Influence of Intraoperative Fluid Management on Postoperative Outcome and Mortality of Cytoreductive Surgery for Advanced Ovarian Cancer—A Retrospective Observational Study
Abstract
1. Introduction
1.1. Background/Rationale
1.2. Objectives
2. Methods
2.1. Study Design
2.2. Variables
- Anastomotic insufficiency;
- Pleural effusion;
- The need for postoperative ventilation.
2.3. Data Sources/Measurement
2.4. Bias
2.5. Statistical Methods
3. Results
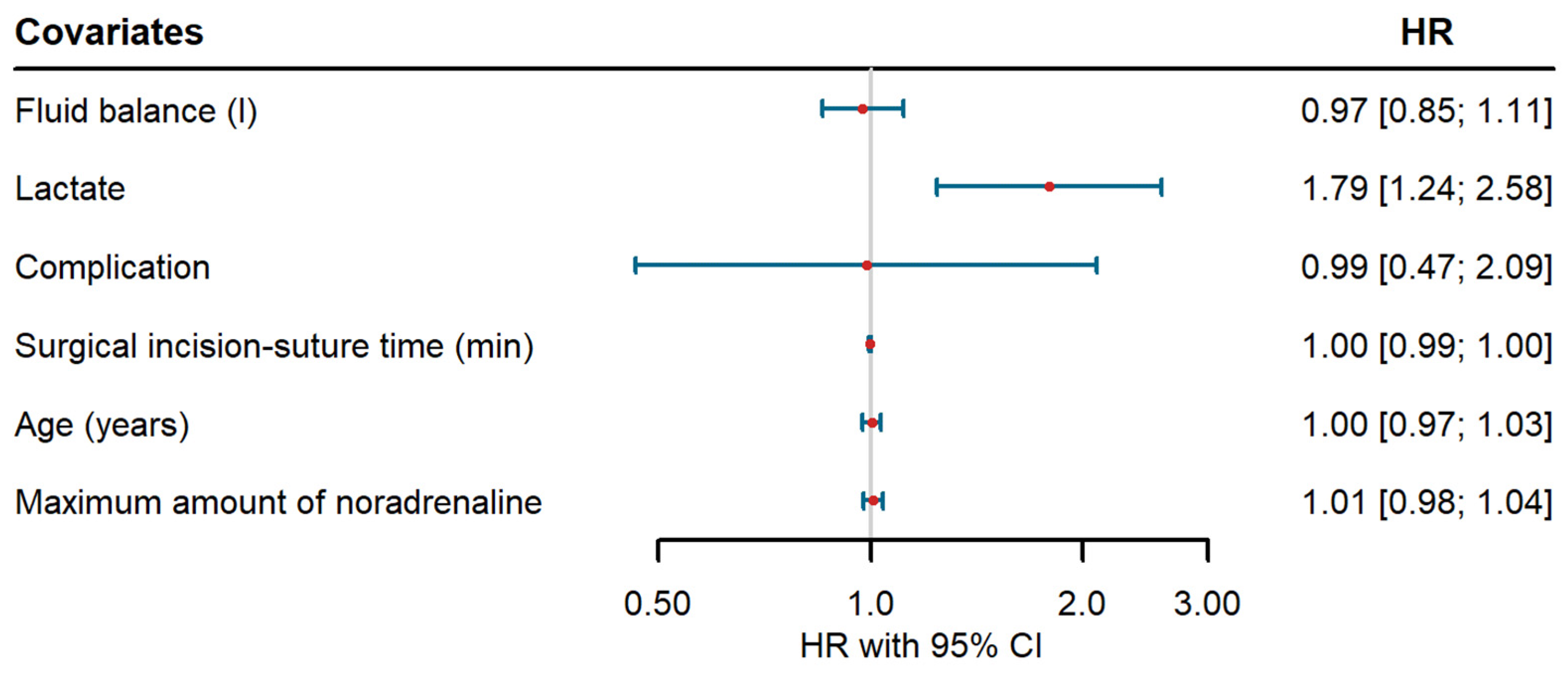
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar]
- Aletti, G.D.; Peiretti, M. Quality control in ovarian cancer surgery. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 96–107. [Google Scholar] [CrossRef]
- Salani, R.; Bristow, R.E. Surgical management of epithelial ovarian cancer. Clin. Obstet. Gynecol. 2012, 55, 75–95. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jamal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef]
- Karam, A.; Ledermann, J.A.; Kim, J.W.; Sehouli, J.; Lu, K.; Gourley, C.; Katsumata, N.; Burger, R.A.; Nam, B.-H.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: First-line interventions. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 711–717. [Google Scholar] [CrossRef]
- Klaschik, S.; Gehlen, J.; Neumann, C.; Keyver-Paik, M.D.; Soehle, M.; Frede, S.; Velter, M.; Hoeft, A.; Hilbert, T. Network of Mediators for Vascular Inflammation and Leakage Is Dysbalanced during Cytoreductive Surgery for Late-Stage Ovarian Cancer. Mediat. Inflamm. 2019, 2019, 5263717. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Gao, C.; Zhou, Y.; Xie, Y. Postoperative pulmonary complications and outcomes in cytoreductive surgery for ovarian cancer: A propensity-matched analysis. BMC Anesthesiol. 2022, 22, 120. [Google Scholar] [CrossRef]
- Palmqvist, C.; Michaëlsson, H.; Staf, C.; Johansson, M.; Albertsson, P.; Dahm-Kähler, P. Complications after advanced ovarian cancer surgery-A population-based cohort study. Acta Obstet. Gynecol. Scand. 2022, 101, 747–757. [Google Scholar] [CrossRef]
- Prys-Roberts, C. The measurement of cardiac output. Br. J. Anaesth. 1969, 41, 751–760. [Google Scholar] [CrossRef]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, S.; Shan, B.; Zhou, S.; Wu, X.; Yang, H.; Ye, S. Serum D-dimer, albumin and systemic inflammatory response markers in ovarian clear cell carcinoma and their prognostic implications. J. Ovarian Res. 2020, 13, 89. [Google Scholar] [CrossRef]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002, 89, 622–632. [Google Scholar] [CrossRef]
- Benes, J.; Chytra, I.; Altmann, P.; Hluchy, M.; Kasal, E.; Svitak, R.; Pradl, R.; Stepan, M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: Results of prospective randomized study. Crit. Care 2010, 14, R118. [Google Scholar] [CrossRef]
- Weinberg, L.; Mackley, L.; Ho, A.; Mcguigan, S.; Ianno, D.; Yii, M.; Banting, J.; Muralidharan, V.; Tan, C.O.; Nikfarjam, M.; et al. Impact of a goal directed fluid therapy algorithm on postoperative morbidity in patients undergoing open right hepatectomy: A single centre retrospective observational study. BMC Anesthesiol. 2019, 19, 135. [Google Scholar] [CrossRef]
- Hasselgren, E.; Hertzberg, D.; Camderman, T.; Björne, H.; Salehi, S. Perioperative fluid balance and major postoperative complications in surgery for advanced epithelial ovarian cancer. Gynecol. Oncol. 2021, 161, 402–407. [Google Scholar] [CrossRef]
- Davies, S.J.; Wilson, R.J.T. Preoperative optimization of the high-risk surgical patient. Br. J. Anaesth. 2004, 93, 121–128. [Google Scholar] [CrossRef]
- Tote, S.P.; Grounds, R.M. Performing perioperative optimization of the high-risk surgical patient. Br. J. Anaesth. 2006, 97, 4–11. [Google Scholar] [CrossRef]
- Hoppenot, C.; Eckert, M.A.; Tienda, S.M.; Lengyel, E. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol. Oncol. 2018, 148, 204–212. [Google Scholar] [CrossRef]
- Clarke, C.L.; Kushi, L.H.; Chubak, J.; Pawloski, P.A.; Bulkley, J.E.; Epstein, M.M.; Burnett-Hartman, A.N.; Powell, B.; Pearce, C.L.; Feigelson, H.S. Predictors of Long-Term Survival among High-Grade Serous Ovarian Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2019, 28, 996–999. [Google Scholar] [CrossRef]
- Som, A.; Maitra, S.; Bhattacharjee, S.; Baidya, D.K. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: A meta-analysis and trial sequential analysis of randomized controlled trials. J. Anesth. 2017, 31, 66–81. [Google Scholar] [CrossRef]
- Meregalli, A.; Oliveira, R.P.; Friedman, G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit. Care 2004, 8, R60–R65. [Google Scholar] [CrossRef]
| Overall (n = 73) | ||
|---|---|---|
| Anaesthesia and surgery times | ||
| Surgical incision-to-suture time (min) | Mean (SD) | 403 (80) |
| Median [IQR] | 405 [335, 460] | |
| Anaesthesia time (min) | Mean (SD) | 529 (106) |
| Median [IQR] | 525 [445, 600] | |
| Ventilation time (min) | Mean (SD) | 2190 (2710) |
| Median [IQR] | 1260 [690, 2730] | |
| Laboratory values | ||
| Max. amount of noradrenaline (µg/min) | Mean (SD) | 11.6 (12.4) |
| Median [IQR] | 9 [3, 15] | |
| Missing | 7 (9.6%) | |
| Lactate (mmol/L) | Mean (SD) | 1.9 (1.04) |
| Median [IQR] | 1.7 [1.06, 2.8] | |
| Missing | 9 (12.3%) | |
| Creatinine (day 3) (mg/dL) | Mean (SD) | 0.73 (0.29) |
| Median [IQR] | 0.63 [0.55, 0.83] | |
| Missing | 10 (13.7%) | |
| Complications | ||
| Any complication | yes | 34 (46.6%) |
| Missing | 1 (1.4%) | |
| Pleural effusion | yes | 25 (34.2%) |
| Missing | 6 (8.2%) | |
| Anastomotic insufficiency | yes | 7 (9.6%) |
| Missing | 2 (2.7%) | |
| Ascites | yes | 4 (5.5%) |
| Missing | 4 (5.5%) | |
| Other complications | yes | 18 (24.7%) |
| Missing | 1 (1.4%) | |
| Overall (n = 73) | ||
|---|---|---|
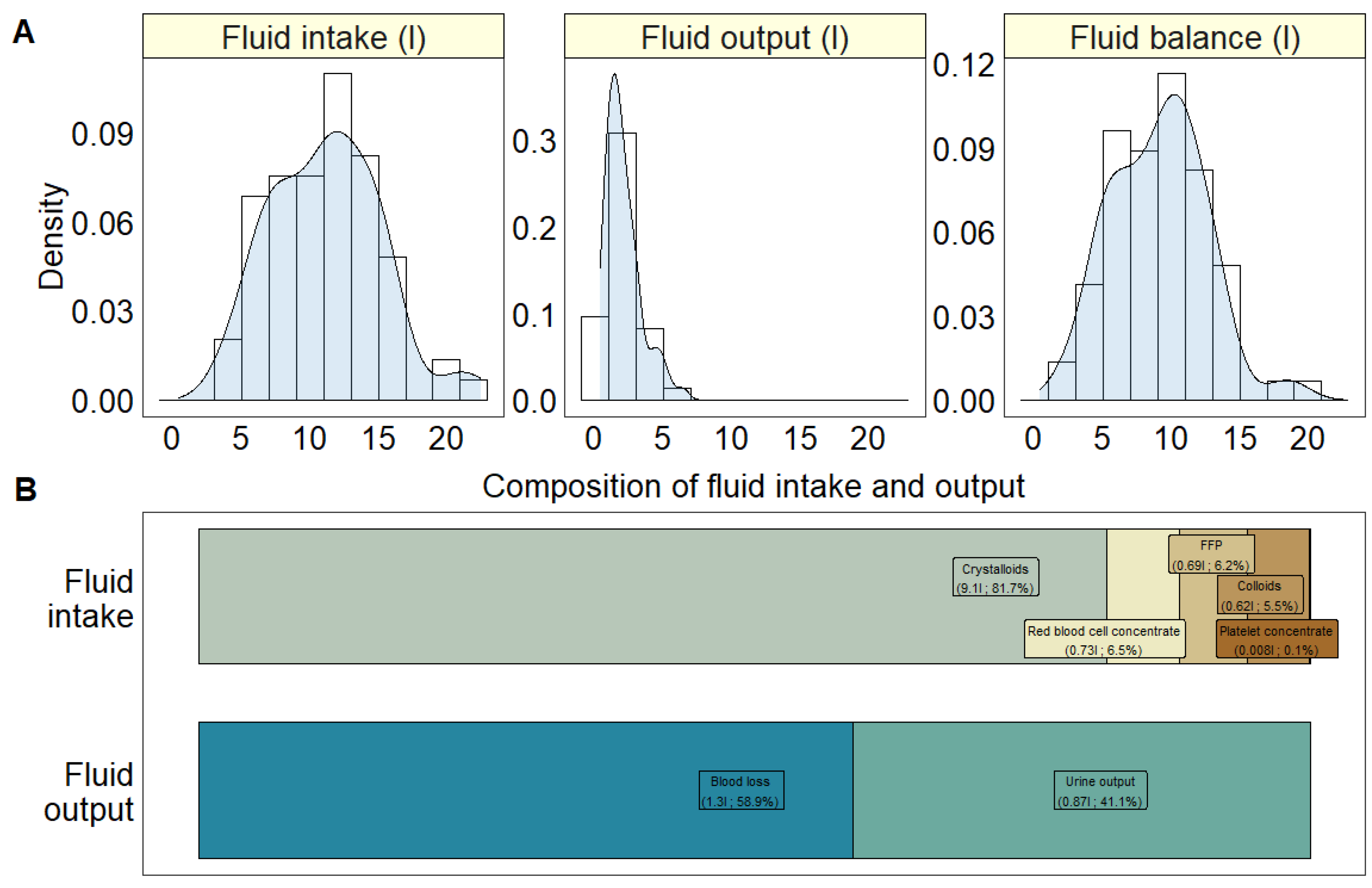
| Total fluid intake (L) | Mean (SD) | 11.2 (4) |
| Median [IQR] | 11.5 [7.9, 14.1] | |
| Red blood cell concentrate (L) | Mean (SD) | 0.73 (0.68) |
| Median [IQR] | 0.58 [0, 1.16] | |
| Fresh frozen plasma (L) | Mean (SD) | 0.69 (0.78) |
| Median [IQR] | 0.58 [0, 1.16] | |
| Platelet concentrate (L) | Mean (SD) | 0.008 (0.05) |
| Median [IQR] | 0 [0, 0] | |
| Colloids (L) | Mean (SD) | 0.62 (0.66) |
| Median [IQR] | 0.5 [0, 1] | |
| Crystalloids (L) | Mean (SD) | 9.1 (3.5) |
| Median [IQR] | 9 [6.5, 11.5] | |
| Total fluid output (L) | Mean (SD) | 2.1 (1.3) |
| Median [IQR] | 1.8 [1.2, 2.7] | |
| Urine output (L) | Mean (SD) | 0.87 (0.67) |
| Median [IQR] | 0.7 [0.48, 1.05] | |
| Missing | 2 (2.7%) | |
| Blood loss (L) | Mean (SD) | 1.3 (0.96) |
| Median [IQR] | 1 [0.6, 1.6] | |
| Fluid balance (L) | Mean (SD) | 9.1 (3.4) |
| Median [IQR] | 9.5 [6.4, 11.4] | |
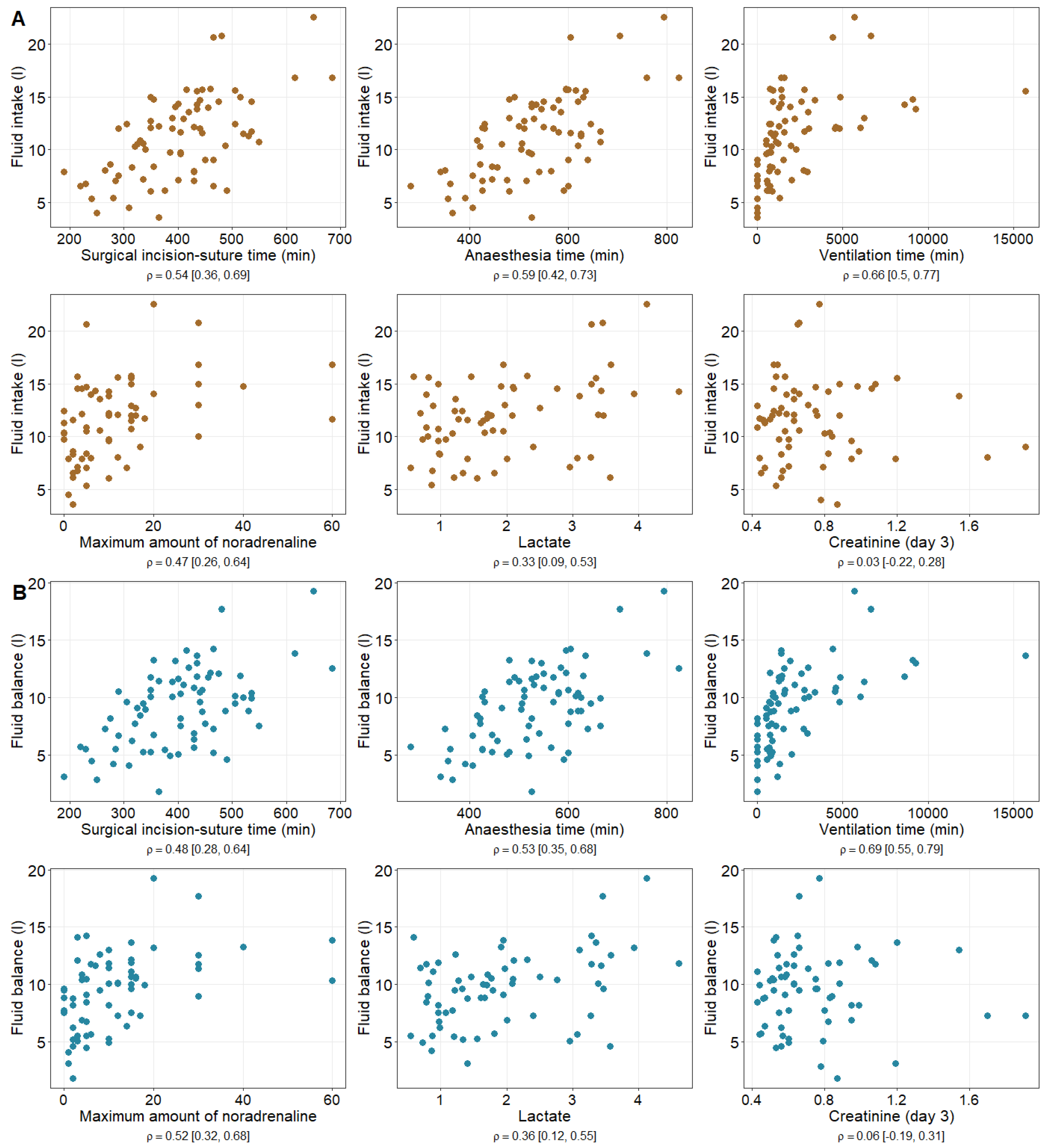
| Fluid Intake | Fluid Balance | |
|---|---|---|
(95%-CI) | (95%-CI) | |
| Surgical incision-to-suture time (min) | 0.54 | 0.48 |
| (0.36, 0.69) | (0.28, 0.64) | |
| Anaesthesia time (min) | 0.59 | 0.54 |
| (0.42, 0.73) | (0.35, 0.68) | |
| Ventilation time (min) | 0.66 | 0.69 |
| (0.50, 0.77) | (0.55, 0.79) | |
| Maximum amount of noradrenaline (µg/min) | 0.47 | 0.52 |
| (0.26, 0.64) | (0.32, 0.68) | |
| Lactate (mmol/L) | 0.33 | 0.36 |
| (0.09, 0.53) | (0.12, 0.55) | |
| Creatinine (Day 3) (mg/dL) | 0.03 | 0.06 |
| (−0.22, 0.28) | (−0.19, 0.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, C.; Kranenberg, E.; Schenk, A.; Kiefer, N.; Hilbert, T.; Klaschik, S.; Keyver-Paik, M.D.; Soehle, M. Influence of Intraoperative Fluid Management on Postoperative Outcome and Mortality of Cytoreductive Surgery for Advanced Ovarian Cancer—A Retrospective Observational Study. Healthcare 2024, 12, 1218. https://doi.org/10.3390/healthcare12121218
Neumann C, Kranenberg E, Schenk A, Kiefer N, Hilbert T, Klaschik S, Keyver-Paik MD, Soehle M. Influence of Intraoperative Fluid Management on Postoperative Outcome and Mortality of Cytoreductive Surgery for Advanced Ovarian Cancer—A Retrospective Observational Study. Healthcare. 2024; 12(12):1218. https://doi.org/10.3390/healthcare12121218
Chicago/Turabian StyleNeumann, Claudia, Eva Kranenberg, Alina Schenk, Nicholas Kiefer, Tobias Hilbert, Sven Klaschik, Mignon Denise Keyver-Paik, and Martin Soehle. 2024. "Influence of Intraoperative Fluid Management on Postoperative Outcome and Mortality of Cytoreductive Surgery for Advanced Ovarian Cancer—A Retrospective Observational Study" Healthcare 12, no. 12: 1218. https://doi.org/10.3390/healthcare12121218
APA StyleNeumann, C., Kranenberg, E., Schenk, A., Kiefer, N., Hilbert, T., Klaschik, S., Keyver-Paik, M. D., & Soehle, M. (2024). Influence of Intraoperative Fluid Management on Postoperative Outcome and Mortality of Cytoreductive Surgery for Advanced Ovarian Cancer—A Retrospective Observational Study. Healthcare, 12(12), 1218. https://doi.org/10.3390/healthcare12121218





