Effectiveness of Osteopathic Manipulative Treatment in Adults with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Eligibility Criteria
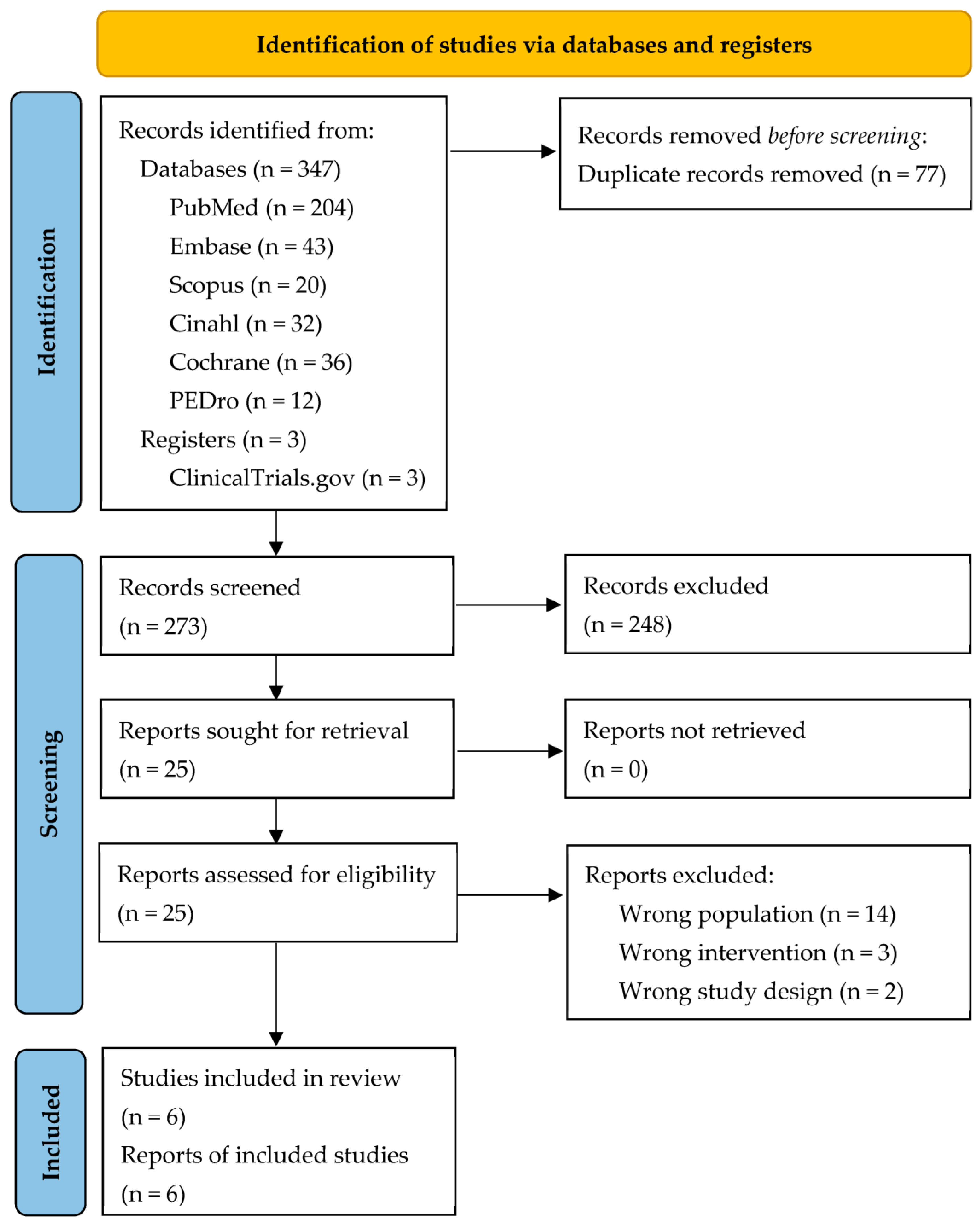
2.4. Study Selection and Data Collection
2.5. Outcomes
2.6. Assessment of Risk of Bias
2.7. Data Synthesis
3. Results
3.1. Studies Selection
3.2. Description of the Studies
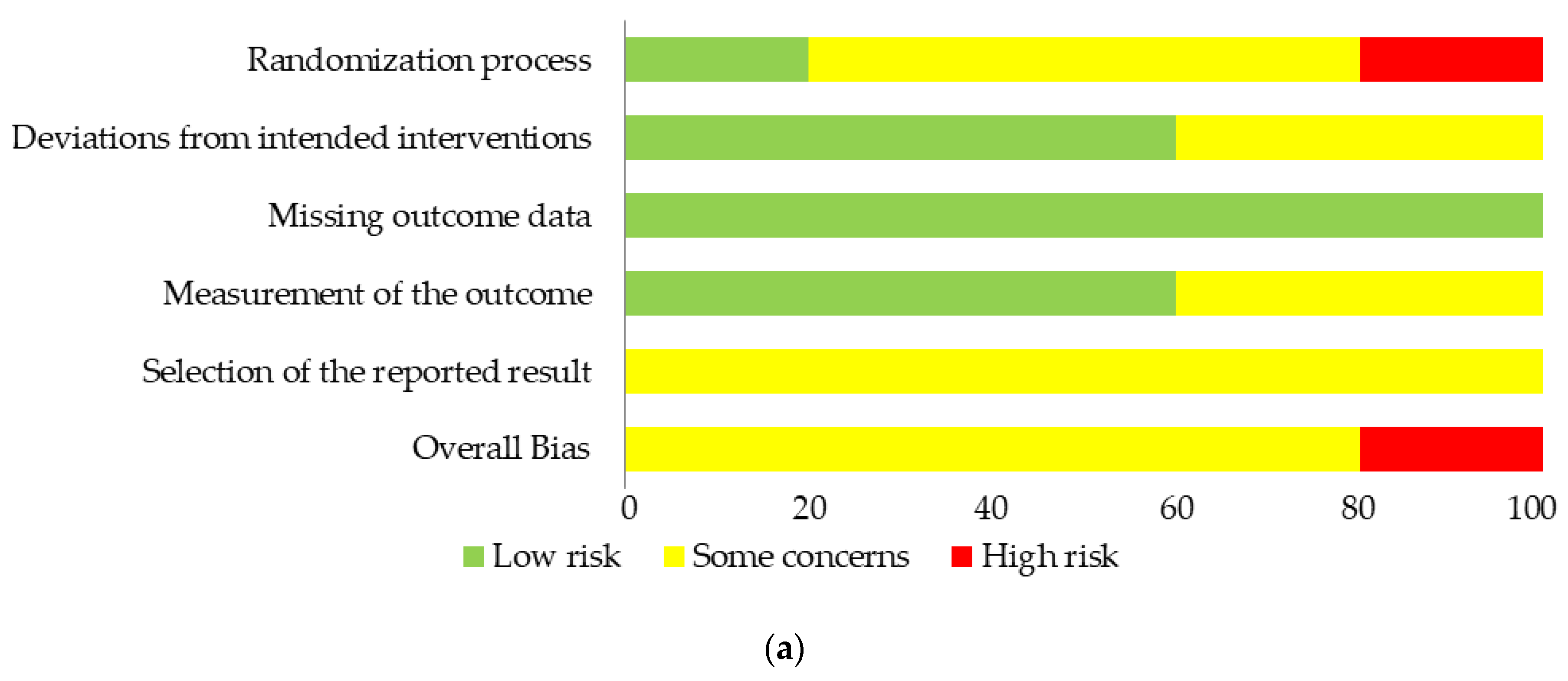
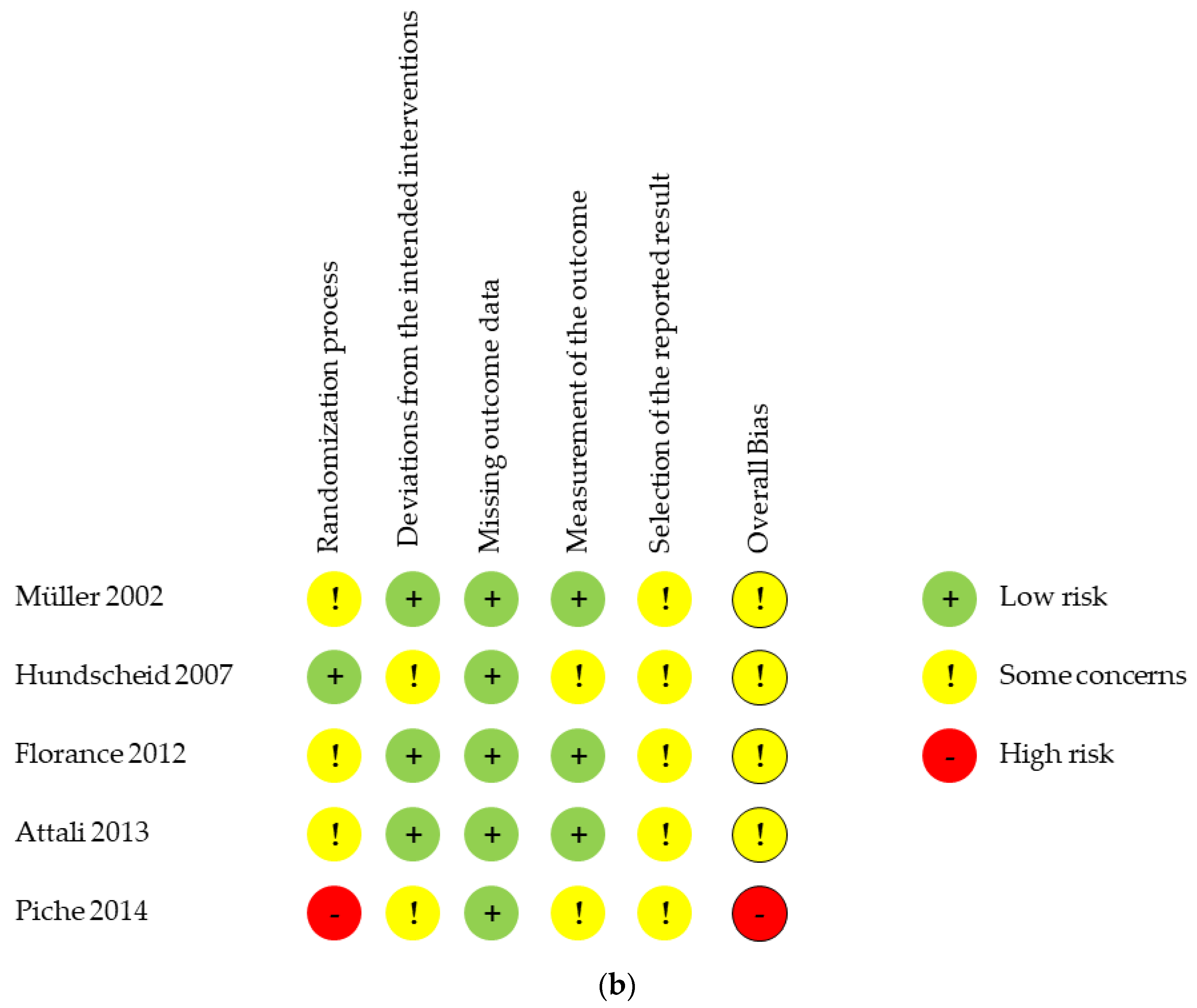
3.3. Risk of Bias
3.4. Description of the Main Results
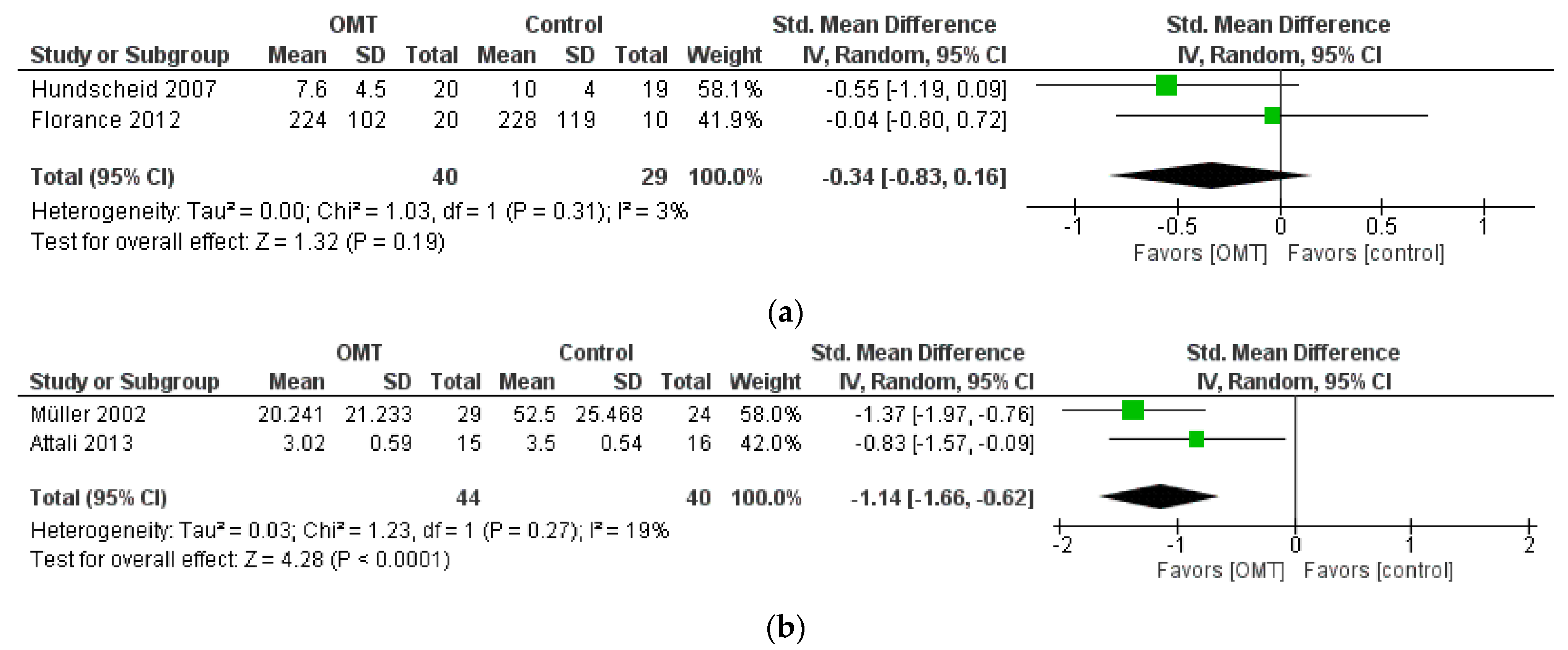
3.5. Effect of Interventions: Quantitative Synthesis
4. Discussion
4.1. Quality of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1.
- PubMed
- 2.
- Embase
- 3.
- Scopus
- 4.
- Cinahl
- 5.
- Cochrane
References
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.; Patel, N. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Bercik, P.; Morgan, D.G.; Bolino, C.; Pintos-Sanchez, M.I.; Moayyedi, P.; Ford, A.C. Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment. Pharmacol. Ther. 2015, 41, 449–458. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef]
- Lacy, B.E.; Everhart, K.K.; Weiser, K.T.; DeLee, R.; Strobel, S.; Siegel, C.; Crowell, M.D. IBS Patients’ Willingness to Take Risks With Medications. Am. J. Gastroenterol. 2012, 107, 804–809. [Google Scholar] [CrossRef]
- Flacco, M.E.; Manzoli, L.; de Giorgio, R.; Gasbarrini, A.; Cicchetti, A.; Bravi, F.; Altini, M.; Caio, G.; Ursini, F. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2986–3000. [Google Scholar] [CrossRef]
- Tack, J.; Fried, M.; Houghton, L.A.; Spicak, J.; Fisher, G. Systematic review: The efficacy of treatments for irritable bowel syndrome—A European perspective. Aliment. Pharmacol. Ther. 2006, 24, 183–205. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Espí-López, G.V.; Inglés, M.; Soliva-Cazabán, I.; Serra-Añó, P. Effect of the soft-tissue techniques in the quality of life in patients with Crohn’s disease: A randomized controlled trial. Medicine 2018, 97, e13811. [Google Scholar] [CrossRef]
- Boas Fernandes, W.V.; Politti, F.; Blanco, C.R.; Garcia Lucareli, P.R.; Gomes, C.A.F.P.; Corrêa, F.I.; Corrêa, J.C.F. Effect of osteopathic visceral manipulation for individuals with functional constipation and chronic nonspecific low back pain: Randomized controlled trial. J. Bodyw. Mov. Ther. 2023, 34, 96–103. [Google Scholar] [CrossRef]
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189. [Google Scholar] [CrossRef]
- Tramontano, M.; Tamburella, F.; Farra, F.D.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11: International Classification of Diseases; 11th Revision. 2019. Available online: https://icd.who.int/ (accessed on 18 March 2023).
- Di Giovanna, E.L.; Schiowitz, S.; Dowling, D.J. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Müller, A.; Franke, H.; Resch, K.L.; Fryer, G. Effectiveness of Osteopathic Manipulative Therapy for Managing Symptoms of Irritable Bowel Syndrome: A Systematic Review. J. Osteopath. Med. 2014, 114, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Bagagiolo, D.; Rosa, D.; Borrelli, F. Efficacy and safety of osteopathic manipulative treatment: An overview of systematic reviews. BMJ Open 2022, 12, e053468. [Google Scholar] [CrossRef]
- Pérez-Montalbán, M.; Ibáñez-Vera, A.J. Efectos de la fisioterapia en sujetos con síndrome de colon irritable: Una revisión sistemática. Fisioterapia 2022, 44, 102–110. [Google Scholar] [CrossRef]
- Amsallem, F.; Sánchez, S.; Armoiry, X.; Mion, F. Efficacy of non-pharmacological interventions for irritable bowel syndrome: A systematic review. Evid. Based Complement. Altern. Med. Ecam 2021, 2021, 4404185. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 66, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Holger, J.; Schünemann Julian, P.T. Completing ‘Summary of Findings’ Tables and Grading the Certainty of the Evidence, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Available online: https://research.bond.edu.au/en/publications/completing-summary-of-findings-tables-and-grading-the-certainty-o (accessed on 6 May 2022).
- Müller, A.; Salomon, J.; Stiedl, M. Osteopathy as a Promising Short-Term Strategy for Irritable Bowel Syndrome: Randomized Controlled Trial; German Academy of Osteopathy: Rohdorf, Germany, 2002; Available online: http://www.osteopathic-research.com/index.php?option=com_jresearch&view=publication&task=show&id=13302&lang=en (accessed on 1 November 2013).
- Hundscheid, H.W.; Pepels, M.J.; Engels, L.G.; Loffeld, R.J. Treatment of irritable bowel syndrome with osteopathy: Results of a randomized controlled pilot study. J. Gastroenterol. Hepatol. 2007, 22, 1394–1398. [Google Scholar] [CrossRef]
- Florance, B.-M.; Frin, G.; Dainese, R.; Nébot-Vivinus, M.-H.; Barjoan, E.M.; Marjoux, S.; Laurens, J.-P.; Payrouse, J.-L.; Hébuterne, X.; Piche, T. Osteopathy improves the severity of irritable bowel syndrome: A pilot randomized sham-controlled study. Eur. J. Gastroenterol. Hepatol. 2012, 24, 944–949. [Google Scholar] [CrossRef]
- Attali, T.V.; Bouchoucha, M.; Benamouzig, R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: Short-term and long-term results of a randomized trial: Visceral osteopathy & IBS. J. Dig. Dis. 2013, 14, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; Pishvaie, D.; Tirouvaziam, D.; Filippi, J.; Dainese, R.; Tonohouhan, M.; DeGalleani, L.; Nébot-Vivinus, M.-H.; Payrouse, J.-L.; Hébuterne, X. Osteopathy decreases the severity of IBS-like symptoms associated with Crohn’s disease in patients in remission. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1392–1398. [Google Scholar] [CrossRef]
- Bouchoucha. Irritable Bowel Syndrome and Osteopathic Manipulative Therapy. Central. Available online: https://clinicaltrials.gov/ct2/show/NCT02932111 (accessed on 6 May 2022).
- Furlan, A.D.; Malmivaara, A.; Chou, R.; Maher, C.G.; Deyo, R.A.; Schoene, M.; Van Tulder, M.W. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine 2015, 40, 1660. [Google Scholar] [CrossRef]
- Henley, C.E.; Ivins, D.; Mills, M.; Wen, F.K.; Benjamin, B.A. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: A repeated measures study. Osteopath. Med. Prim. Care 2008, 2, 7. [Google Scholar] [CrossRef]
- Schaub, N.; Degen, L. Funktionelle Diarrhoe—Pathophysiologie, Abklärung und Therapie. Ther. Umsch. 2014, 71, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Lombardi, L.; Fornari, M.; Sgoifo, A. Exploring the Effects of Osteopathic Manipulative Treatment on Autonomic Function through the Lens of Heart Rate Variability. Front. Neurosci. 2020, 14, 579365. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2020.579365 (accessed on 18 March 2023).
- Qin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Benchmarks for Training in Traditional/Complementary and Alternative Medicine: Benchmarks for Training in Osteopathy. Available online: https://www.who.int/publications-detail-redirect/9789241599665 (accessed on 18 March 2023).
- Cicchitti, L.; Martelli, M.; Cerritelli, F. Chronic Inflammatory Disease and Osteopathy: A Systematic Review. PLoS ONE 2015, 10, e0121327. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, F.; Chiacchiaretta, P.; Gambi, F.; Perrucci, M.G.; Barassi, G.; Visciano, C.; Bellomo, R.G.; Saggini, R.; Ferretti, A. Effect of manual approaches with osteopathic modality on brain correlates of interoception: An fMRI study. Sci. Rep. 2020, 10, 3214. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Cerritelli, F.; Cortelli, P. Sensitization and Interoception as Key Neurological Concepts in Osteopathy and Other Manual Medicines. Front. Neurosci. 2016, 10, 100. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2016.00100 (accessed on 18 March 2023). [CrossRef]
- Tramontano, M.; Cerritelli, F.; Piras, F.; Spanò, B.; Tamburella, F.; Piras, F.; Caltagirone, C.; Gili, T. Brain Connectivity Changes after Osteopathic Manipulative Treatment: A Randomized Manual Placebo-Controlled Trial. Brain Sci. 2020, 10, 969. [Google Scholar] [CrossRef]
- Cerritelli, F.; Chiacchiaretta, P.; Gambi, F.; Saggini, R.; Perrucci, M.G.; Ferretti, A. Osteopathy modulates brain–heart interaction in chronic pain patients: An ASL study. Sci. Rep. 2021, 11, 4556. [Google Scholar] [CrossRef]
- Moher, D.; Schulz, K.F.; Altman, D.; for the CONSORT Group. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. JAMA 2001, 285, 1987–1991. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; A Berlin, J.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
- Panjabi, M.M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J. Spinal Disord. 1992, 5, 390–396; discussion 397. [Google Scholar] [CrossRef]
- Sun Freeman, B.; Natanson, C. Meta-analysis of Clinical Trials. In Principles and Practice of Clinical Research, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 361–370. [Google Scholar]
| Author/Year | Design | Objective | Outcomes | Population | Intervention and Comparison |
|---|---|---|---|---|---|
| Müller et al. (2002) [29] | Parallel RCT | Efficacy of OMT on IBS symptoms |
| N = 60 Male = 17% Age = 48.64 ± 2.79 Pain = 63.63 ± 2.96 IBS duration: at least 1 year | OMT (n = 29) Description: 5 sessions every two weeks; duration up to 45 min. Follow-up at 12 weeks (2 weeks after the last session) CG (n = 24) Description: 5 sessions every two weeks of sham therapy; duration up to 30 min |
| Hundscheid et al. (2007) [30] | Parallel pilot RCT | Efficacy of OMT on IBS symptoms and general well-being |
| N = 39 Male= 41% Age: 43.75 FBDSI: 172.5 | OMT (n = 20) Description: 5 sessions once per 2–3 weeks. Follow-up 6 months CG (n = 19) Description: standard care |
| Florance et al. (2012) [31] | Parallel pilot RCT | Efficacy of OMT on IBS severity |
| N = 30 Male = 23% Age: 47.5 ± 16.8 Severity of IBS: 287 ± 81.5 IBS duration (years): 11.35 ± 13.85 | OMT (n = 20) Description: 2 sessions with a 7 day interval; duration each session: 60 min CG (n = 10) Description: sham OMT |
| Attali et al. (2013) [32] | Crossover RCT | Efficacy of visceral OMT on symptoms, colonic transit time and rectal sensitivity in refractory IBS patients |
| N = 31 Male = 26% Age: 50 ± 2 Abdominal pain: 6.29 ± 0.47 IBS duration (years): 10 ± 4 | OMT (n = 15) Description: 3 sessions of OMT separated by 2-week intervals, and 3 sessions of placebo separated by 2-week intervals; duration of each session: 45 min CG (n = 16) Description: 3 sessions of placebo separated by 2-week intervals, and 3 sessions of OMT separated by 2-week intervals; duration of each session: 45 min |
| Piche et al. (2014) [33] | Parallel RCT | Efficacy of OMT on the severity of IBS-like symptoms in quiescent CD patients |
| N = 37 Male = 35% Age: 41.75 (35.1–49) Severity of IBS: 71.5 (45.5–119.0) IBS duration (years): 7.5 (2–12) | OMT (n = 25) Description: 3 sessions of OMT at day 15, 30, and 45, after the last infusion of TNF-α (infliximab) at day 0; duration of each session: 60 min CG (n = 12) Description: 3 sessions of caring attention and listening without manipulation at day 15, 30 and 45, after the last infusion of TNF-α at day 0 |
| Bouchoucha et al. (2018) [34] NCT02932111 | Parallel RCT, ongoing study (estimated completion date: 12 November 2023) | Efficacy of OMT in IBS symptoms |
| N = 210 Age: ≥18 years Sexes: both | OMT Description: 3 sessions of OMT over a 6-week treatment period. CG Description: 3 sessions of sham OMT over a 6-week treatment period. |
| Author/Year | Description of Interventions | Main Results |
|---|---|---|
| Müller et al. (2002) [29] | OMT group: specific OMT During each session an osteopath performed an evaluation of the suture occipito mastoidea, epigastric zone, and colon in relation to small intestine and to parietal planes. According to the severity (0–2), the osteopath treated the dysfunctional areas. Each technique was performed for about 8 min. CG: sham OMT The patients underwent an osteopathic examination of the spine from T11 to L5, ribs 11 and 12, symphysis, sacroiliac joint and coccygeum. Both groups were allowed to take medications, except during the 48 h before the treatment | Abdominal pain (VAS) OMT: baseline—30 days (p = 0.0002) baseline—45 days (p = 0.0103) baseline—60 days (p < 0.0001) baseline—75 days (p < 0.0001) CG: baseline—75 days (p = 0.017) OMT vs. CG: at 75 days in favor of OMT (p < 0.0001) Diarrhea OMT vs. CG: at 60 days in favor of OMT (p = 0.0225) at 75 days in favor of OMT(p = 0.0165) Constipation OMT vs. CG: at 60 days in favor of OMT (p = 0.0183) at 75 days in favor of OMT (p = 0.0080) |
| Hundscheid et al. (2007) [30] | OMT group: black box method OMT was performed adapting the treatment to the clinical history of each patient. Patients could not to take any medication used in the standard care of IBS, and they did not nor receive the advice to consume more fiber. CG: standard care Patients were advised to have a diet rich in fiber. They could take extra fiber or laxatives in case of constipation, loperamide for predominant diarrhea, and mebeverine for cramps. | IBSQOL OMT: baseline—month 3: from 111 ± 22 to 125 ± 20 (p < 0.009) baseline—month 6: from 111 ± 22 to 129 ± 19 (p < 0.009) CG: no statistically significant differences (p > 0.05) Symptom score OMT: no statistically significant differences (p > 0.05) CG: no statistically significant differences (p > 0.05) OMT vs. CG: OMT was superior to CG (p = 0.02) FBDSI OMT: baseline—month 6: from 174 ± 36 to 74 ± 64 (p < 0.0001) CG: baseline—month 6: from 171 ± 31 to 119 ± 48 (p < 0.0001) OMT vs. CG: OMT was superior to CG (p = 0.02) AEs None. OMT: slight increase in the severity of the symptoms after the first session which resolved quickly. |
| Florance et al. (2012) [31] | OMT group: standardized OMT During each session an osteopath performed a physical examination and treatment of the spine and the abdomen. The operator used both direct techniques (hand pressure on each segment of the spine for 90 s) and an indirect technique (pressure on the segment using hands, knees, or the chest). Each session was completed by visceral osteopathy. CG: sham OMT The osteopath touched the same areas treated in the OMT group (spine and abdomen) with a gentle massage. | IBS severity score OMT: baseline—day 7: 196 ± 88 (p < 0.01) baseline—day 28: 224 ± 102 (p < 0.01) CG: baseline—day 7: 244 ± 75 (p = 0.04) baseline—day 28: no statistically significant differences (p = 0.07). OMT vs. CG: at day 7 in favor of OMT (p = 0.01) at day 28 no differences (p = 0.8). Stool frequency and consistency No statistically significant differences (p > 0.05) AEs None. |
| Attali et al. (2013) [32] | OMT group: standardized OMT The osteopath performed abdominal and sacral gentle manipulations. At the beginning of the session, the operator applied a global visceral technique, performing gentle vibrations with both hands. Then, the areas addressed as highly sensitive by the patients were treated by pressing and vibrating the fingers. Eventually, the osteopath performed a sacral technique. CG: sham OMT Superficial massage in the same points of the OMT. Movements were similar in variety and duration. After 3 sessions of either OMT or sham OMT, the two groups switched to the other intervention: group A received sham OMT in Phase 1 and OMT in Phase 2, while group B received OMT in Phase 1 and sham OMT in Phase 2. | IBS symptoms Phase 1 OMT (group B): baseline—week 5: constipation (p = 0.022) diarrhea (p = 0.016) abdominal distension (p = 0.001) abdominal pain (p = 0.005) CG (group A): baseline—week 5: abdominal distension (p = 0.026) abdominal pain (p = 0.001) OMT vs. CG: at week 5 no statistically significant differences (p > 0.05). Phase 2 OMT (group A): week 5—week 11: abdominal distension (p = 0.002) abdominal pain (p = 0.003) CG (group B): week 5—week 11: no differences (p > 0.05) All patients: baseline—week 11: constipation (p < 0.001) diarrhea (p = 0.003) abdominal distension (p < 0.001) abdominal pain (p < 0.001) baseline—1 year: diarrhea (p = 0.029) abdominal distension (p = 0.001) abdominal pain (p < 0.001) |
| Piche et al. (2014) [33] | OMT group: standardized OMT During each session an osteopath performed a physical examination and treatment of the spine and the abdomen. The operator used both direct techniques (hand pressure on each segment of the spine for 90 s) and an indirect technique (pressure on the segment using hands, knees, or the chest). Each session was completed by visceral osteopathy. CG: no intervention The osteopath offered caring attention and listening without manipulation. | IBS severity score OMT: baseline—15 days: no statistically significant differences (p = 0.3) baseline—30 days: 67 (16–116), p = 0.05 baseline—45 days: 50 (12–99), p = 0.01 baseline—60 days: 32 (15–106), p = 0.01 CG: no statistically significant differences (p > 0.05) OMT vs. CG: at 15 days no statistically significant differences (p > 0.05) at 30 days in favor of OMT (p = 0.01) at 45 days in favor of OMT (p = 0.04) at 60 days in favor of OMT (p = 0.05) IBDq OMT: baseline—15 days: no statistically significant differences (p = 0.6) baseline—30 days: 200 (177–213), p = 0.01 baseline—45 days: 204 (174–216), p = 0.01 baseline—60 days: 196 (174–209), p = 0.05 CG: no statistically significant differences (p > 0.05) OMT vs. CG: at 15, 30 and 60 days no statistically significant differences (p > 0.05) at 45 days in favor of OMT (p = 0.05) AEs None. OMT: brief sensation of fatigue immediately after intervention. |
| Bouchoucha et al. (2018) [34] NCT02932111 | OMT group: standardized OMT Friction in the hourly sense, vibration, inhibitions or rebounds in the abdominal projection of the junction, where there is the trigger zone. CG: sham OMT The osteopath will mimic the techniques provided in the OMT group without any intention of treatment. | Ongoing study, hence no reported results. |
| Outcome | SMD (95% CI) | N. of Subjects (Studies) | Comments | Quality of Evidence |
|---|---|---|---|---|
| IBS symptoms (IBS Severity Score and Likert) | −0.34 [−0.83, 0.16] | 69 (2 RCT) | Downgraded by 1 level for RoB Downgraded by 1 level for Imprecision | ⊕⊕◯◯ LOW |
| Abdominal pain (VAS) | −1.14 [−1.66, −0.62] | 84 (2 RCT) | Downgraded by 1 level for RoB Downgraded by 1 level for Imprecision | ⊕⊕◯◯ LOW |
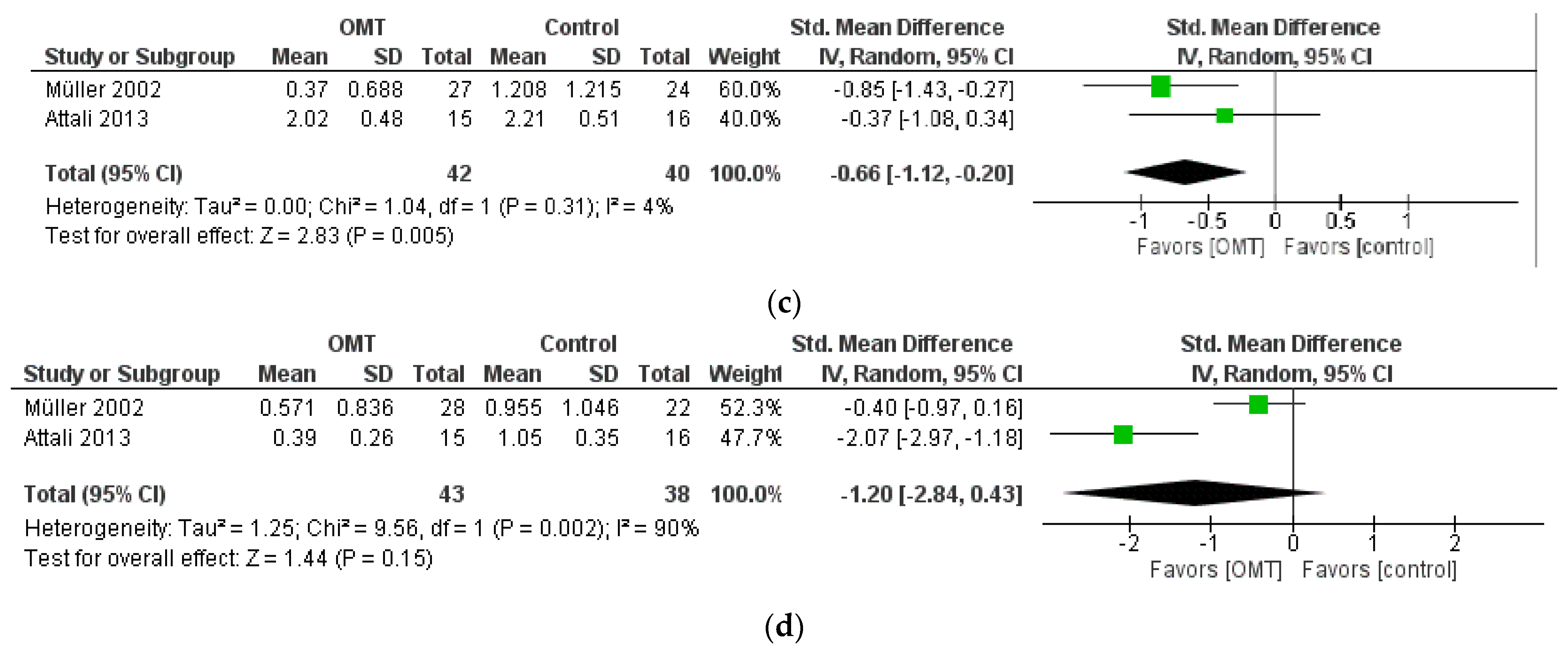
| Constipation | −0.66 [−1.12, −0.20] | 82 (2 RCT) | Downgraded by 1 level for RoB Downgraded by 1 level for Imprecision | ⊕⊕◯◯ LOW |
| Diarrhea | −1.20 [−2.84, 0.43] | 81 (2 RCT) | Downgraded by 1 level for RoB Downgraded by 2 levels for Inconsistency (I2 = 90%) Downgraded by 1 level for Imprecision | ⊕◯◯◯ VERY LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffone, F.; Tarantino, A.G.; Belloni, F.; Spadafora, A.; Bolzoni, G.; Bruini, I.; Bergna, A.; Vismara, L. Effectiveness of Osteopathic Manipulative Treatment in Adults with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 2442. https://doi.org/10.3390/healthcare11172442
Buffone F, Tarantino AG, Belloni F, Spadafora A, Bolzoni G, Bruini I, Bergna A, Vismara L. Effectiveness of Osteopathic Manipulative Treatment in Adults with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Healthcare. 2023; 11(17):2442. https://doi.org/10.3390/healthcare11172442
Chicago/Turabian StyleBuffone, Francesca, Andrea Gianmaria Tarantino, Federico Belloni, Andrea Spadafora, Giorgio Bolzoni, Irene Bruini, Andrea Bergna, and Luca Vismara. 2023. "Effectiveness of Osteopathic Manipulative Treatment in Adults with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis" Healthcare 11, no. 17: 2442. https://doi.org/10.3390/healthcare11172442
APA StyleBuffone, F., Tarantino, A. G., Belloni, F., Spadafora, A., Bolzoni, G., Bruini, I., Bergna, A., & Vismara, L. (2023). Effectiveness of Osteopathic Manipulative Treatment in Adults with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Healthcare, 11(17), 2442. https://doi.org/10.3390/healthcare11172442






