Herbal Medicine for Postpartum Pain: A Systematic Review of Puerperal Wind Syndrome (Sanhupung)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Sources and Searches
2.3. Study Selection
2.3.1. Types of Studies
2.3.2. Participants
2.3.3. Types of Interventions
2.3.4. Types of Comparisons
2.3.5. Types of Outcome Measures
2.4. Data Extraction
2.5. Assessment of Risk of Bias (ROB)
2.6. Data Analyses
3. Results
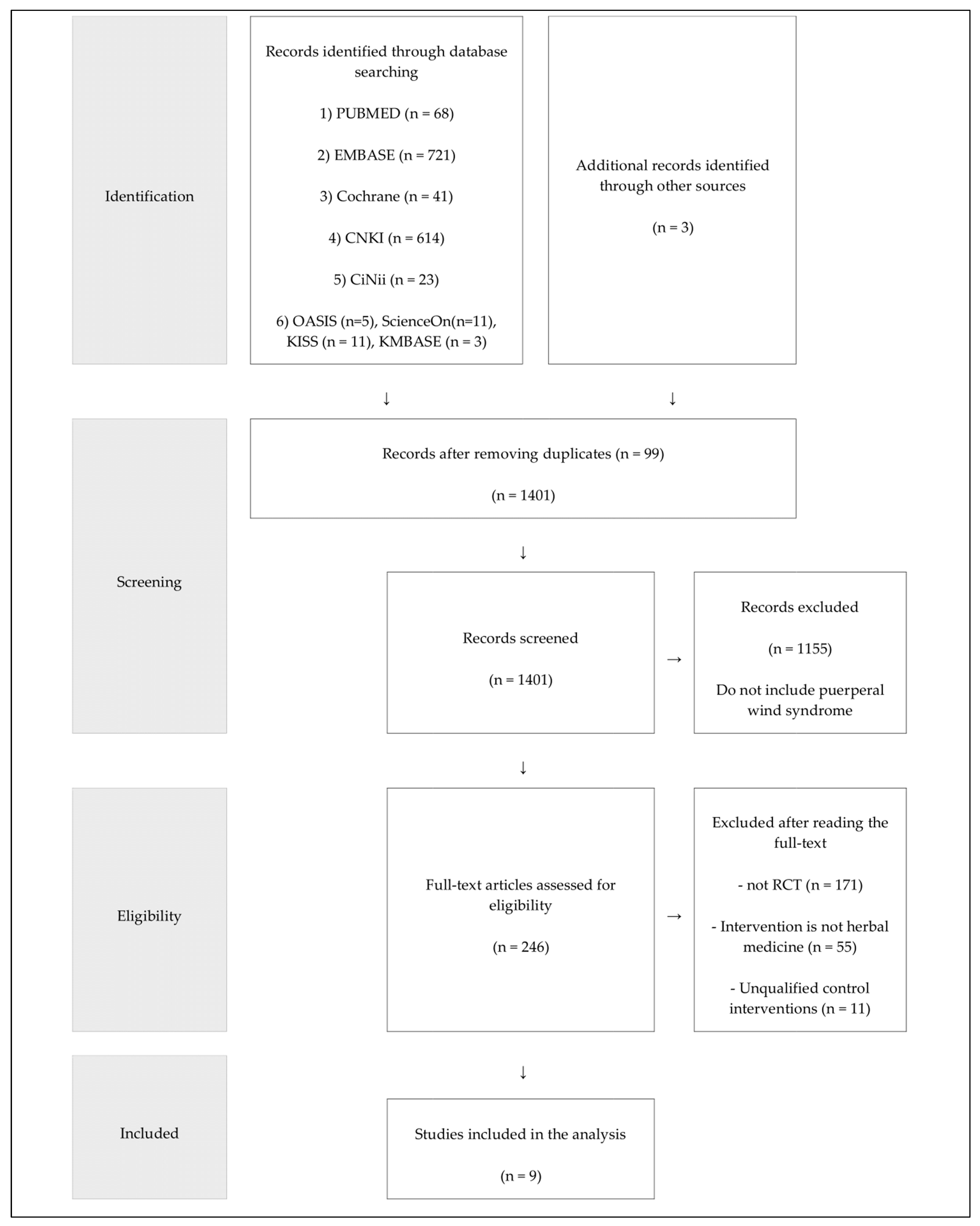
3.1. Study Selection and Description
3.2. Participants
3.3. Intervention
3.3.1. Name of Prescription
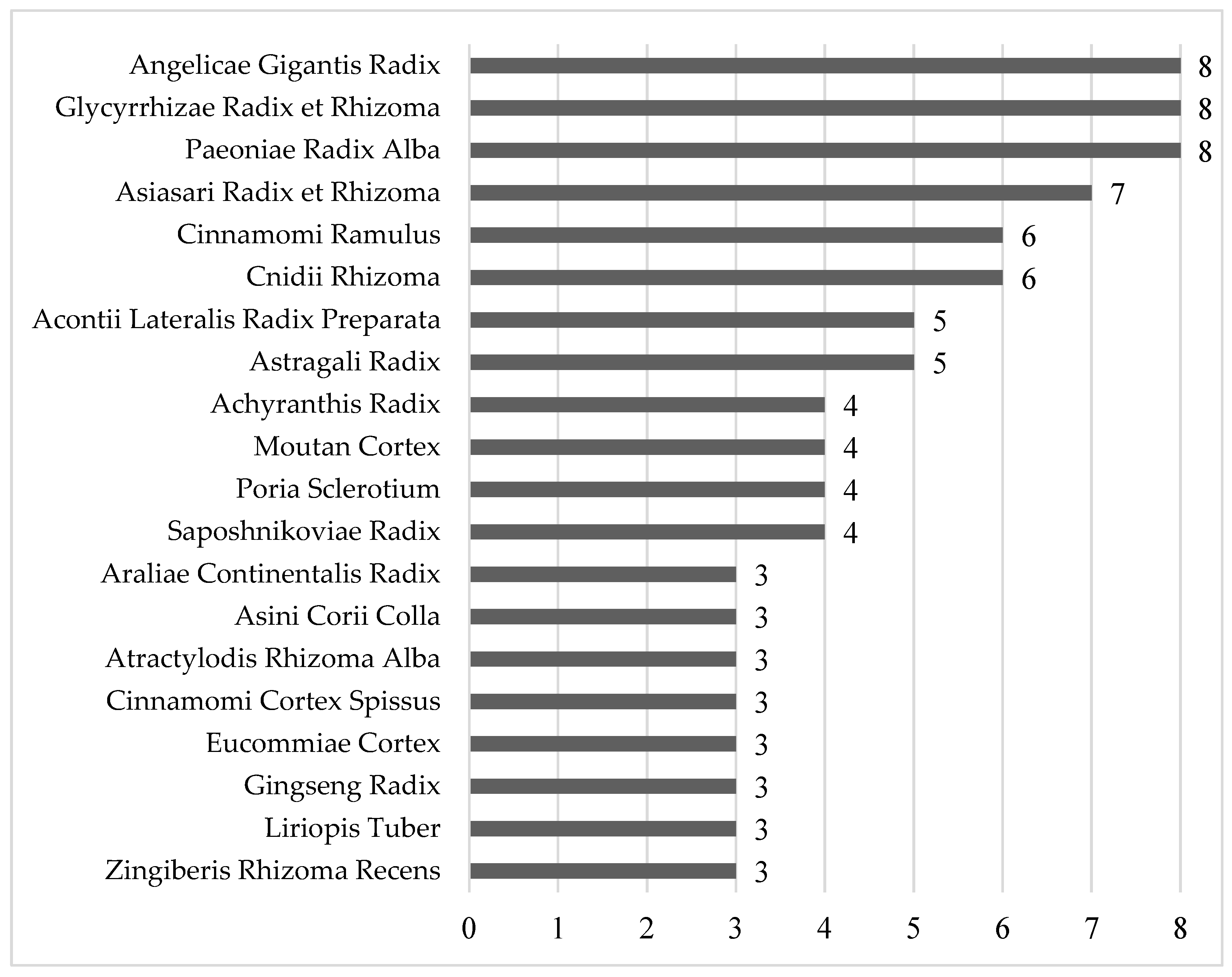
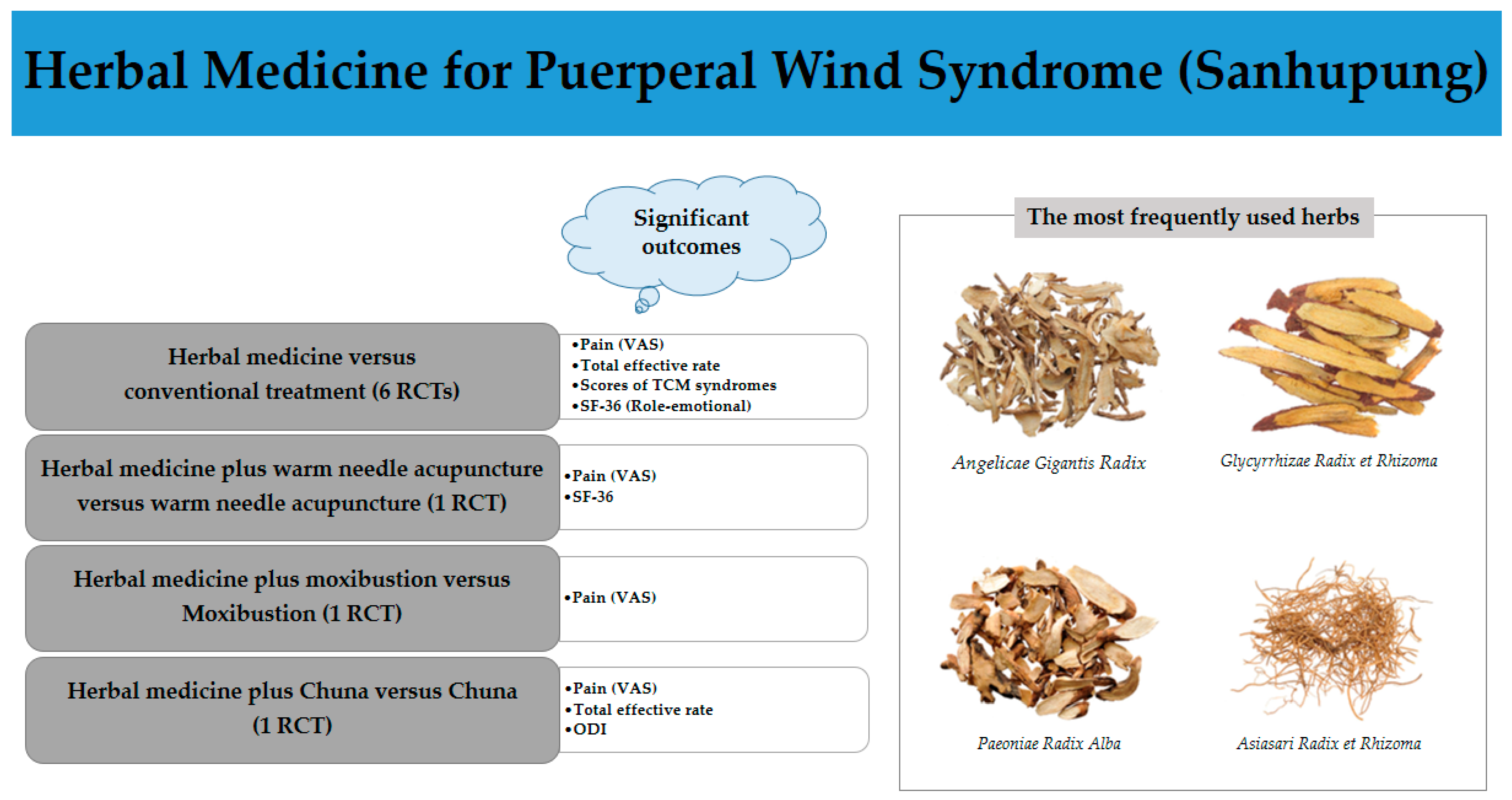
3.3.2. Herbs Used in the Included Studies
3.3.3. Modified Herbs
3.3.4. Duration and Frequency of Taking Herbal Medicine
3.3.5. Formulation of Herbal Medicine
3.4. Outcomes
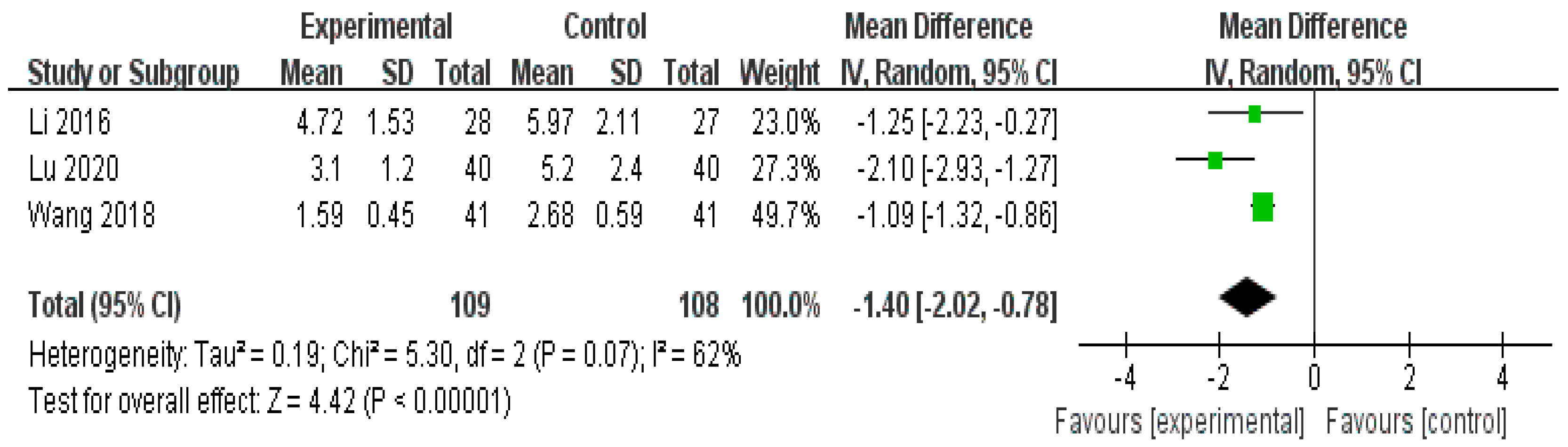
3.4.1. Pain (Visual Analog Scale [VAS])
- (1)
- Herbal medicine versus conventional treatment
- (2)
- Herbal medicine plus warm needle acupuncture versus warm needle acupuncture
- (3)
- Herbal medicine plus moxibustion versus moxibustion
- (4)
- Herbal medicine plus Chuna versus Chuna
3.4.2. Total Effective Rate
- (1)
- Herbal medicine versus conventional treatment
- (2)
- Herbal medicine plus warm needle acupuncture versus warm needle acupuncture
- (3)
- Herbal medicine plus moxibustion versus moxibustion
- (4)
- Herbal medicine plus Chuna versus Chuna
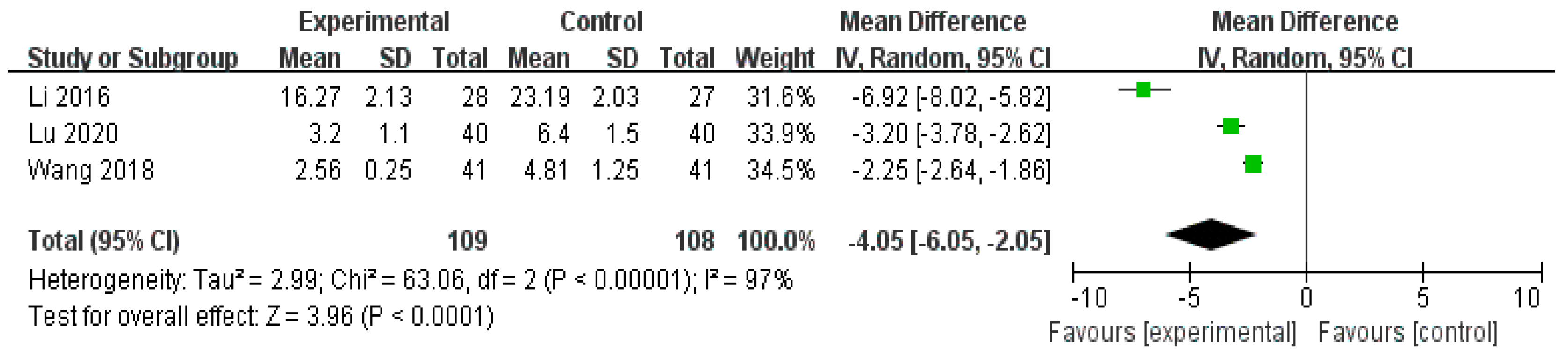
3.4.3. Scores of TCM syndromes
3.4.4. Oswestry Disability Index (ODI)
3.4.5. QOL
- (1)
- Herbal medicine versus conventional treatment
- (2)
- Herbal medicine plus conventional treatment versus conventional treatment alone
- (3)
- Herbal medicine plus warm needle acupuncture versus warm needle acupuncture
3.4.6. Adverse Events
4. Assessment of ROB
5. Publication Bias
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montgomery, K.S.; Best, M.; Aniello, T.B.; Phillips, J.D.; Hatmaker-Flanigan, E. Postpartum weight loss: Weight struggles, eating, exercise, and breast-feeding. J. Holist. Nurs. 2013, 31, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Franke, J.D.; Belz, S.; Fryer, G. Osteopathic manipulative treatment for low back pain and pelvic girdle pain during and after pregnancy: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2017, 21, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Sockol, L.E.; Epperson, C.N.; Barber, J.P. Preventing postpartum depression: A meta-analytic review. Clin. Psychol. Rev. 2013, 33, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Suh, H.W.; Park, H.S.; Youn, I.A.; Park, M.J.; Seo, J.H. Mothers’ experience of childbirth and perspectives on korena medicine-based postpartum care in korena: A qualitative study. Int. J. Environ. Res. Public Health 2022, 19, 5332. [Google Scholar] [CrossRef]
- Cush, J.J. Rheumatoid arthritis: Early diagnosis and treatment. Med. Clin. N. Am. 2021, 105, 355–365. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, D.I. A Delphi Survey for the Revision of the Diagnostic Criteria for Sanhupung (Puerperal Wind Disorder, U32.7). J. Korean Obstet. Gynecol. 2022, 35, 42–53. [Google Scholar] [CrossRef]
- Peng, C.C.; Pearce, E.N. An update on thyroid disorders in the postpartum period. J. Endocrinol. Investig. 2022, 45, 1497–1506. [Google Scholar] [CrossRef]
- Edlow, J.A.; Caplan, L.R.; O’Brien, K.; Tibbles, C.D. Diagnosis of acute neurological emergencies in pregnant and post-partum women. Lancet Neurol. 2013, 12, 175–185. [Google Scholar] [CrossRef]
- Wuytack, F.; O’Donovan, M. Outcomes and outcomes measurements used in intervention studies of pelvic girdle pain and lumbopelvic pain: A systematic review. Chiropr. Man. Ther. 2019, 27, 62. [Google Scholar] [CrossRef]
- Wiezer, M.; Hage-Franse, M.A.H.; Otto, A.; Wieffer-Platvoet, M.S.; Slotman, M.H.; Sanden, M.W.G.N.; Pool-goudzwaard, A.L. Risk factors for pelvic girdle pain postpartum and pregnancy related low back pain postpartum; a systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2020, 48, 102154. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Committee opinion No. 736: Optimizing postpartum care. Obstet. Gynecol. 2018, 131, e140–e150. [Google Scholar] [CrossRef] [PubMed]
- Prosser, R.; Hancock, M.J.; Nicholson, L.L.; Harvey, L.A.; LaStayo, P.; Hargreaves, I.; Herbert, R. Prognosis and prognostic factors for patients with persistent wrist pain who proceed to wrist arthroscopy. J. Hand Ther. 2012, 25, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Makeen, M.; Farrell, L.M.; LaSorda, K.R.; Deng, Y.; Altamirano, V.; Jarvis, O.; Kenkre, T.; Lim, G. Associations between postpartum pain, mood, and maternal–infant attachment and parenting outcomes. Sci. Rep. 2022, 12, 17814. [Google Scholar] [CrossRef] [PubMed]
- Baird, H.; Harris, H.B.; Santos, H.P., Jr. The Effects of Maternal Perinatal Depression on Child IQ: A Systematic Review. Matern. Child Health J. 2023, 27, 1489–1502. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.Z.; Sawadogo, R.; Tan, T.; Yuan, C.S. Effects of Herbal Medicines on Pain Management. Am. J. Chin. Med. 2020, 48, 1–16. [Google Scholar] [CrossRef]
- Eshkevari, L.; Trout, K.K.; Damore, J. Management of postpartum pain. J. Midwifery Women’s Health 2013, 58, 622–631. [Google Scholar] [CrossRef]
- Vermani, E.; Mittal, R.; Weeks, A. Pelvic girdle pain and low back pain in pregnancy: A review. Pain Pract. 2020, 10, 60–71. [Google Scholar] [CrossRef]
- Allaire, A.D.; Moos, M.K.; Wells, S.R. Complementary and alternative medicine in pregnancy: A survey of North Carolina certified nurse-midwives. Obstet. Gynecol. 2000, 95, 19–23. [Google Scholar] [CrossRef]
- Fung, F.Y.; Linn, Y.C. Developing traditional Chinese medicine in the era of evidence-based medicine: Current evidences and challenges. Evid. Based Complement. Altern. Med. 2015, 2015, 425037. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.Z.; Hesse-Fong, J.; Lin, J.G.; Yuan, C.S. Application of Chinese medicine in acute and critical medical conditions. Am. J. Chin. Med. 2019, 47, 1223–1235. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, N.Y. The Study to Actual Condition on Postpartum Care using Korean Health Panel Data. Asia-Pac. J. Multimed. Serv. Converg. Art Humanit. Sociol. 2017, 7, 533–543. [Google Scholar] [CrossRef]
- Fu, Y.; Tai, Y.; Yang, Y. Description and Treatment Progress of Postpartum Body Pain in Chinese Medicine. Curr. Res. Med. Sci. 2023, 2, 31–39. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Q.; Zhang, Y. Research Progress of Traditional Chinese Medicine in the Treatment of Postpartum Apoplexy Based on Syndrome Differentiation. Guangming Tradit. Chin. Med. 2022, 37, 2854–2858. [Google Scholar] [CrossRef]
- Lin, M.J.; Chen, H.W.; Liu, P.H.; Cheng, W.J.; Kuo, S.L.; Kao, M.C. The prescription patterns of traditional Chinese medicine for women with polycystic ovary syndrome in Taiwan: A nationwide population-based study. Medicine 2019, 98, e15890. [Google Scholar] [CrossRef]
- Ried, K. Chinese herbal medicine for female infertility: An updated meta-analysis. Complement. Ther. Med. 2015, 23, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Lee, H.S.; Lee, D.H.; Jo, H.G. Dangguijagyag-san for primary dysmenorrhea: A PRISMA-compliant systematic review and meta-analysis of randomized-controlled trials. Medicine 2020, 99, e22761. [Google Scholar] [CrossRef]
- Peng, F. The Intervention of DunHuang Postpartum Prescription on the Patients after Delivery. West. J. Tradit. Chin. Med. 2014, 27, 21–23. [Google Scholar]
- Chang, P.J.; Lin, C.C.; Chen, Y.C.; Chuang, C.H.; Tseng, Y.C.; Hseish, W.S.; Lin, S.J.; Chen, P.C. Use of herbal dietary supplement Si-Wu-Tang and health-related quality of life in postpartum women: A population-based correlational study. Evid. Based Complement. Altern. Med. 2013, 2013, 790474. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (Updated February 2022). Available online: www.training.cochrane.org/handbook (accessed on 8 August 2023).
- Lu, X.Q. Clinical observation on modified Wenjing Decoction in treating post-partum body pain, cold and Qi-Stagnation. Sci. Tech. Inf. Gansu 2020, 49, 15–17. [Google Scholar] [CrossRef]
- Zhang, P.H. Application and effectiveness of Wenjing Decoction in treating Postpartum body pain. Psychol. Mon. 2019, 9, 142. [Google Scholar] [CrossRef]
- Chen, X.; Ma, D. Clinical Study on Xiaoxuming Tang for Postpartum Impediment Syndrome. J. New Chin. Med. 2021, 4, 39–41. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhou, M. Influence of Huangqi Guizhi Wuwu decoction on joint pain and sleep quality in patients with postpartum arthralgia. Mod. Med. J. 2018, 46, 611–615. [Google Scholar] [CrossRef]
- Li, N.; Lin, C.; Liu, Q.; Jiang, Y.; Lu, J. Therapeutic Evaluation of Chanhoubi Decoction in Treatment of Postpartum Paralysis. Liaoning J. Tradit. Chin. Med. 2016, 43, 333–334. [Google Scholar] [CrossRef]
- Zhao, G.C. Analysis on the Treatment of Postpartum Joint Pain. Cap. Med. 2018, 11, 24. [Google Scholar]
- Mao, B.; Zhang, L.; Wei, W. Warming Needle Moxibustion Combined with Yangyuan Huoxue Decoction for the Treatment of Postpartum Backache. World Chin. Med. 2020, 15, 2317–2320. [Google Scholar] [CrossRef]
- Zhang, F.; Du, G.; Zhang, C. Clinical observation on grain-moxibustion combined with Duhuo Jisheng decoction in treatment of postpartum pain of exogenous syndrome. China Med. Pharm. 2020, 10, 55–58. [Google Scholar]
- Wu, H.; Sun, J.; Li, Y. Clinical Study of Duhuo Jisheng Decoction Combined with American Chiropractic Therapy in Treatment of Postpartum Low Back Pain. Chin. Foreign Med. Res. 2019, 17, 38–40. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Chen, W. Clinical Observation of Radix Astragali Cassia Twigs Five Decoction in the Treatment of Postpartum Body Pain. Chin. J. Med. Guide 2014, 16, 1219–1220. [Google Scholar]
- Weiner, D.K.; Ernst, E. Complementary and alternative approaches to the treatment of persistent musculoskeletal pain. Clin. J. Pain 2004, 20, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wu, Y.; Zhang, W.; Deng, P.; Huang, F.S.; Du, X.; Chen, Z.J.; Ma, Y.F. Efficacy and Safety of Acupuncture Combined with Herbal Medicine in Treating Gouty Arthritis: Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2021, 2021, 8161731. [Google Scholar] [CrossRef] [PubMed]
- Aitken, Z.; Garrett, C.C.; Hewitt, B.; Keogh, L.; Hocking, J.S.; Kavanagh, A.M. The maternal health outcomes of paid maternity leave: A systematic review. Soc. Sci. Med. 2015, 130, 32–41. [Google Scholar] [CrossRef]
- Hewitt, B.; Strazdins, L.; Martin, B. The benefits of paid maternity leave for mothers’ post-partum health and wellbeing: Evidence from an Australian evaluation. Soc. Sci. Med. 2017, 182, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, M.S.; Bhatia, R.; Riano, N.S.; Faria, L.D.; Catapano-Friedman, S.; Ravven, S.; Weissman, B.; Nzodom, C.; Alexander, A.; Budde, K.; et al. The Impact of Paid Maternity Leave on the Mental and Physical Health of Mothers and Children: A Review of the Literature and Policy Implications. Harv. Rev. Psychiatry 2020, 28, 113–126. [Google Scholar] [CrossRef]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and decursinol angelate: Molecular mechanism and therpeutic potential in inflammatory diseas. Inflamm. Res. 2017, 67, 209–218. [Google Scholar] [CrossRef]
- Oh, Y.C.; Jeong, Y.H.; Li, W.; Go, Y.H. Angelicae Gigantis Radix Regulates LPS-Induced Neuroinflammation in BV2 Microglia by Inhibiting NF-κB and MAPK Activity and Inducing Nrf-2 Activity. Molecules 2019, 24, 3755. [Google Scholar] [CrossRef]
- Li, X.Y.; Lin, S.; Lin, Y.; Huang, L.Q.; Xie, X.M.; Tian, G.H. Medication rules of traditional Chinese medicine compounds for pain. Zhongguo Zhong Yao Za Zhi 2023, 48, 3386–3393. [Google Scholar] [CrossRef]
- Yan, T.; Wang, H.; Cao, L.; Wang, Q.; Takahashi, S.; Yagai, T.; Li, G.; Krausz, K.W.; Wang, G.; Gonzalez, F.J.; et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab. Dispos. 2018, 46, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, B.P.P. Liquoric (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Wang, Q.S.; Gao, T. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol. 2014, 52, 1189–1195. [Google Scholar] [CrossRef]
- Yan, B.; Shen, M.; Fang, J.; Wei, D.; Qin, L. Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J. Pharm. Biomed. Anal. 2018, 160, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Suzuki, Y.; Yuzurihara, M.; Kase, Y.; Takeda, S.; Watanabe, S.; Aburada, M.; Miyamoto, K. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesi in mice. Eur. J. Pharmacol. 2006, 553, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.F.; Sherriff, J.; Hattingh, H.L.; Parsons, R.; Tee, L.B.G. The use of herbal medicines during breastfeeding: A population-based survey in western australia. BMC Complement. Altern. Med. 2013, 13, 317. [Google Scholar] [CrossRef]
- Al-Sawalha, N.A.; Tahaineh, L.; Sawalha, A.; Almomani, B.A. Medication use in breastfeeding women: A national study. Breastfeed. Med. 2016, 11, 386–391. [Google Scholar] [CrossRef]
- James, B.P.; Kaikai, A.I.; Bah, A.J.; Steel, A.; Wardle, J. Herbal medicine use during breastfeeding: A cross-sectional study among mothers visiting public heallth facilities in the western area of Sierra Leone. BMC Complement. Altern. Med. 2019, 19, 66. [Google Scholar] [CrossRef]
- Bazzano, A.N.; Hofer, R.; Thibeau, S.; Gillispie, V.; Jacobs, M.; Theall, K.P. A review of herbal and pharmaceutical galactagogues for breast-feeding. Ochsner J. 2016, 16, 511–524. [Google Scholar]
- Chen, Y.C. Chinese Values, Health and Nursing. J. Adv. Nurs. 2001, 36, 270–273. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, H.Y.; Lou, Y.Q. Syndrome differentiation and treatment of postpartum arthralgia. China J. Tradit. Chin. Med. Pharm. 2020, 35, 726–728. [Google Scholar]
- Yu, W.; Song, C.; Lei, Z.; Li, Y.; He, X.; Yu, J.; Yang, X. Anti-fatigue effect of traditional Chinese medicines: A review. Saudi Pharm. J. 2023, 31, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.J.; Tseng, Y.C.; Chuang, C.H.; Chen, Y.C.; Hsieh, W.S.; Hurng, B.S.; Lin, S.J.; Chen, P.C. Use of Sheng-Hua-Tang and health-related quality of life in postpartum women: A population-based cohort study in Taiwan. Int. J. Nurs. Stud. 2010, 47, 13–19. [Google Scholar] [CrossRef] [PubMed]



| Author, Year | Sample Size | Average Age (Experimental Group/Control Group) | Primipara/Multipara (Experimental Group/Control Group) | Experimental Group (No. of Participants Analyzed/ Randomized) | Control Group (No. of Participants Analyzed/ Randomized) | Outcome Measures | Main Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Lu (2020) [31] | 80 | 27.5 ± 2.4 28.2 ± 3.2 | 23/17 21/19 | Modified Wenjing decoction (40/40) | Ibuprofen (40/40) | (1) TER | (1) No significant difference (p = 0.08) | TG < CG (p < 0.05) |
| (2) VAS | (2) Positive (p < 0.05) | |||||||
| (3) Scores of TCM syndromes | (3) Positive (p < 0.05) | |||||||
| (4) SF-36 ① Physical functioning ② Bodily pain ③ Social functioning ④ Role-emotional | (4) ① Positive (p < 0.00001) ② Positive (p < 0.00001) ③ Positive (p < 0.00001) ④ Positive (p < 0.00001) | |||||||
| Zhang (2019) [32] | 60 | 29.3 29.2 | 18/12 19/11 | Wenjing decoction (30/30) | Ibuprofen (30/30) | (1) TER | (1) No significant difference (p = 0.05) | TG < CG (p < 0.05) |
| Chen (2021) [33] | 92 | 33.29 ± 3.45 33.09 ± 3.31 | 12/34 13/33 | Xiaoxuming Tang (46/46) | Indomethacin cataplasm (46/46) | (1) TER | (1) Positive (p < 0.05) | TG < CG (p < 0.05) |
| (2) SF-36 ① Role-physical ② General health ③ Vitality ④ Social Functioning ⑤ Role-emotional ⑥ Mental health | (2) ① Positive (p = 0.001) ② Positive (p = 0.002) ③ Positive (p < 0.0001) ④ Positive (p = 0.002) ⑤ Positive (p = 0.0008) ⑥ Positive (p = 0.001) | |||||||
| Wang (2018) [34] | 82 | 29.02 ± 4.41 28.79 ± 4.25 | 31/10 33/8 | Huangqi Guizhi Wuwu decoction (41/41) | Indomethacin cataplasm (41/41) | (1) TER | (1) No significant difference (p = 0.05) | N.R. |
| (2) VAS | (2) Positive (p < 0.05) | |||||||
| (3) Scores of TCM syndromes | (3) Positive (p < 0.05) | |||||||
| (4) PSQI | (4) Positive (p < 0.05) | |||||||
| (5) Number of painful joints | (5) Positive (p < 0.05) | |||||||
| (6) Duration of morning stiffness | (6) Positive (p < 0.05) | |||||||
| Li (2016) [35] | 60 | 30.16 ± 7.87 29.73 ± 7.49 | N.R. | Chanhoubi decoction (28/30) | Indomethacin cataplasm (27/30) | (1) TER | (1) Positive (p < 0.05) | No significant difference |
| (2) VAS | (2) Positive (p < 0.05) | |||||||
| (3) Scores of TCM syndromes | (3) Positive (p < 0.01) | |||||||
| (4) WHOQOL-BREF | (4) Positive (p < 0.05) | |||||||
| (5) Number of painful joints | (5) No significant difference | |||||||
| (6) Duration of morning stiffness | (6) Positive (p < 0.01) | |||||||
| Zhao (2018) [36] | 96 | N.R. | N.R. | Chanhoubi decoction + indomethacin cataplasm (48/48) | Indomethacin cataplasm (48/48) | (1) Pain improvement rate | (1) No significant difference (p = 0.31) | N.R. |
| (2) Period to improvement in symptoms (weeks) | (2) Positive (p < 0.00001) | |||||||
| (3) Scores of quality of life | (3) Positive (p < 0.00001) | |||||||
| Mao (2020) [37] | 64 | 28.34 ± 3.21 28.14 ± 3.17 | N.R. | Yangyuan Huoxue decoction + warm needle acupuncture (32/32) | Warm needle acupuncture (32/32) | (1) TER | (1) No significant difference (p = 0.11) | N.R. |
| (2) VAS | (2) Positive (p < 0.00001) | |||||||
| (3) ODI | (3) Positive (p < 0.00001) | |||||||
| (4) SF-36 ① Sleep ② Mental health ③ Diet ④ Social functioning | (4) ① Positive (p < 0.00001) ② Positive (p < 0.00001) ③ Positive (p < 0.00001) ④ Positive (p < 0.00001) | |||||||
| (5) Serum cytokine level ① Serum 6-Keto-PGFlα ② IL-1β ③ CGRP ④ TXB2 | (5) ① Positive (p = 0.004) ② Positive (p < 0.00001) ③ Positive (p < 0.00001) ④ Positive (p < 0.00001) | |||||||
| Zhang (2020) [38] | 60 | 27.1 ± 4.0 26.3 ± 3.7 | N.R. | Duhuo Jisheng decoction + moxibustion (30/30) | Moxibustion (30/30) | (1) TER | (1) No significant difference (p = 0.28) | N.R. |
| (2) VAS | (2) Positive (p = 0.0006) | |||||||
| Wu (2019) [39] | 58 | 35.41 ± 5.24 36.02 ± 5.09 | N.R. | Duhuo Jisheng decoction + Chuna (29/29) | Chuna (29/29) | (1) TER | (1) Positive (p = 0.04) | TG < CG (p < 0.05) |
| (2) VAS | (2) Positive (p < 0.0001) | |||||||
| (3) ODI | (3) Positive (p < 0.0001) | |||||||
| (4) Recurrence rate | (4) Positive (p = 0.04) |
| Author, Year | Duration | Frequency | Composition of Herbal Medicine | Modified Herb |
|---|---|---|---|---|
| Lu (2020) [31] | 1 month | Twice a day | Glycyrrhizae Radix et Rhizoma 6 g, Zingiberis Rhizoma Recens 3 pieces, Asini Corii Colla 10 g, Moutan Cortex 8 g, Liriopis Tuber 20 g, Asiasari Radix et Rhizoma 1 g, Cnidii Rhizoma 10 g, Angelicae Gigantis Radix 15 g, Schizonepetae Spica 15 g, Evodiae Fructus 8 g, Cinnamomi Ramulus 15 g, Atractylodis Rhizoma alba 10 g, Paeoniae Radix Alba 30 g, Acontii Lateralis Radix Preparata 8 g, Astragali Radix 15 g, Astragali Radix Preparata 20 g, Gingseng Radix 8 g |
|
| Zhang (2019) [32] | 1 month | Twice a day | Astragali Radix 15 g, Angelicae Gigantis Radix 15 g, Schizonepetae Spica 15 g, Astragali Radix Preparata 20 g, Liriopis Tuber 20 g, Gingseng Radix 8 g, Acontii Lateralis Radix Preparata 8 g, Moutan Cortex 8 g, Evodiae Fructus 8 g, Paeoniae Radix Alba 30 g, Asini Corii Colla 10 g, Atractylodis Rhizoma alba 10 g, Cnidii Rhizoma 10 g, Cinnamomi Ramulus 15 g, Asiasari Radix et Rhizoma 1 g, Glycyrrhizae Radix et Rhizoma 6 g, Zingiberis Rhizoma Recens 3 pieces |
|
| Chen (2021) [33] | 3 months | Twice a day | Sinomeni Caulis et Rhizoma 10 g, Paeoniae Radix rubra 10 g, Armeniacae Semen 10 g, Saposhnikoviae Radix 10 g, Cnidii Rhizoma 10 g, Cinnamomi Cortex 15 g, Scutellariae Radix 15 g, Codonopsis Pilosulae Radix 15 g, Acontii Lateralis Radix Preparata 6 g, Ephedrae Herba 6 g, Glycyrrhizae Radix et Rhizoma 6 g | |
| Wang (2018) [34] | 1 month | Three times a day | Astragali Radix 45 g, Cinnamomi Ramulus 15 g, Paeoniae Radix Alba 20 g, Zingiberis Rhizoma Recens 18 g, Zizyphi Fructus 4 pieces, Cocicis Semen 30 g, Mucunae Caulis 30 g, Salviae Miltiorrhizae Radix 15 g, Atractyodis Rhizoma 15 g, Achyranthis Radix 15 g, Citri Unshius Pericarpium 12 g, Angelicae Gigantis Radix 12 g, Scorpio 10 g, Carthami Flos 10 g, Persicae Semen 10 g, Atractylodis Rhizoma alba 10 g, Saposhnikoviae Radix 10 g |
|
| Li (2016) [35] | 8 weeks | Three times a day | Cinnamomi Ramulus 10 g, Poria Sclerotium 20 g, Moutan Cortex 10 g, Paeoniae Radix Alba 15 g, Persicae Semen 10 g, Curcumae Longae Rhizoma 15 g, Acontii Lateralis Radix Preparata 10 g, Asiasari Radix et Rhizoma 6 g, Angelicae Gigantis Radix 10 g, Zingiberis Rhizoma 10 g, Scorpio 5 g, Astragali Radix 20 g, Glycyrrhizae Radix et Rhizoma 6 g | |
| Zhao (2018) [36] | 10 weeks | Once a day | Poria Sclerotium 20 g, Cinnamomi Ramulus 10 g, Paeoniae Radix Alba 15 g, Curcumae Longae Rhizoma 15 g, Moutan Cortex 10 g, Asiasari Radix et Rhizoma 6 g, Zingiberis Rhizoma 10 g, Angelicae Gigantis Radix 10 g, Astragali Radix 20 g, Glycyrrhizae Radix et Rhizoma 6 g | |
| Mao (2020) [37] | 14 days | Once a day | Gingseng Radix Rubra 15 g, Astragali Radix Preparata 20 g, Acontii Lateralis Radix Preparata 8 g, Cinnamomi Ramulus 15 g, Asiasari Radix et Rhizoma 3 g, Osterici Radix 9 g, Araliae Continentalis Radix 12 g, Eucommiae Cortex 15 g, Achyranthis Radix 15 g, Liriopis Tuber 20 g, Angelicae Gigantis Radix 15 g, Cnidii Rhizoma 10 g, Mucunae Caulis 15 g, Asini Corii Colla 10 g, Piperis Kandsurae Caulis 15 g, Corydalis Tuber 15 g, Clematidis Radix 20 g, Paeoniae Radix Alba 30 g, Bupleuri Radix 9 g, Chaenomelis Fructus 9 g, Citri Unshius Pericarpium 8 g, Glycyrrhizae Radix et Rhizoma 6 g |
|
| Zhang (2020) [38] | 10 days | Twice a day | Araliae Continentalis Radix 9 g, Loranthi Ramulus 6 g, Eucommiae Cortex 6 g, Achyranthis Radix 6 g, Asiasari Radix et Rhizoma 6 g, Gentianae Macrophyllae Radix 6 g, Poria Sclerotium 6 g, Cinnamomi Cortex 6 g, Saposhnikoviae Radix 6 g, Cnidii Rhizoma 6 g, Gingseng Radix 6 g, Glycyrrhizae Radix et Rhizoma 6 g, Angelicae Gigantis Radix 6 g, Paeoniae Radix Alba 6 g, Rehmanniae Radix 6 g | - |
| Wu (2019) [39] | 7 days | Twice a day | Loranthi Ramulus 20 g, Achyranthis Radix 20 g, Rehmanniae Radix Preparata 20 g, Eucommiae Cortex 20 g, Gentianae Macrophyllae Radix 15 g, Araliae Continentalis Radix 15 g, Saposhnikoviae Radix 15 g, Paeoniae Radix Alba 15 g, Angelicae Gigantis Radix 15 g, Cnidii Rhizoma 15 g, Codonopsis Pilosulae Radix 15 g, Poria Sclerotium 15 g, Cinnamomi Cortex 10 g, Asiasari Radix et Rhizoma 10 g, Glycyrrhizae Radix et Rhizoma 10 g | - |
| Author, Year | Ingredient | Dose | Administration Route | Frequency | Duration |
|---|---|---|---|---|---|
| Lu (2020) [31] | Ibuprofen | 1 capsule (0.3 g) | Oral | Twice a day | 1 month |
| Zhang (2019) [32] | Ibuprofen | 1 capsule (0.3 g) | Oral | Twice a day | 1 month |
| Chen (2021) [33] | Indomethacin | N.R. | Cataplasm | Once a day | 3 months |
| Wang (2018) [34] | Indomethacin | N.R. | Cataplasm | Once a day | 1 month |
| Li (2016) [35] | Indomethacin | N.R. | Cataplasm | Once a day | 8 weeks |
| Mao (2020) [36] | Indomethacin | N.R. | Cataplasm | Once a day | 10 weeks |
| Author, Year | Scale of TER | Symptoms Included in the TER Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|
| Pain | Discomfort | Coldness | Numbness | Swelling | Functional Activity | Fatigue | ||
| Lu (2020) [31] | 3-point scale | O | O | X | O | O | O | X |
| Zhang (2019) [32] | 3-point scale | O | X | X | O | X | O | X |
| Chen (2021) [33] | 4-point scale | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. |
| Wang (2018) [34] | 4-point scale | O | X | O | O | X | O | O |
| Li (2016) [35] | 4-point scale | O | X | O | O | X | X | O |
| Mao (2020) [37] | 3-point scale | O | X | X | X | X | O | X |
| Zhang (2020) [38] | 4-point scale | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. |
| Wu (2019) [39] | 3-point scale | O | X | X | X | X | O | X |
| Outcome | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95%) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Assumed Risk | |||||
| Placebo | Risk Difference with Pain (VAS) | ||||
| Herbal medicine versus conventional treatment | 217 (3 studies) | ⨁⨁⨁◯ Moderate | - | - | MD 1.4 lower (2.02 lower to 0.78 lower) |
| Herbal medicine plus warm needle acupuncture versus warm needle acupuncture | 64 (1 study) | ⨁⨁◯◯ Low | - | - | MD 1.4 lower (2.02 lower to 0.78 lower) |
| Herbal medicine plus moxibustion versus moxibution | 60 (1 study) | ⨁⨁◯◯ Low | - | - | MD 1.4 lower (2.02 lower to 0.78 lower) |
| Herbal medicine plus Chuna versus Chuna | 58 (1 study) | ⨁⨁◯◯ Low | - | - | MD 1.28 lower (1.85 lower to 0.71 lower) |
| Outcome | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95%) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Assumed Risk | |||||
| Placebo | Risk Difference with TER | ||||
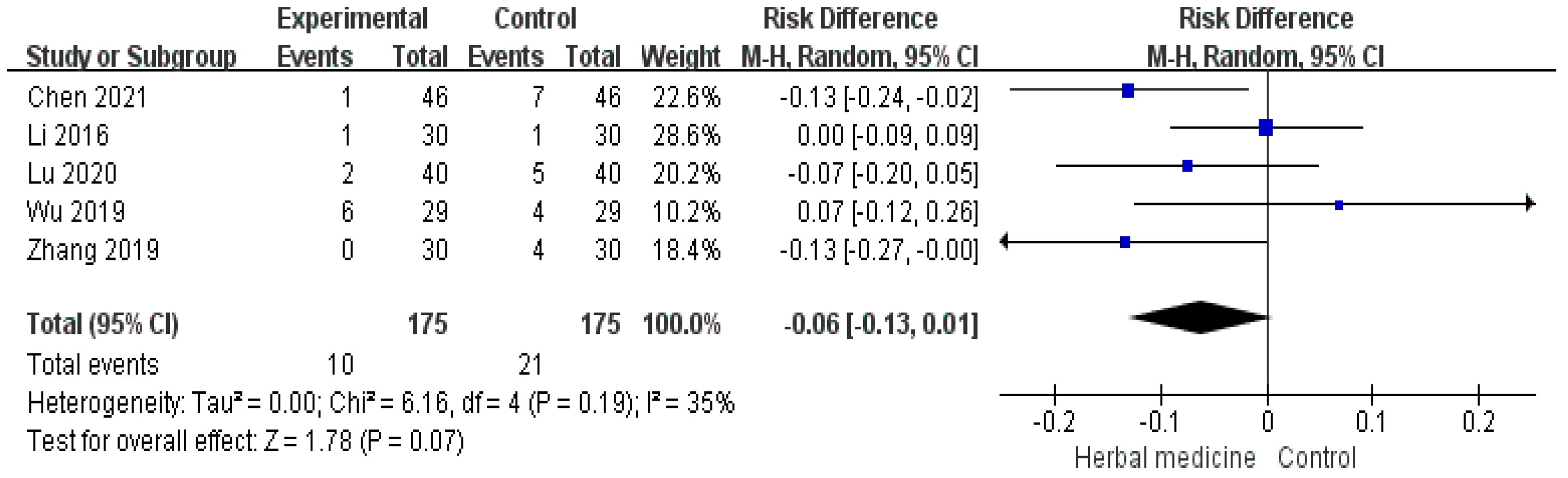
| Herbal medicine versus conventional treatment | 369 (5 studies) | ⨁⨁⨁◯ Moderate | RR 1.19 (1.10 to 1.29) | 772 per 1000 | 147 more per 1000 (77 more to 224 more) |
| Herbal medicine plus warm needle acupuncture versus warm needle acupuncture | 64 (1 study) | ⨁⨁◯◯ Low | RR 1.21 (0.96 to 1.52) | 750 per 1000 | 157 more per 1000 (30 fewer to 390 more) |
| Herbal medicine plus moxibustion versus moxibution | 60 (1 study) | ⨁⨁◯◯ Low | RR 1.13 (0.91 to 1.39) | 800 per 1000 | 104 more per 1000 (72 fewer to 312 more) |
| Herbal medicine plus Chuna versus Chuna | 58 (1 study) | ⨁⨁◯◯ Low | RR 1.29 (1.01 to 1.64) | 724 per 1000 | 210 more per 1000 (7 more to 463 more) |
| Outcome | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95%) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Assumed Risk | |||||
| Placebo | Risk Difference with Scores of TCM Syndromes | ||||
| Herbal medicine versus conventional treatment | 217 (3 studies) | ⨁⨁⨁◯ Moderate | - | - | MD 4.05 lower (6.05 lower to 2.05 lower) |
| Outcome | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95%) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Assumed Risk | |||||
| Placebo | Risk Difference with ODI | ||||
| Herbal medicine plus Chuna versus Chuna | 58 (1 study) | ⨁⨁◯◯ Low | - | - | MD 4.92 lower (7.32 lower to 2.52 lower) |
| Outcome | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95%) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Assumed Risk | |||||
| Placebo | Risk Difference with QOL | ||||
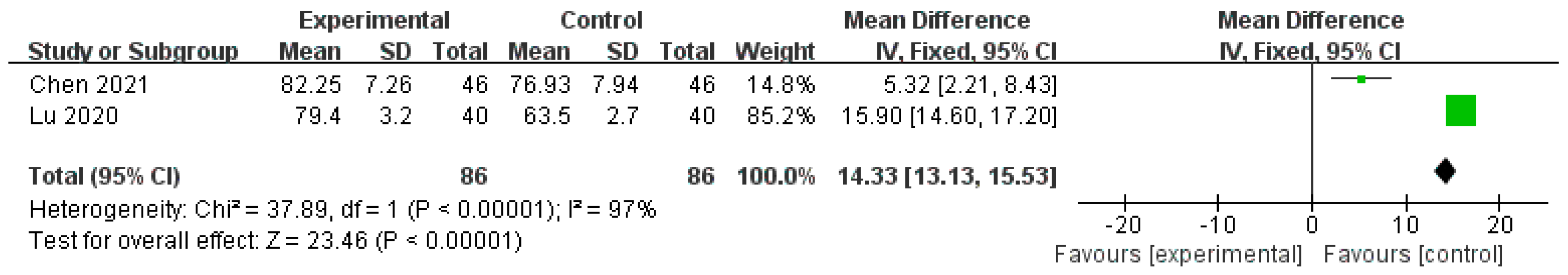
| SF-36 (Role-emotional) | 172 (2 studies) | ⨁⨁⨁◯ Moderate | - | - | MD 14.33 higher (13.13 higher to 15.53 higher) |
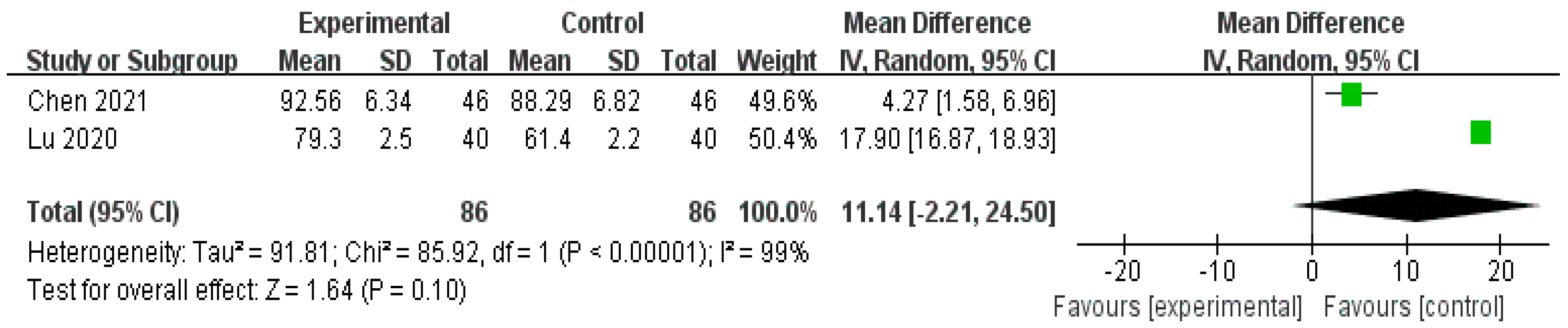
| SF-36 (Social functioning) | 172 (2 studies) | ⨁⨁⨁◯ Moderate | - | - | MD 11.14 higher (2.21 lower to 24.5 higher) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, N.-Y.; Lee, H.-Y.; Hwang, S.-I.; Sung, S.-H.; Cho, S.-J.; Yoon, Y.-J.; Park, J.-K. Herbal Medicine for Postpartum Pain: A Systematic Review of Puerperal Wind Syndrome (Sanhupung). Healthcare 2023, 11, 2743. https://doi.org/10.3390/healthcare11202743
Kwon N-Y, Lee H-Y, Hwang S-I, Sung S-H, Cho S-J, Yoon Y-J, Park J-K. Herbal Medicine for Postpartum Pain: A Systematic Review of Puerperal Wind Syndrome (Sanhupung). Healthcare. 2023; 11(20):2743. https://doi.org/10.3390/healthcare11202743
Chicago/Turabian StyleKwon, Na-Yoen, Hee-Yoon Lee, Su-In Hwang, Soo-Hyun Sung, Su-Jin Cho, Young-Jin Yoon, and Jang-Kyung Park. 2023. "Herbal Medicine for Postpartum Pain: A Systematic Review of Puerperal Wind Syndrome (Sanhupung)" Healthcare 11, no. 20: 2743. https://doi.org/10.3390/healthcare11202743
APA StyleKwon, N.-Y., Lee, H.-Y., Hwang, S.-I., Sung, S.-H., Cho, S.-J., Yoon, Y.-J., & Park, J.-K. (2023). Herbal Medicine for Postpartum Pain: A Systematic Review of Puerperal Wind Syndrome (Sanhupung). Healthcare, 11(20), 2743. https://doi.org/10.3390/healthcare11202743





