) | 0.89 |
| Outcome | Studies | Sample Size Telehealth | Sample Size Face-to-Face | Outcome Measure | Mean Difference between Groups (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Range of motion—active (degrees) | ||||||
| Dias Correia 2019 [41] | 35 | 31 | Hip Supine flexion Supine abduction Standing flexion Standing hyperextension Standing abduction | 26.4 (13.32, 39.5) 15.1 (6.91, 23.25) 12.0 (1.81, 22.33) −10.1 (−15.75, −4.38) 14.1 (7.51, 20.76) | <0.001 * <0.001 * 0.02 * 0.001 * <0.001 * | |
| Dias Correia 2022 [42] | 27 | 23 | Shoulder Scapular elevation Shoulder flexion Shoulder abduction External rotation | 6.69 (−15.00, 28.38) 6.10 (−13.32, 25.52) 25.70 (−2.18, 49.22) 2.92 (−6.78, 12.63) | 0.53 0.53 0.03 * 0.54 | |
| Moffet 2015 [53] | 104 | 101 | Knee Flexion Extension | 1.0 (−2.4, 4.3) 0.1 (1.0, 1.2) | >0.05 >0.05 | |
| Piqueras 2013 [29] | 72 | 70 | Knee Flexion Extension | −0.82 (−3.03, 1.39) 0.70 (−0.38, 1.78) | 0.47 0.2 | |
| Strength | ||||||
| Piqueras 2013 [29] | 72 | 70 | Knee Extension Flexion | −1.34 (−2.29, −0.39) −0.48 (−1.27, 0.31) | 0.006 * 0.24 | |
| Plaza 2023 [58] | 23 | 22 | Knee extension (kg) Right Left | 1.0 (−1.66, 3.66) 1.80 (−0.78, 4.38) | 0.46 0.17 | |
| Function | Doiron-Cadrin 2020 [43] | 11 | 11 | Self-paced walk (seconds) | 0.80 (−3.58, 5.18) | 0.72 |
| Doiron-Cadrin 2020 [43] | 11 | 11 | Stair test | 0.10 (−3.60, 3.80) | 0.96 | |
| Outcome | Studies | Sample Size | Outcome Measure | Mean Difference between Groups (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| Telehealth | Face-to-Face | |||||
| Cognition | Meltzer 2017 [79] | CLCD 5 | 6 | Cognitive-Linguistic Quick Test Language Memory Executive Attention Visuospatial | 1.0 (−3.20, 5.20) −2.30 (−22.99, 18.39) −1.50 (−5.18, 2.18) −23.10 (−49.97, 3.77) NR | 0.64 0.83 0.42 0.09 NR |
| Communication | Meltzer 2017 [79] | Aphasia 14 CLCD 4 | 14 5 | Communication Confidence Rating Scale for Aphasia Communication Effectiveness Index (partner rating) | 2.61 (0.48, 4.74) 2.90 (0.37, 5.43) 6.38 (5.76) | 0.02 * 0.02 * 0.87 |
| Theodoros 2016 [83] | 15 | 16 | Communication Partner Rating Easy to Understand Repetition Initiate with familiar Initiate with unfamiliar Overall rating | 0.4 (−0.16, 0.96) 0.60 (−0.23, 1.43) 0.30 (−0.51, 1.11) 0.40 (−0.55, 1.35) 0.60 (−0.33, 1.53) | 0.212 0.418 0.632 0.289 0.263 | |
| Rietdijk 2020 [80] | Measures of Support in Conversation Reveal Competence CC Reveal Competence PC Acknowledge Competence CC Acknowledge Competence PC Measures of Participation in Conversation Interaction CC Interaction PC Transaction CC Transaction PC | −0.33 (−0.66, 0.00) −0.10 (−0.36, 0.16) −0.17 (−0.54, 0.02) −0.23 (−0.61, 0.15) −0.12 (−0.61, 0.37) −0.04 (−0.51, 0.43) −0.13 (−0.55, 0.,29) −0.12 (−0.32, 0.56) | 0.05 0.45 0.37 0.24 0.63 0.87 0.54 0.59 | |||
| Meltzer 2017 [79] | 14 | 14 | Western Aphasia Battery | −2.61 (−4.74, −0.48) | 0.02 * | |
| Woolf 2016 [84] | 5/5 ‡ † | 5 | Clinical Site Content words per turn Nouns per turn University Site Content words per turn Nouns per turn | 0.72 (−2.72, 1.28) −0.05 (−0.58, 0.48) −1.19 (−5.54, 3.16) −1.0 (−3.09, 1.09) | 0.48 0.85 0.59 0.35 | |
| Voice Function | Theodoros 2016 [83] | 15 | 16 | Acoustic Measures Sustained Phonation (dB) Reading (dB) Monologue (dB) Maximum F0 range (Hz) | 3.50 (1.22, 5.78) 2.30 (−1.19, 5.79) 0.70 (−3.00, 4.40) 42.20 (−5.32, 89.72) | 0.443 0.388 0.596 0.296 |
| Carey 2010 [78] | 20 | 20 | Stuttering frequency Speech naturalness Self-reported stuttering severity Daily Talking to friends/family members | NR NR NR NR | 0.9 0.24 0.7 0.2 | |
| Quality of Life | Theodoros 2016 [83] | 15 | 16 | Dysarthria Impact Profile Effect on person Acceptance Others’ reactions Communication with others Total score Parkinson’s Disease Questionnaire-39 Communication Activities of daily living Cognition Emotion Social support Stigma Bodily discomfort Mobility PQD-39 Summary Index | 0.40 (−0.16, 0.96) 0.60 (−0.23, 1.43) 0.30 (−0.51, 1.11) 0.40 (−0.55, 1.35) 0.60 (−0.33, 1.53) −2.40 (−15.01, 10.21) −5.60 (−18.44, 7.24) 5.40 (−9.95, 20.75) 3.60 (−6.49, 13.69) 3.80 (−3.31, 10.91) −1.50 (−12.29, 9.29) 0.70 (−14.05, 15.45) −6.20 (−20.48, 8.08) −0.30 (−8.52, 7.92) | 0.766 0.354 0.775 0.812 0.746 0.71 0.39 0.49 0.48 0.29 0.79 0.93 0.39 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymond, M.J.; Christie, L.J.; Kramer, S.; Malaguti, C.; Mok, Z.; Gardner, B.; Giummarra, M.J.; Alves-Stein, S.; Hudson, C.; Featherston, J.; et al. Delivery of Allied Health Interventions Using Telehealth Modalities: A Rapid Systematic Review of Randomized Controlled Trials. Healthcare 2024, 12, 1217. https://doi.org/10.3390/healthcare12121217
Raymond MJ, Christie LJ, Kramer S, Malaguti C, Mok Z, Gardner B, Giummarra MJ, Alves-Stein S, Hudson C, Featherston J, et al. Delivery of Allied Health Interventions Using Telehealth Modalities: A Rapid Systematic Review of Randomized Controlled Trials. Healthcare. 2024; 12(12):1217. https://doi.org/10.3390/healthcare12121217
Chicago/Turabian StyleRaymond, Melissa J., Lauren J. Christie, Sharon Kramer, Carla Malaguti, Zaneta Mok, Betina Gardner, Melita J. Giummarra, Serena Alves-Stein, Claire Hudson, Jill Featherston, and et al. 2024. "Delivery of Allied Health Interventions Using Telehealth Modalities: A Rapid Systematic Review of Randomized Controlled Trials" Healthcare 12, no. 12: 1217. https://doi.org/10.3390/healthcare12121217
APA StyleRaymond, M. J., Christie, L. J., Kramer, S., Malaguti, C., Mok, Z., Gardner, B., Giummarra, M. J., Alves-Stein, S., Hudson, C., Featherston, J., Holland, A. E., & Lannin, N. A. (2024). Delivery of Allied Health Interventions Using Telehealth Modalities: A Rapid Systematic Review of Randomized Controlled Trials. Healthcare, 12(12), 1217. https://doi.org/10.3390/healthcare12121217








