The Bayesian-Based Area under the Curve of Vancomycin by Using a Single Trough Level: An Evaluation of Accuracy and Discordance at Tertiary Care Hospital in KSA
Abstract
1. Introduction
2. Materials and Methods
2.1. Place of Study
2.2. Study Design, Setting, and Patient Population
2.3. Vancomycin Dosing and Monotiring
2.4. Pharmacokinetic Parameters Estimation
2.5. Criteria of Inclusion and Exclusion
2.6. Endpoints
2.6.1. Primary Endpoints
- To estimate the vancomycin AUC0–24 using Bayesian software and determine the probability (%) of patients who achieved the targeted AUC0–24 of 400–600 mg·h/L.
- To evaluate the accuracy and precision of the PrecisePK Bayesian platform in determining the trough level.
2.6.2. Secondary Endpoints
- To compare the association between the attainment of AUC0–24 ≥ 600 mg·h/L and trough levels of 10–14.9 mg/L, and 15–20 mg/L, respectively.
- To identify factors for achieving target AUC0–24.
2.7. Statistical Analysis
3. Results
3.1. Estimation of AUC0–24 and Determine the Probability of Patients Who Achieved Optimal AUC0–24
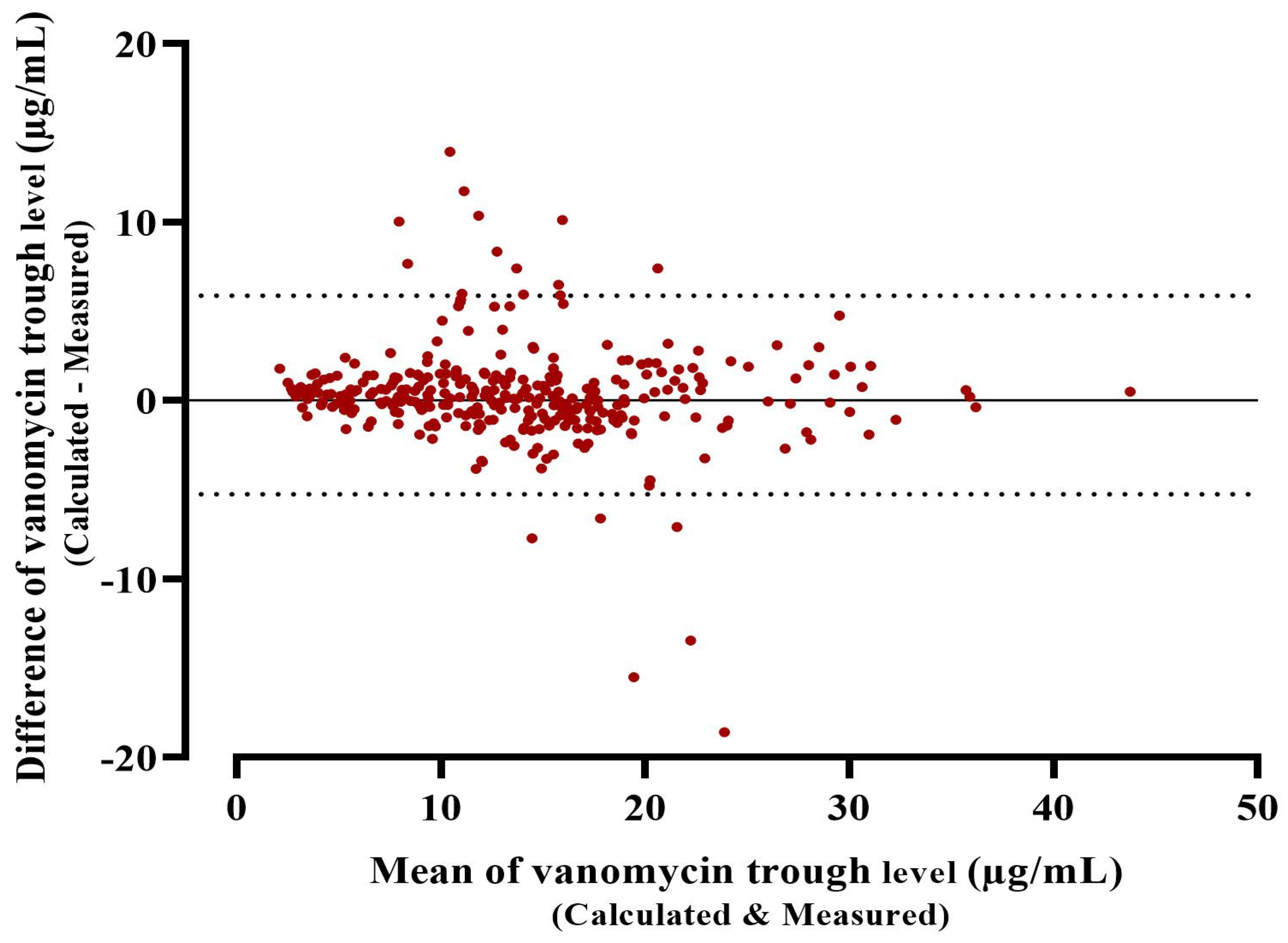
3.2. Accuracy and Precision of Bayesian Software (Posterior) in Determining the Trough Level
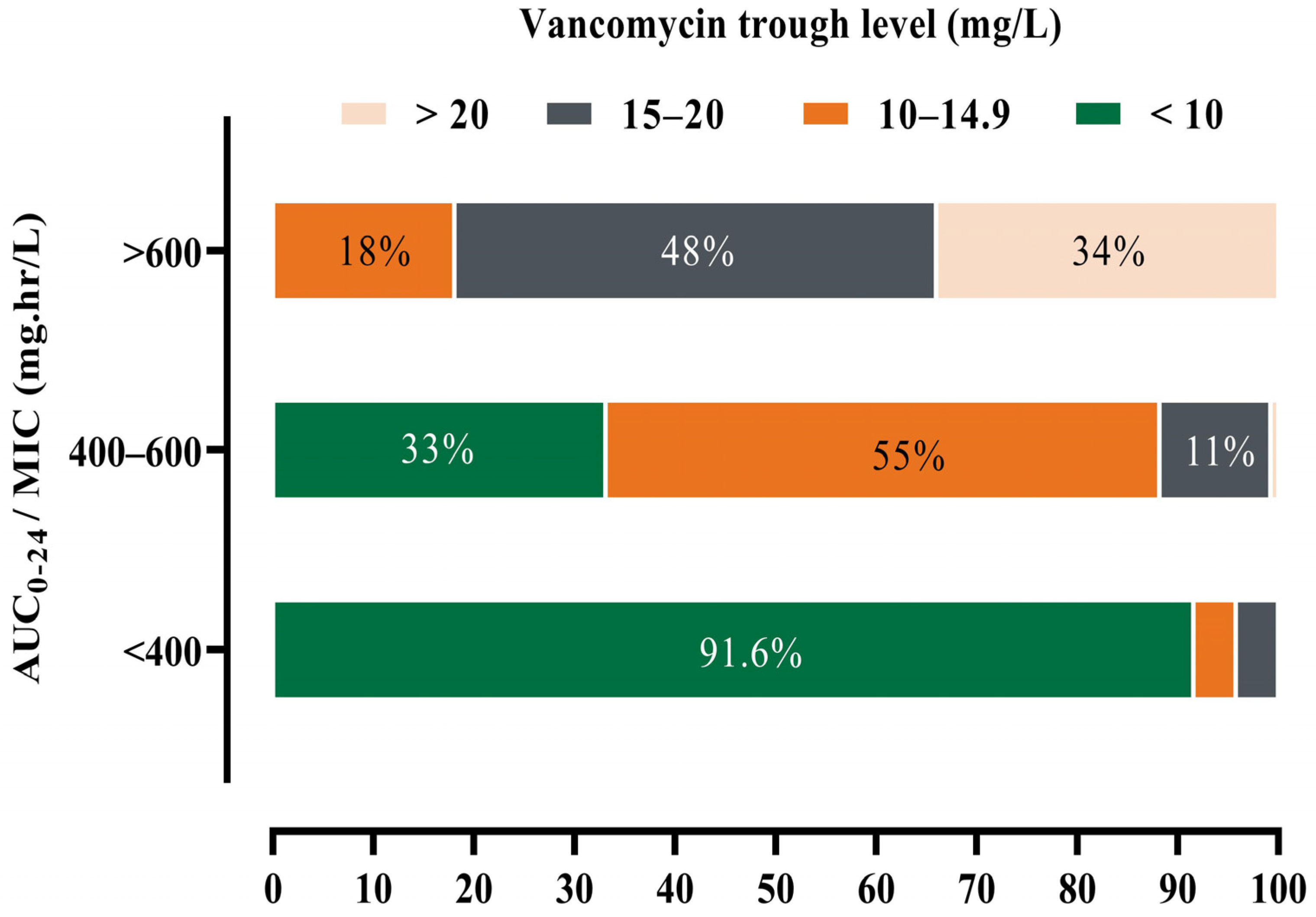
3.3. Association of AUC0–24 ≥600 mg·h/L with Normal Trough Level
3.4. Factors for Achieving Target AUC0–24
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, Prevalence, and Management of MRSA Bacteremia across Patient Populations—A Review of Recent Developments in MRSA Management and Treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P.; Reilly, C. Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Holubar, M.; Meng, L.; Deresinski, S. Bacteremia Due to Methicillin-Resistant Staphylococcus Aureus: New Therapeutic Approaches. Infect. Dis. Clin. N. Am. 2016, 30, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; DiazGranados, C.A. High Vancomycin Minimum Inhibitory Concentration and Clinical Outcomes in Adults with Methicillin-Resistant Staphylococcus Aureus Infections: A Meta-Analysis. Int. J. Infect. Dis. 2013, 17, e93–e100. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus Aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatr. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [CrossRef]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S.; et al. The Monitoring of Vancomycin: A Systematic Review and Meta-Analyses of Area under the Concentration-Time Curve-Guided Dosing and Trough-Guided Dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef] [PubMed]
- Donagher, J.; Martin, J.H.; Barras, M.A. Individualised Medicine: Why We Need Bayesian Dosing. Intern. Med. J. 2017, 47, 593–600. [Google Scholar] [CrossRef]
- Neely, M.N.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; Van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective Trial on the Use of Trough Concentration versus Area under the Curve to Determine Therapeutic Vancomycin Dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.J. Questioning the Accuracy of Trough Concentrations as Surrogates for Area under the Curve in Determining Vancomycin Safety. Ther. Adv. Drug Saf. 2014, 5, 118–120. [Google Scholar] [CrossRef]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Yamada, K.; Tsuchida, T.; Otani, N.; Takahashi, Y.; Ishihara, M.; et al. Validation of Vancomycin Area under the Concentration—Time Curve Estimation by the Bayesian Approach Using One-Point Samples for Predicting Clinical Outcomes in Patients with Methicillin-Resistant Staphylococcus Aureus Infections. Antibiotics 2022, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.M.; Naeem, A.; Alwadie, A.F.; Albogami, K.; Alzhrani, R.M.; Basudan, S.S.; Alzahrani, Y.A. Causes of Vancomycin Dosing Error; Problem Detection and Practical Solutions; A Retrospective, Single-Center, Cross-Sectional Study. Saudi Pharm. J. 2021, 29, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Blum, R.A.; Fischer, J.H.; Zokufa, H.Z.; Rotschafer, J.C.; Crossley, K.B.; Riff, L.J. Vancomycin Pharmacokinetics in Patients with Various Degrees of Renal Function. Antimicrob. Agents Chemother. 1988, 32, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.B.; Beal, S.L. Some Suggestions for Measuring Predictive Performance. J. Pharmacokinet. Biopharm. 1981, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Hashiguchi, Y.; Kimura, T.; Tsuji, Y.; Shoji, K.; Takahashi, Y.; Matsumoto, K.; Kawamura, H.; Saito, H.; Takesue, Y. Performance of Area under the Concentration-Time Curve Estimations of Vancomycin with Limited Sampling by a Newly Developed Web Application. Pharm. Res. 2021, 38, 637–646. [Google Scholar] [CrossRef]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are Vancomycin Trough Concentrations Adequate for Optimal Dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Kojiro, K.; Shephard, E.A.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area under the Curve in Critically Ill Patients. Pharmacotherapy 2018, 38, 1174–1183. [Google Scholar] [CrossRef]
- Olney, K.B.; Wallace, K.L.; Mynatt, R.P.; Burgess, D.S.; Grieves, K.; Willett, A.; Mani, J.; Flannery, A.H. Comparison of Bayesian-Derived and First-Order Analytic Equations for Calculation of Vancomycin Area under the Curve. Pharmacotherapy 2022, 42, 284–291. [Google Scholar] [CrossRef]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative Approaches to Optimizing the Delivery of Vancomycin in Individual Patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef]
- Patel, N.; Pai, M.P.; Rodvold, K.A.; Lomaestro, B.; Drusano, G.L.; Lodise, T.P. Vancomycin: We Can’t Get There from Here. Clin. Infect. Dis. 2011, 52, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.; Zasowski, E.; Murray, K.; Mynatt, R.; Zhao, J.; Yost, R.; Pogue, J.; Rybak, M. A Quasi-Experiment to Study the Impact of Vancomycin Area under the Concentration-Time Curve-Guided Dosing on Vancomycin-Associated Nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01293-17. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-H.; Kim, H.B.; Kim, H.; Lee, M.J.; Jung, Y.; Kim, G.; Hwang, J.-H.; Kim, N.-H.; Kim, M.; Kim, C.-J.; et al. Impact of Area under the Concentration-Time Curve to Minimum Inhibitory Concentration Ratio on Vancomycin Treatment Outcomes in Methicillin-Resistant Staphylococcus Aureus Bacteraemia. Int. J. Antimicrob. Agents 2015, 46, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.V.; Fong, G.; Bolaris, M.; Neely, M.; Minejima, E.; Kang, A.; Lee, G.; Gong, C.L. Cost-Benefit Analysis Comparing Trough, Two-Level AUC and Bayesian AUC Dosing for Vancomycin. Clin. Microbiol. Infect. 2021, 27, 1346.e1–1346.e7. [Google Scholar] [CrossRef]
- Ghosh, N.; Chavada, R.; Maley, M.; van Hal, S.J. Impact of Source of Infection and Vancomycin AUC0-24/MICBMD Targets on Treatment Failure in Patients with Methicillin-Resistant Staphylococcus Aureus Bacteraemia. Clin. Microbiol. Infect. 2014, 20, O1098–O1105. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.M.; Seabury, R.W.; Steele, J.M.; Darko, W.; Miller, C.D. Are Vancomycin Trough Concentrations of 15 to 20 Mg/L Associated with Increased Attainment of an AUC/MIC ≥ 400 in Patients with Presumed MRSA Infection? J. Pharm. Pract. 2017, 30, 329–335. [Google Scholar] [CrossRef]
- Suzuki, A.; Hamada, Y.; Ikeda, H.; Tanaka, H.; Yanagihara, M.; Namiki, M.; Watanabe, T.; Sasaki, T. Comparison of Trough Concentration and Area under the Curve of Vancomycin Associated with the Incidence of Nephrotoxicity and Predictors of a High Trough Level. J. Infect. Chemother. 2021, 27, 455–460. [Google Scholar] [CrossRef]

| Variables | n (%) |
|---|---|
| Gender | |
| Male | 181 (52.9) |
| Vancomycin Indication | |
| Documented infection | 98 (28.7) |
| Empirical therapy | 244 (71.3) |
| Type of infection | |
| Pneumonia, unspecified | 14 (14.3) |
| Bacteremia | 11 (11.2) |
| Bone and skin infection | 29 (29.6) |
| Mean ± SD | |
| Age (years) | 57 ± 19.2 |
| BMI (Kg/m2) | 26.6 ± 7.6 |
| Weight (kg) | 68.9 ± 20 |
| CrCl (mL/min) | 85.6 ± 42.7 |
| TDD (mg/day) | 1857 ± 590 |
| Estimated AUC0–24 (mg. hr/L) * | 573 ± 199.6 |
| Trough level mg/L mean ± SD | 13.69 ± 6.9 |
| Calculated trough mg/L * | 13.38 ± 7.29 |
| Independent Factors | Low AUC0–24 vs. Normal AUC0–24 | High AUC0–24 vs. Normal AUC0–24 | ||||||
|---|---|---|---|---|---|---|---|---|
| β | Adj. OR | (95% CI) | p-Value | β | Adj. OR | (95% CI) | p-Value | |
| Trough level | −0.591 | 0.554 | 0.463–0.662 | <0.05 | 0.741 | 2.09 | 1.724–2.551 | <0.05 |
| CrCl | 0.015 | 1.015 | 1.001–1.030 | <0.05 | −0.025 | 0.976 | 0.959–0.992 | <0.05 |
| BMI | −0.144 | 0.866 | 0.79–0.949 | <0.05 | 0.119 | 1.126 | 1.057–1.2 | <0.05 |
| TDD | −0.014 | 0.866 | 0.802–0.936 | <0.05 | 0.122 | 1.13 | 1.052–1.213 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.M.; Naeem, A.; Alzhrani, R.M.; Harbi, M.A.; Alghamdi, S.A.; Karim, S.; Ali, A.S.; Alsenaini, G.; Hasan, H.; Alkatheeri, A.A.; et al. The Bayesian-Based Area under the Curve of Vancomycin by Using a Single Trough Level: An Evaluation of Accuracy and Discordance at Tertiary Care Hospital in KSA. Healthcare 2023, 11, 362. https://doi.org/10.3390/healthcare11030362
Alzahrani AM, Naeem A, Alzhrani RM, Harbi MA, Alghamdi SA, Karim S, Ali AS, Alsenaini G, Hasan H, Alkatheeri AA, et al. The Bayesian-Based Area under the Curve of Vancomycin by Using a Single Trough Level: An Evaluation of Accuracy and Discordance at Tertiary Care Hospital in KSA. Healthcare. 2023; 11(3):362. https://doi.org/10.3390/healthcare11030362
Chicago/Turabian StyleAlzahrani, Abdullah M., Anjum Naeem, Rami M. Alzhrani, Manar A. Harbi, Sarah A. Alghamdi, Shahid Karim, Ahmed S. Ali, Ghusun Alsenaini, Hani Hasan, Ayed A. Alkatheeri, and et al. 2023. "The Bayesian-Based Area under the Curve of Vancomycin by Using a Single Trough Level: An Evaluation of Accuracy and Discordance at Tertiary Care Hospital in KSA" Healthcare 11, no. 3: 362. https://doi.org/10.3390/healthcare11030362
APA StyleAlzahrani, A. M., Naeem, A., Alzhrani, R. M., Harbi, M. A., Alghamdi, S. A., Karim, S., Ali, A. S., Alsenaini, G., Hasan, H., Alkatheeri, A. A., Basudan, S. S., & Alzahrani, Y. A. (2023). The Bayesian-Based Area under the Curve of Vancomycin by Using a Single Trough Level: An Evaluation of Accuracy and Discordance at Tertiary Care Hospital in KSA. Healthcare, 11(3), 362. https://doi.org/10.3390/healthcare11030362






