Activities of Clinical Expertise and Research in a Rare Disease Referral Centre: A Place for Telemedicine beyond the COVID-19 Pandemic?
Abstract
:1. Introduction
2. Methods
2.1. Survey of Expertise Activity
2.1.1. Census of Opinions Given
2.1.2. Quality of the Census
2.1.3. Medico-Economic Evaluation of the Expert Opinion Activity if Performed as TLM Acts
2.2. Survey of the Clinical Research Activity
2.2.1. Physician Survey
2.2.2. Patient Survey
3. Results
3.1. Characterisation of the Expertise Activity of Our Department
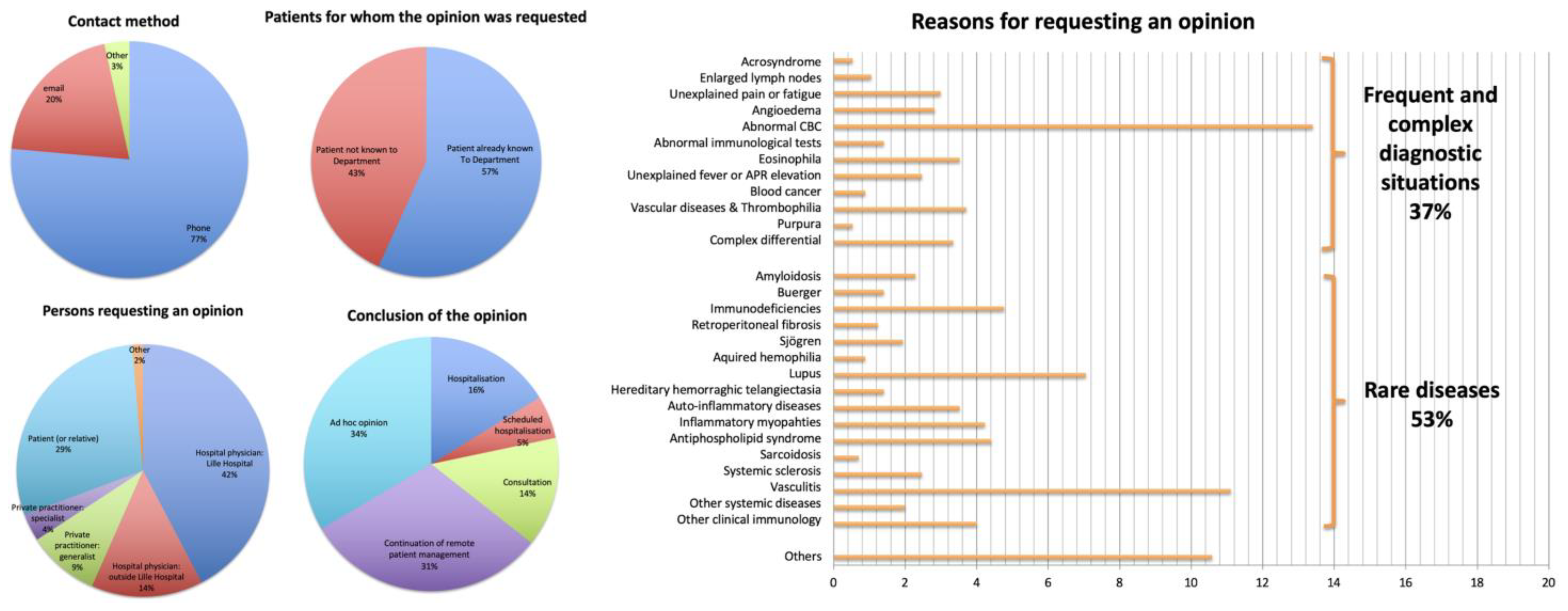
3.1.1. Survey of the Opinions Delivered
3.1.2. Quality of the Census Carried Out
3.1.3. Medico-Economic Evaluation of the Expert Opinion Activity if Performed as TLM Acts
3.2. Acceptability of Protocol Follow-Up TLCs to Physician-Investigators and to Patients Included in Clinical Research Protocols
3.2.1. Physician Survey (Figure 2)

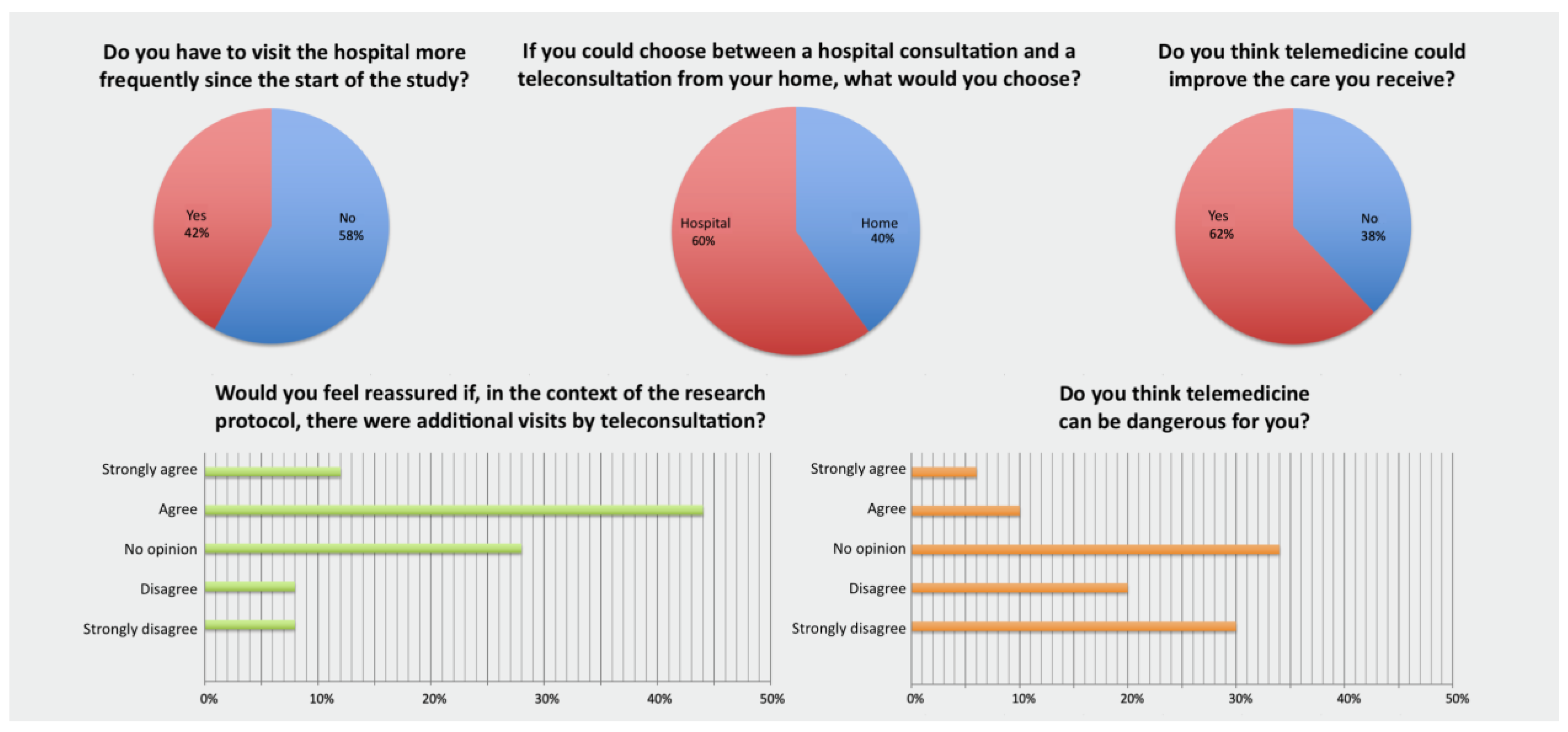
3.2.2. Patient Survey (Figure 3)
4. Discussion
4.1. Providing Clinical Expertise Is a Time-Consuming and Uncompensated Activity: Could TLM Be a Solution?
4.2. Clinical Trial TLCs: Towards Tele-Trials?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union Committee of Experts on Rare Diseases (EUCERD). 2014 Report on the State of the Art of Rare Disease Activities in Europe; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Donnart, A.; Viollet, V.; Roinet-Tournay, M. [Rare diseases, a public health issue]. Soins Pediatr. Pueric. 2013, 274, 17–19. [Google Scholar]
- Amselem, S.; Gueguen, S.; Weinbach, J.; Clement, A.; Paul Landais for the RaDiCo Program. RaDiCo, the French National Research Program on Rare Disease Cohorts. Orphanet J. Rare Dis. 2021, 16, 454. [Google Scholar] [CrossRef]
- Ferreira, C.R. The Burden of Rare Diseases. Am. J. Med. Genet. Part A 2019, 179, 885–892. [Google Scholar] [CrossRef]
- Code de La Santé Publique—Article L6316-1; Volume L6316-1. Available online: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000038887059 (accessed on 11 August 2020).
- Décret N° 2010-1229 Du 19 Octobre 2010 Relatif à La Télémédecine. 2010. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000022932449 (accessed on 11 August 2020).
- L’essor de La Télémédecine, Une Bascule Soudaine Rendue Possible Par Un Investissement Préalable Sur La Durée. In Améliorer la Qualité du Système de Santé et Maîtriser les Dépenses—Propositions de L’Assurance Maladie Pour 2021. 2020. Available online: https://assurance-maladie.ameli.fr/sites/default/files/2020-07_rapport-propositions-pour-2021_assurance-maladie_1.pdf (accessed on 11 August 2020).
- Décret N° 2021-707 Du 3 Juin 2021 Relatif à La Télésanté. 2021. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000043596730 (accessed on 21 December 2022).
- Arrêté du 22 Septembre 2021 Portant Approbation de L’avenant n° 9 à la Convention Nationale Organisant les Rapports Entre les Médecins Libéraux et L’assurance Maladie Signée le 25 août 2016; Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000044097701 (accessed on 21 December 2022).
- Walkowiak, D.; Domaradzki, J. Needs Assessment Study of Rare Diseases Education for Nurses and Nursing Students in Poland. Orphanet J. Rare Dis. 2020, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, R.; Nasirzadeh, A.; Farzan, M.; Domaradzki, J.; Jouybari, L.; Sanagoo, A.; Farzan, M.; Aghazadeh-Habashi, K.; Fallah Faraghe, A.; Bagheri, S.; et al. Iranian Future Healthcare Professionals’ Knowledge and Opinions about Rare Diseases: Cross-Sectional Study. Orphanet J. Rare Dis. 2022, 17, 366. [Google Scholar] [CrossRef]
- Sanges, S.; Farhat, M.-M.; Assaraf, M.; Galland, J.; Rivière, E.; Roubille, C.; Lambert, M.; Yelnik, C.; Maillard, H.; Sobanski, V.; et al. Raising Rare Disease Awareness Using Red Flags, Role Play Simulation and Patient Educators: Results of a Novel Educational Workshop on Raynaud Phenomenon and Systemic Sclerosis. Orphanet J. Rare Dis. 2020, 15, 159. [Google Scholar] [CrossRef]
- Soussand, L.; Kuchenbuch, M.; Messiaen, C.; Sandrin, A.; Jannot, A.-S.; Nabbout, R. Impact of the COVID-19 Pandemic on the Care of Rare and Undiagnosed Diseases Patients in France: A Longitudinal Population-Based Study. Orphanet J. Rare Dis. 2022, 17, 430. [Google Scholar] [CrossRef]
- Resseguier, A.S.; Gerbaud, L.; De Ruffray, P.; Vaillant-Roussel, H.; Pereira, B.; Ruivard, M. Évaluation d’une plateforme de télé-expertise spécifique à la médecine interne. Rev. Med. Interne 2017, 38S, A55. [Google Scholar] [CrossRef]
- Coppo, P.; Corre, E.; Rondeau, E.; Benhamou, Y.; Bachet, A.; Stépanian, A.; Veyradier, A.; Centre de référence des Microangiopathies Thrombotiques. [Telemedicine in thrombotic microangiopathies: A way forward in rare diseases requiring emergency care]. Rev. Med. Interne 2016, 37, 514–520. [Google Scholar] [CrossRef]
- Devadula, S.; Langbecker, D.; Vecchio, P.; Tesiram, J.; Meiklejohn, J.; Benham, H. Tele-Rheumatology to Regional Hospital Outpatient Clinics: Patient Perspectives on a New Model of Care. Telemed. J. E-Health 2020, 26, 912–919. [Google Scholar] [CrossRef]
- Piga, M.; Cangemi, I.; Mathieu, A.; Cauli, A. Telemedicine for Patients with Rheumatic Diseases: Systematic Review and Proposal for Research Agenda. Semin. Arthritis Rheum. 2017, 47, 121–128. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.A.; Ferucci, E.D.; Glover, J.; Fraenkel, L. Telerheumatology: A Systematic Review. Arthritis Care Res. 2017, 69, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Gkrouzman, E.; Wu, D.D.; Jethwa, H.; Abraham, S. Telemedicine in Rheumatology at the Advent of the COVID-19 Pandemic. HSS J. 2020, 16, 108–111. [Google Scholar] [CrossRef]
- Cavagna, L.; Zanframundo, G.; Codullo, V.; Pisu, M.G.; Caporali, R.; Montecucco, C. Telemedicine in Rheumatology: A Reliable Approach beyond the Pandemic. Rheumatology 2021, 60, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Lampe, C.; Dionisi-Vici, C.; Bellettato, C.M.; Paneghetti, L.; van Lingen, C.; Bond, S.; Brown, C.; Finglas, A.; Francisco, R.; Sestini, S.; et al. The Impact of COVID-19 on Rare Metabolic Patients and Healthcare Providers: Results from Two MetabERN Surveys. Orphanet J. Rare Dis. 2020, 15, 341. [Google Scholar] [CrossRef]
- Clark, J.M.; Heifetz, L.J.; Palmer, D.; Brown, L.M.; Cooke, D.T.; David, E.A. Telehealth Allows for Clinical Trial Participation and Multimodality Therapy in a Rural Patient with Stage 4 Non-Small Cell Lung Cancer. Cancer Treat. Res. Commun. 2016, 9, 139–142. [Google Scholar] [CrossRef]
- Sabesan, S.; Zalcberg, J. Telehealth Models Could Be Extended to Conducting Clinical Trials-a Teletrial Approach. Eur. J. Cancer Care 2018, 27, e12587. [Google Scholar] [CrossRef]
- Doolittle, G.C.; Caracione, A.; Coulter, J.; Olson, K.; Knoebber-Carr, K. Using Telemedicine to Increase Access to Cancer Clinical Trials for Patients in Rural Areas: A Feasibility Study. J. Clin. Oncol. 2018, 36, e18884. [Google Scholar] [CrossRef]
- Laggis, C.W.; Williams, V.L.; Yang, X.; Kovarik, C.L. Research Techniques Made Simple: Teledermatology in Clinical Trials. J. Investig. Dermatol. 2019, 139, 1626–1633.e1. [Google Scholar] [CrossRef]
- Lee, J.J.; Burbury, K.; Underhill, C.; Harris, S.; Shackleton, K.; McBurnie, J.; McPhee, N.; Osmond, F.; Wilkins, K.; Baden, P.; et al. Exploring Australian Regional Cancer Patients’ Experiences of Clinical Trial Participation via Telemedicine Technology. J. Telemed. Telecare 2020, 28, 508–516. [Google Scholar] [CrossRef]
- Gonçalves, B.T.; Baiocchi, G. Telemedicine and Cancer Research during the COVID-19 Pandemic. J. Surg. Oncol. 2020, 123, 359. [Google Scholar] [CrossRef] [PubMed]
- Takeda, C.; Guyonnet, S.; Ousset, P.J.; Soto, M.; Vellas, B. Toulouse Alzheimer’s Clinical Research Center Recovery after the COVID-19 Crisis: Telemedicine an Innovative Solution for Clinical Research during the Coronavirus Pandemic. J. Prev. Alzheimers Dis. 2020, 7, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Izmailova, E.S.; Ellis, R.; Benko, C. Remote Monitoring in Clinical Trials During the COVID-19 Pandemic. Clin. Transl. Sci. 2020, 13, 838–841. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducrocq, Q.; Guédon-Moreau, L.; Launay, D.; Terriou, L.; Morell-Dubois, S.; Maillard, H.; Lefèvre, G.; Sobanski, V.; Lambert, M.; Yelnik, C.; et al. Activities of Clinical Expertise and Research in a Rare Disease Referral Centre: A Place for Telemedicine beyond the COVID-19 Pandemic? Healthcare 2023, 11, 2447. https://doi.org/10.3390/healthcare11172447
Ducrocq Q, Guédon-Moreau L, Launay D, Terriou L, Morell-Dubois S, Maillard H, Lefèvre G, Sobanski V, Lambert M, Yelnik C, et al. Activities of Clinical Expertise and Research in a Rare Disease Referral Centre: A Place for Telemedicine beyond the COVID-19 Pandemic? Healthcare. 2023; 11(17):2447. https://doi.org/10.3390/healthcare11172447
Chicago/Turabian StyleDucrocq, Quentin, Laurence Guédon-Moreau, David Launay, Louis Terriou, Sandrine Morell-Dubois, Hélène Maillard, Guillaume Lefèvre, Vincent Sobanski, Marc Lambert, Cécile Yelnik, and et al. 2023. "Activities of Clinical Expertise and Research in a Rare Disease Referral Centre: A Place for Telemedicine beyond the COVID-19 Pandemic?" Healthcare 11, no. 17: 2447. https://doi.org/10.3390/healthcare11172447
APA StyleDucrocq, Q., Guédon-Moreau, L., Launay, D., Terriou, L., Morell-Dubois, S., Maillard, H., Lefèvre, G., Sobanski, V., Lambert, M., Yelnik, C., Farhat, M.-M., Garcia Fernandez, M. J., Hachulla, E., & Sanges, S. (2023). Activities of Clinical Expertise and Research in a Rare Disease Referral Centre: A Place for Telemedicine beyond the COVID-19 Pandemic? Healthcare, 11(17), 2447. https://doi.org/10.3390/healthcare11172447





