Cardiac Troponin Release after Exercise in Healthy Young Athletes: A Systematic Review
Abstract
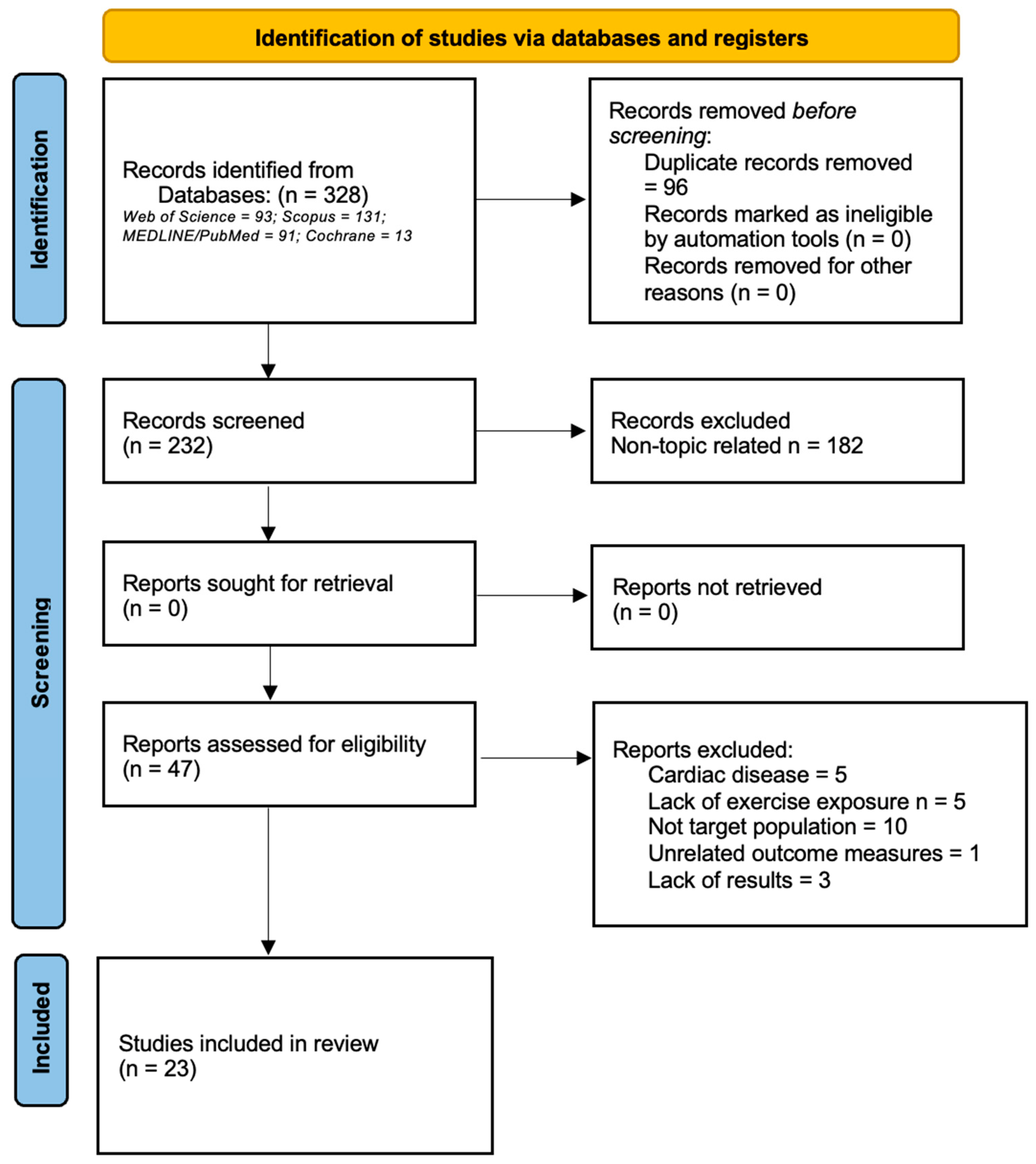
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Data Extraction
3.2. Quality Assessment and Risk of Publication Bias
3.3. Variables
3.3.1. Sex
3.3.2. Age
3.3.3. Training History
3.3.4. Exercise Modality
4. Discussion
4.1. Sex
4.2. Age
4.3. Training History
4.4. Exercise Modality
4.5. cTn Results above Reference Range
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sigurdardottir, F.D.; Lyngbakken, M.N.; Holmen, O.L.; Dalen, H.; Hveem, K.; Røsjø, H.; Omland, T. Relative Prognostic Value of Cardiac Troponin I and C-Reactive Protein in the General Population (from the Nord-Trøndelag Health [HUNT] Study). Am. J. Cardiol. 2018, 121, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, I.; Aspelund, T.; Gudmundsson, E.; Eiriksdottir, G.; Harris, T.B.; Launer, L.J.; Gudnason, V.; Venge, P. High-Sensitivity Cardiac Troponin I Is a Strong Predictor of Cardiovascular Events and Mortality in the AGES-Reykjavik Community-Based Cohort of Older Individuals. Clin. Chem. 2016, 62, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Collinson, P.; Boa, F.G.; Gaze, D.C. Measurement of cardiac troponins. Ann. Clin. Biochem. 2001, 38, 423–449. [Google Scholar] [CrossRef]
- Alquézar Arbé, A.; Santaló Bel, M.; Sionis, A. Interpretación clínica de la determinación de troponina T de elevada sensibilidad. Med. Clin. 2015, 145, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Smith, S.W.; Love, S.A.; Sexter, A.; Schulz, K.; Apple, F.S. Single High-Sensitivity Cardiac Troponin I to Rule Out Acute Myocardial Infarction. Am. J. Med. 2017, 130, 1076–1083.e1. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Storti, S.; Salvadori, S.; Pecori, A.; Bernardini, S.; Romeo, F.; Paolo, G.; Aldo, C. Cardiac troponins: Are there any differences between T and I? J. Cardiovasc. Med. 2021, 22, 797–805. [Google Scholar] [CrossRef]
- Sandoval, Y.; Smith, S.W.; Apple, F.S. Present and Future of Cardiac Troponin in Clinical Practice: A Paradigm Shift to High-Sensitivity Assays. Am. J. Med. 2015, 129, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Klemenz, T.; Fiedler, G.M.; Nakas, C.T.; Exadaktylos, A.K.; Leichtle, A.B. High sensitive cardiac troponin T: Testing the test. Int. J. Cardiol. 2017, 228, 779–783. [Google Scholar] [CrossRef]
- Meigher, S.; Thode, H.C.; Peacock, W.F.; Bock, J.L.; Gruberg, L.; Singer, A.J. Causes of Elevated Cardiac Troponins in the Emergency Department and Their Associated Mortality. Acad. Emerg. Med. 2016, 23, 1267–1273. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Shave, R.; George, K.P.; Atkinson, G.; Hart, E.; Middleton, N.; Whyte, G.; Gaze, D.C.; Collinson, P.O. Exercise-induced cardiac troponin T release: A meta-analysis. Med. Sci. Sports Exerc. 2007, 39, 2099–2106. [Google Scholar] [PubMed]
- Gresslien, T.; Agewall, S. Troponin and exercise. Int. J. Cardiol. 2016, 221, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Legaz-Arrese, A.; Carranza-García, L.E.; Navarro-Orocio, R.; Valadez-Lira, A.; Mayolas-Pi, C.; Munguía-Izquierdo, D.; Reverter-Masía, J.; George, K. Cardiac Biomarker Release after Endurance Exercise in Male and Female Adults and Adolescents. J. Pediatr. 2017, 191, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Egger, M.; Peer, E.; Dieplinger, B. 5th generation cardiac troponin I and T assays in clinical routine—A head-to-head comparison with data from the Linz troponin (LITROP) study. Clin. Chim. Acta 2018, 485, 195–204. [Google Scholar] [CrossRef]
- Caselli, C.; Cangemi, G.; Masotti, S.; Ragusa, R.; Gennai, I.; Del Ry, S.; Prontera, C.; Clerico, A. Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a high sensitive immunoassay method: High sensitive troponin I in pediatric age. Clin. Chim. Acta. 2016, 458, 68–71. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Fernandez, A.B.; Thompson, P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol. Rev. 2016, 96, 99–125. Available online: www.prv.org (accessed on 1 May 2022).
- Shave, R.; Baggish, A.; George, K.; Wood, M.; Scharhag, J.; Whyte, G.; Gaze, D.; Thompson, P.D. Exercise-Induced Cardiac Troponin Elevation: Evidence, Mechanisms, and Implications. J. Am. Coll. Cardiol. 2010, 56, 169–176. [Google Scholar] [CrossRef]
- Legaz-Arrese, A.; George, K.; Carranza-García, L.E.; Munguía-Izquierdo, D.; Moros-García, T.; Serrano-Ostáriz, E. The impact of exercise intensity on the release of cardiac biomarkers in marathon runners. Eur. J. Appl. Physiol. 2011, 111, 2961–2967. [Google Scholar] [CrossRef]
- Mingels, A.; Jacobs, L.; Michielsen, E.; Swaanenburg, J.; Wodzig, W.; van Dieijen-Visser, M. Reference Population and Marathon Runner Sera Assessed by Highly Sensitive Cardiac Troponin T and Commercial Cardiac Troponin T and I Assays. Clin. Chem. 2009, 55, 101–108. [Google Scholar] [CrossRef]
- Shave, R.; Oxborough, D. Exercise-Induced Cardiac Injury: Evidence From Novel Imaging Techniques and Highly Sensitive Cardiac Troponin Assays. Prog. Cardiovasc. Dis. 2012, 54, 407–415. [Google Scholar] [CrossRef]
- Janssen, S.L.; Berge, K.; Luiken, T.; Aengevaeren, V.L.; Eijsvogels, T.M. Cardiac troponin release in athletes: What do we know and where should we go? Curr. Opin. Physiol. 2023, 31, 100629. [Google Scholar] [CrossRef]
- Vilela, E.M.; Bastos, J.C.C.; Rodrigues, R.P.; Nunes, J.P.L. High-sensitivity troponin after running--a systematic review. Neth. J. Med. 2014, 72, 5–9. [Google Scholar] [PubMed]
- Legaz-Arrese, A.; López-Laval, I.; George, K.P.; Puente-Lanzarote, J.J.; Mayolas-Pi, C.; Serrano-Ostáriz, E.; Revilla-Martí, P.; Moliner-Urdiales, D.; Reverter-Masià, J. Impact of an endurance training program on exercise-induced cardiac biomarker release. Am. J. Physiol. Circ. Physiol. 2015, 308, H913–H920. [Google Scholar] [CrossRef] [PubMed]
- Cirer-Sastre, R.; Corbi, F.; López-Laval, I.; Carranza-García, L.E.; Reverter-Masià, J. Exercise-Induced Release of Cardiac Troponins in Adolescent vs. Adult Swimmers. Int. J. Environ. Res. Public Health 2021, 18, 1285. [Google Scholar] [CrossRef]
- Neilan, T.G.; Januzzi, J.L.; Lee-Lewandrowski, E.; Ton-Nu, T.-T.; Yoerger, D.M.; Jassal, D.S.; Lewandrowski, K.B.; Siegel, A.J.; Marshall, J.E.; Douglas, P.S.; et al. Myocardial Injury and Ventricular Dysfunction Related to Training Levels Among Nonelite Participants in the Boston Marathon. Circulation 2006, 114, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Fortescue, E.B.; Shin, A.Y.; Greenes, D.S.; Mannix, R.C.; Agarwal, S.; Feldman, B.J.; Shah, M.I.; Rifai, N.; Landzberg, M.J.; Newburger, J.W.; et al. Cardiac Troponin Increases Among Runners in the Boston Marathon. Ann. Emerg. Med. 2007, 49, 137–143.e1. [Google Scholar] [CrossRef]
- López-Laval, I.; Legaz-Arrese, A.; George, K.; Serveto-Galindo, O.; González-Rave, J.M.; Reverter-Masia, J.; Munguía-Izquierdo, D. Cardiac troponin I release after a basketball match in elite, amateur and junior players. Clin. Chem. Lab. Med. 2016, 54, 333–338. [Google Scholar] [CrossRef]
- Fu, F.H.; Nie, J.; George, K.; Tong, T.K.; Lin, H.; Shi, Q. Impact of a 21-km Run on Cardiac Biomarkers in Adolescent Runners. J. Exerc. Sci. Fit. 2010, 8, 61–66. [Google Scholar] [CrossRef]
- Nie, J.; George, K.P.; Tong, T.K.; Tian, Y.; Shi, Q. Effect of Repeated Endurance Runs on Cardiac Biomarkers and Function in Adolescents. Med. Sci. Sports Exerc. 2011, 43, 2081–2088. [Google Scholar] [CrossRef]
- Nie, J.; George, K.P.; Tong, T.K.; Gaze, D.; Tian, Y.; Lin, H.; Shi, Q. The Influence of a Half-Marathon Race Upon Cardiac Troponin T Release in Adolescent Runners. Curr. Med. Chem. 2011, 18, 3452–3456. [Google Scholar] [CrossRef]
- Legaz-Arrese, A.; López-Laval, I.; George, K.; Puente-Lanzarote, J.J.; Moliner-Urdiales, D.; Ayala-Tajuelo, V.J.; Mayolas-Pi, C.; Reverter-Masià, J. Individual variability in cardiac biomarker release after 30 min of high-intensity rowing in elite and amateur athletes. Appl. Physiol. Nutr. Metab. 2015, 40, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Aakre, K.M. Cardiac Troponin Increase after Endurance Exercise: A New Marker of Cardiovascular Risk? Circulation 2019, 140, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Gaze, D.; Mohan, S.; Williams, K.L.; Sprung, V.; George, K.; Jeffries, R.; Hudson, Z.; Perry, M.; Shave, R. Post-Exercise Cardiac Troponin Release is Related to Exercise Training History. Int. J. Sports Med. 2012, 33, 333–337. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; George, K.; Nie, J.; Carranza-García, L.E.; Reverter-Masià, J. Cardiac Biomarker Release After Exercise in Healthy Children and Adolescents: A Systematic Review and Meta-Analysis. Pediatr. Exerc. Sci. 2019, 31, 28–36. [Google Scholar] [CrossRef]
- Cantinotti, M.; Clerico, A.; Giordano, R.; Assanta, N.; Franchi, E.; Koestenberger, M.; Marchese, P.; Storti, S.; D’Ascenzi, F. Cardiac Troponin-T Release After Sport and Differences by Age, Sex, Training Type, Volume, and Intensity: A Critical Review. Clin. J. Sport. Med. 2021, 32, e230–e242. [Google Scholar]
- Papamichail, A.; Androulakis, E.; Xanthopoulos, A.; Briasoulis, A. Effect of Training Load on Post-Exercise Cardiac Biomarkers in Healthy Children and Adolescents: A Systematic Review of the Existing Literature. J. Clin. Med. 2023, 12, 2419. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Tian, Y.; Nie, J.; Tong, T.K.; Cao, J.; Gao, Q.; Man, J.; Shi, Q.; Liu, W. Changes in serum cardiac troponins following a 21-km run in junior male runners. J. Sports Med. Phys. Fit. 2006, 46, 481–488. [Google Scholar]
- Nie, J.; Tong, T.; Shi, Q.; Lin, H.; Zhao, J.; Tian, Y. Serum Cardiac Troponin Response in Adolescents Playing Basketball. Int. J. Sports Med. 2008, 29, 449–452. [Google Scholar] [CrossRef]
- Fu, F.; Nie, J.; Tong, T. Serum Cardiac Troponin T in Adolescent Runners: Effects of Exercise Intensity and Duration. Int. J. Sports Med. 2009, 30, 168–172. [Google Scholar] [CrossRef]
- Nie, J.; Tong, T.K.; George, K.; Fu, F.H.; Lin, H.; Shi, Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports 2011, 21, 625–629. [Google Scholar] [CrossRef]
- Traiperm, N.; Gatterer, H.; Wille, M.; Burtscher, M. Cardiac Troponins in Young Marathon Runners. Am. J. Cardiol. 2012, 110, 594–598. [Google Scholar] [CrossRef]
- Ma, G.; Liu, Y.; Liu, K. Influence of Repeated Bouts of Table Tennis Training on Cardiac Biomarkers in Children. Pediatr. Cardiol. 2014, 35, 711–718. [Google Scholar] [CrossRef]
- Kong, Z.; Nie, J.; Lin, H.; George, K.; Zhao, G.; Zhang, H.; Tong, T.K.; Shi, Q. Sex differences in release of cardiac troponin T after endurance exercise. Biomarkers 2016, 22, 345–350. [Google Scholar] [CrossRef]
- Peretti, A.; Mauri, L.; Masarin, A.; Annoni, G.; Corato, A.; Maloberti, A.; Giannattasio, C.; Vignati, G. Cardiac Biomarkers Release in Preadolescent Athletes After an High Intensity Exercise. High. Blood Press. Cardiovasc. Prev. 2017, 25, 89–96. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Azizi, M.; Samadi, A.; Talebi, N.; Gatterer, H.; Burtscher, M. Impact of a Soccer Game on Cardiac Biomarkers in Adolescent Players. Pediatr. Exerc. Sci. 2018, 30, 90–95. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; López-Laval, I.; Puente-Lanzarote, J.; Hernández-González, V.; Reverter-Masià, J. Effect of Training Load on Post-Exercise Cardiac Troponin T Elevations in Young Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 4853. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; López-Laval, I.; George, K.; Reverter-Masia, J. Influence of maturational status in the exercise-induced release of cardiac troponin T in healthy young swimmers. J. Sci. Med. Sport 2021, 24, 116–121. [Google Scholar] [CrossRef]
- Birat, A.; Bourdier, P.; Dodu, A.; Grossoeuvre, C.; Blazevich, A.J.; Amiot, V.; Dupont, A.-C.; Nottin, S.; Ratel, S. Effect of Long-Duration Adventure Races on Cardiac Damage Biomarker Release and Muscular Function in Young Athletes. Front. Physiol. 2020, 11, 10. [Google Scholar] [CrossRef]
- Tian, Y.; Nie, J.; George, K.P.; Huang, C. Reproducibility of cardiac biomarkers response to prolonged treadmill exercise. Biomarkers 2014, 19, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wedin, J.O.; Henriksson, A.E. Postgame elevation of cardiac markers among elite floorball players. Scand. J. Med. Sci. Sports 2015, 25, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Cirer-Sastre, R.; Jiménez-Gaytán, R.; Carranza-García, L.E.; George, K.; Apple, F.S.; Navarro-Orocio, R.; López-García, R.; Reverter-Masía, J.; Mayolas-Pi, C.; Morales-Corral, P.G.; et al. A comparison of modelled serum cTnT and cTnI kinetics after 60 min swimming. Biomarkers 2022, 27, 619–624. [Google Scholar] [CrossRef]
- Tian, Y.; Nie, J.; Huang, C.; George, K.P.; Eijsvogels, T.M.H.; Fernandez, A.B.; Thompson, P.D.; Legaz-Arrese, A.; López-Laval, I.; Puente-Lanzarote, J.J.; et al. The kinetics of highly sensitive cardiac troponin T release after prolonged treadmill exercise in adolescent and adult athletes. J. Appl. Physiol. 2012, 113, 418–425. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Corbi, F.; López-Laval, I.; Puente-Lanzarote, J.J.; Hernández-González, V.; Reverter-Masia, J. Cardiac Troponin T Release after Football 7 in Healthy Children and Adults. Int. J. Environ. Res. Public. Health 2020, 17, 956. [Google Scholar] [CrossRef]
- Kimenai, D.M.; Shah, A.S.V.; McAllister, D.A.; Lee, K.K.; Tsanas, A.; Meex, S.J.R.; Porteous, D.J.; Hayward, C.; Campbell, A.; Sattar, N.; et al. Sex Differences in Cardiac Troponin I and T and the Prediction of Cardiovascular Events in the General Population. Clin. Chem. 2021, 67, 1351–1360. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, H.; Kong, Z.; Wang, C.; Liu, Y.; Shi, Q.; George, K. The impact of exercise modality and menstrual cycle phase on circulating cardiac troponin T. J. Sci. Med. Sport 2020, 23, 309–314. [Google Scholar] [CrossRef]
- Li, L.I. Oxidative stress during exercise: Implication of Antioxidant Nutrients. Free Radic. Biol. Med. 1995, 18, 1079–1086. [Google Scholar]
- Bjørkavoll-Bergseth, M.; Kleiven, Ø.; Auestad, B.; Eftestøl, T.; Oskal, K.; Nygård, M.; Skadberg, Ø.; Aakre, K.M.; Melberg, T.; Gjesdal, K.; et al. Duration of Elevated Heart Rate Is an Important Predictor of Exercise-Induced Troponin Elevation. J. Am. Heart Assoc. 2020, 9, e014408. [Google Scholar] [CrossRef]
- Babuin, L.; Jaffe, A. Troponin: The biomarker of choice for the detection of cardiac injury. Can. Med. Assoc. J. 2005, 173, 1191–1202. [Google Scholar] [CrossRef]
- Apple, F.S.; Quist, H.E.; Doyle, P.J.; Otto, A.P.; Murakami, M.M. Plasma 99th Percentile Reference Limits for Cardiac Troponin and Creatine Kinase MB Mass for Use with European Society of Cardiology/American College of Cardiology Consensus Recommendations. Clin. Chem. 2003, 49, 1331–1336. [Google Scholar] [CrossRef]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical Validation of a High-Sensitivity Cardiac Troponin T Assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hubble, K.M.; Fatovich, D.M.; Grasko, J.M.; Vasikaran, S.D. Cardiac troponin increases among marathon runners in the Perth Marathon: The Troponin in Marathons (TRIM) study. Med. J. Aust. 2009, 190, 91–93. [Google Scholar] [CrossRef] [PubMed]
| Author | Biomarker | Participants Male/Female | Mean Age (Years) | Training Level (Years) | Timing of Sample Collection (Bolded Timepoint Giving Highest Prevalence) | cTn Results above Reference Range | Exercise Modality |
|---|---|---|---|---|---|---|---|
| Tian, Y., Nie, J., et al. (2006) [39] | cTnT cTnI | 10/0 | 16.2 +/− 0.6 | 2.4 +/− 1.9 | Baseline, 2, 4, and 24 h after the exercise | cTnT 6/10 (60%) MI cut off (0.03 ng/mL) 4/10 (40%) AMI cut off (0.1 ng/mL) cTnI 6/10 (60%) MI cut off (0.09 ng/L) 1/10 (10%) AMI cut off (0.5 ng/mL) | Half marathon |
| Nie, J., Tong, T.K., et al. (2008) [40] | cTnT cTnI | 10/0 | 15 +/− 0.7 Tanner: 3.6 +/− 0.5 | 2.7 +/− 1.2 | Baseline, 2, 4, and 24 h after the exercise | cTnT 4/10 (40%) MI cut off (0.01 ng/mL) 2/10 (20%) AMI cut off (0.05 ng/mL) cTnI 3/10 (30%) MI cut off (0.06 ng/mL) 0/10 (0%) AMI cut off (0.5 ng/mL) | Basketball game (4 × 12′) |
| Fu, F., Nie, J. & Tong, T.K. (2009) [41] | cTnT | 13/0 | 14.8 +/− 1.6 | 3.1 +/− 1.5 | Baseline, immediately, and 5 h after the exercise | MI cut off (0.01 ng/mL) AMI cut off (0.05 ng/mL) Group 1: 0/13 Group 2: MI 2/13 (15%); AMI 1/13 (8%) Group 3: MI 8/13 (62%); AMI 3/13 (23%) Group 4: MI 12/13 (92%); AMI 8/13 (62%) | Running (treadmill): Group 1—80% VTh 45′ Group 2—80% VTh 90′ Group 3—100% VTh 45′ Group 4—100% VTh 90′ |
| Fu, F., Nie, J., George, K., et al. (2010) [28] | cTnT | 17/0 | 16.5 +/− 1.6 | 2.6 +/− 1 | Baseline, immediately, and 4 h after the exercise | 13/17 (77%) MI cut off (0.03 ng/mL) 0/17 (0%) AMI cut off (0.05 ng/mL) | Half marathon |
| Nie, J., Geoge, K., Tong, T.K., Tian, Y. & Shi, Q. (2011) [29] | cTnT | 12/0 | 14.5 +/− 1.5 | 3.4 +/− 1.5 | Baseline, immediately, POST1+4/PRE2, POST2, and POST2+4 | POST1+4/PRE2: 8/12 (67%) MI cut off (0.01 ng/mL) 3/12 (25%) AMI cut off (0.05 ng/mL) | Running (treadmill): 2 × 45′ VTh. 255′ rest between sets |
| Nie, J., Tong, T.K., George, K., Fu, F., Lin, H. & Shi, Q. (2011) [42] | cTnT cTnI | 12/0 | 16.2 +/− 0.6 | 3.2 +/− 1.8 | Baseline, 2, 4, and 24 h after the exercise | cTnT 8/12 (67%) MI cut off (0.03 ng/mL) 8/12 (67%) AMI cut off (0.05 ng/mL) cTnI 11/12 (92%) MI cut off (0.06 ng/mL) 3/12 (25%) AMI cut off (0.5 ng/mL) | Half marathon |
| Nie, J., Geoge, K., Tong, TK., Gaze, D., Tian, Y., Lin, H. & Shi, Q (2011) [30] | cTnT | 53/10 | 16.4 +/− 1.5 | 2.4 +/− 1.3 | Baseline, 4, and 24 h after the exercise | 57/63 (90%) MI cut off (0.01 ng/mL) 44/63 (70%) AMI cut off (0.05 ng/mL) | Half marathon |
| Traiperm, N., Gatterer, H., Wile, M. & Burtscher, M. (2012) [43] | cTnT cTnI | 19/18 | M: 16,7 +/− 0.5 F: 14,7 +/− 1.3 | 2.9 +/− 1.3 2.0 +/− 1.7 | Baseline, immediately, and 24 h after the exercise | cTnT 30/37 (81%) MI cut off (0.01 ng/mL) 1/37 (3%) AMI cut off (0.1 ng/mL) cTnI 30/37 (81%) MI cut off (0.1 ng/mL) 2/37 (5%) AMI cut off (0.5 ng/mL) | Marathon |
| Ma, G., Liu, Y. & Liu, K. (2014) [44] | cTnT cTnI | 28/0 | 7.2 +/− 1.1 | 6 months to 1 year | Baseline, immediately, 4, 24, and 48 h after the exercise | cTnT 9/28 (32%) MI cut off (0.03 ng/mL) 5/28 (18%) AMI cut off (0.05 ng/mL) cTnI 6/28 (21%) MI cut off (0.06 ng/mL) 2/28 (7%) AMI cut off (0.5 ng/mL) | Table Tennis (forehand exercises): 6 × 10′; resting 5′ every two sets. |
| Kong, Z., Nie, J., Lin, H., George, K., Zhao, G., Zhang, H., Tong, TK. & Shi, Q. (2016) [45] | cTnT | 19/19 | M: 16.1 +/− 1.2 Tanner: F: 3.7 +/− 0.6 Tanner: 4.0 +/− 0.4 15.9 +/− 1.4 | M: 2.3 +/− 1.0 F: 2.2 +/− 1.0 | Baseline and 4 h after the exercise | Male 19/19 (100%) MI cut off (0.01 ng/mL) 18/19 (95%) AMI cut off (0.05 ng/mL) Female 18/19 (95%) MI cut off (0.01 ng/mL) 12/19 (63%) AMI cut off (0.05 ng/mL) | Half marathon |
| Peretti, A., Mauri, L., Masarin, A., Annoni, G., Corato, A., Maloberti, A., Giannattasio, C. & Vignati, G. (2017) [46] | Hs-cTnT | 20/0 | 9.2 +/− 1.7 | At least 1 year. | 2.5 h after the exercise | 6/20 (30%) above URL (14 ng/L) | Cycling: “A single, maximal intensity cycling exercise prolonged until muscular exhaustion” |
| Hosseini, S.M., Azizi, M., Samadi, A., Talebi, N., Hannes, G. & Burtscher, M. (2017) [47] | cTnI | 22/0 | 15.4 +/− 0.4 | At least 1 year. | Baseline, immediately, 2, and 24 h after the exercise cTnI: MI 0 | 0/22 (0%) above URL (0.035 ng/mL) | Soccer game (90′) |
| Cirer, R., Legaz, A., Corbi, F., López, I., Puente, J., Hernández, V. & Reverter, J. (2019) [48] | Hs-cTnT | 20/0 T2: n = 8 T3: n = 8 T4: n = 4 | 11.9 +/− 2 | 5.9 +/− 1.7 | Baseline, immediately, and 3 h after the exercise | 4/20 (20%) above URL (14 ng/L) | Soccer: SSG (5 vs. 5) 16′ of effort (4 × 4′) (3′ passive rest between parts) |
| Cirer, R., Legaz, A., Corbi, F., López, I., George, K. & Reverter, J. (2020) [49] | Hs-cTnT | 70/0 T1: n = 14 T2: n = 15 T3: n = 15 T4: n = 13 T5: n = 13 | 7–18 years | 1–11 years | Baseline, immediately, and 3 h after the exercise | T1: 2/14 (14%) above URL (14 ng/L) T2: 4/15 (27%) above URL (14 ng/L) T3: 6/15 (40%) above URL (14 ng/L) T4: 6/13 (46%) above URL (14 ng/L) T5: 7/13 (54%) above URL (14 ng/L) | Swimming: 6 × 25 m maximal sprints (10” of recovery between efforts) |
| Birat, A., Bourdier, P., Dodu, A., Grossoeuvre, C., Blazevich, AJ., Amiot, V., Duppont, AC., Nottin, S. & Ratel, S. (2020) [50] | cTnI | 12/0 | 14.4 +/− 0.5 | 2 | Baseline, immediately, D+1, and D+2 | Race 1 8/12 (80%) MI cut off (0.5 ng/mL) Race 2 6/12 (50%) MI cut off (0.5 ng/mL) | Adventure race: Race 1: 48.2 km Day 1: 5.5 km trail running Day 2: 7.1 km trail running, 27 km mountain biking, 4.4 km kayaking, 4.2 km line skating. 10 days between both races. Race 2: 66 km Day 1: 7 km trail running Day 2: 14 km trail running, 27 km mountain biking, 18 km kayaking |
| Author | Biomarker | Participants Male/Female | Mean Age (Years) | Training Level (Years) | Timing of Sample Collection (Bolded Timepoint Giving Highest Prevalence) | cTn Results above Reference Range | Exercise Modality |
|---|---|---|---|---|---|---|---|
| Tian, Y., Nie, J., George, K. & Huang, C. (2014) [51] | Hs-cTnT | 10/0 | 20.4 +/− 5.4 | 2.4 +/− 0.9 | Baseline, immediately, 1, and 3 h after the exercise | Test 1 10/10 (100%) above URL (14 ng/L) Test 2 9/10 (90%) above URL (14 ng/L) | Treadmill Running 90′: Test 1: 95% VTh Separated by 3 weeks Test 2: 95% VTh |
| Wedin, JO. & Henriksson, AE. (2014) [52] | Hs-cTnT | Game 1: 23/0 Game 2: 16/0 | 19 (16 to 34 years) | “Elite floorball players” | Baseline, immediately, and 2 h after the exercise | Game 1 6/23 (26%) above URL (14 ng/L) Game 2 7/16 (43%) above URL (14 ng/L) | Floorball Game 1: 3 periods of 20′ Separated by 3 weeks Game 2: 3 periods of 20′ |
| Cirer, R., Jiménez, R., Carranza, LE., George, K., Apple, A., Navarro, R., López, R., Reverter, J., Mayolas, C., Morales, PG & Legaz, A. (2022) [53] | Hs-cTnT Hs-cTnI | 24/32 T3: 13 T4: 17 T5: 26 | 15 (14–22) | 2.67 (1.75–5.08) | Baseline, immediately, 1, 3, 6, 12, and 24 h after the exercise | cTnT 35/62(55%) above URL (14 ng/L) cTnI 28/62 (45%) above URL (11.8 ng/L) | Swimming: 60′ distance trial test. |
| Author | Biomarker | Participants Male/Female | Mean Age (Years) | Training Level (Years) | Timing of Sample Collection (Bolded Timepoint Giving Highest Prevalence) | cTn Results above Reference Range | Exercise Modality |
|---|---|---|---|---|---|---|---|
| Tian, Y., Nie, J., Huang, C. & George, K. (2012) [54] | Hs-cTnT | C: 13/0 A: 13/0 | C:14.1 +/− 1.1 T2: n = 8 T3: n = 5 A: 24 +/−3.6 | C: 2.7 +/− 1.3 A: 2.5 +/− 1.1 | Baseline, immediately, 1, 2, 3, 4, 5, 6, and 24 h after the exercise | Adults 11/13 (85%) above URL (14 ng/L) Children: Tanner 2 8/13 (62%) above URL (14 ng/L) Children: Tanner 3 4/13 (31%) above URL (14 ng/L) | Running (treadmill): 90-min 95% VTh. |
| López, I., Legaz, A., George, K., Serveto, O., González, J.M., Reverter, J. & Munguía, D. (2016) [27] | cTnI | PBA: 12/0 ABA: 12/0 JBA: 11/0 | 27.3 +/− 4.1 29.6 +/− 2.9 16.6 +/− 0.9 | 17.0 +/− 5.0 13.0 +/− 5.0 8.0 +/− 4.0 | Baseline, immediately, 1, 3, 6, 12, and 24 h after the exercise | PBA 3/12 (25%) above URL (0.04 ng/mL) ABA 0/12 (0%) above URL (0.04 ng/mL) JBA 6/11 (55%) above URL (0.04 ng/mL) | Basketball: 32′ of the 40′. “Every team made a change every 4 min of actual game time”. |
| Legaz, A., Carranza, L.E., Navarra, R., Valadez, A., Mayolas, C., Munguía, D., Reverter, J. & George, K. (2017) [13] | Hs-cTnT | T3: 4/10 T4: 11/11 T5: 10/4 A: 7/9 | 14.8 +/− 1.8 15.1 +/− 1.3 16.4 +/− 1.6 31.1 +/− 7.9 | 2.3 +/− 1.6 2.7 +/− 2.0 4.8 +/− 3.6 7.1 +/− 6.4 | Baseline, immediately, 1, 3, 6, and 12 h after the exercise | T3 6/14 (43%) above URL (14 ng/L) T4 17/22 (77%) above URL (14 ng/L) T5 10/14 (71%) above URL (14 ng/L) Adults 8/16 (50%) above URL (14 ng/L) | Swimming: 5′ Warm up (<60% Hrmax) followed by a 60 min “all out” test. |
| Cirer, R., Legaz, A., Corbi, F., López, I., Puente, J., Hernández, V. & Reverter, J. (2020) [55] | Hs-cTnT | C: 24/0 A: 12/0 | 10.7 +/− 1.6 37.5 +/− 12.7 | 4.6 +/− 1.7 23.6 +/− 14.5 | Baseline, immediately, and 3 h after the exercise | Children 17/24 (71%) above URL (14 ng/L) Adult 8/24 (33%) above URL (14 ng/L) | Soccer: SSG (7 vs. 7) 60′. 4 × 15′ (2′-10′-2′ rest between quarts) |
| Cirer, R., Corbi, F., López, I., Carranza, LE. & Reverter, J. (2021) [24] | Hs-cTnT | C: 18/0 A: 14/0 | 14.0 +/− 3 35.0 +/− 9 | 7.0 +/− 2 6.0 +/− 2 | Baseline, immediately, and 3 h after the exercise | Children 6/18 (33%) above URL (14 ng/L) Adult 8/14 (57%) above URL (14 ng/L) | Swimming: 45′ distance trial test. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa-Milian, E.; Cirer-Sastre, R.; Hernández-González, V.; Legaz-Arrese, A.; Corbi, F.; Reverter-Masia, J. Cardiac Troponin Release after Exercise in Healthy Young Athletes: A Systematic Review. Healthcare 2023, 11, 2342. https://doi.org/10.3390/healthcare11162342
Conesa-Milian E, Cirer-Sastre R, Hernández-González V, Legaz-Arrese A, Corbi F, Reverter-Masia J. Cardiac Troponin Release after Exercise in Healthy Young Athletes: A Systematic Review. Healthcare. 2023; 11(16):2342. https://doi.org/10.3390/healthcare11162342
Chicago/Turabian StyleConesa-Milian, Enric, Rafel Cirer-Sastre, Vicenç Hernández-González, Alejandro Legaz-Arrese, Francisco Corbi, and Joaquin Reverter-Masia. 2023. "Cardiac Troponin Release after Exercise in Healthy Young Athletes: A Systematic Review" Healthcare 11, no. 16: 2342. https://doi.org/10.3390/healthcare11162342
APA StyleConesa-Milian, E., Cirer-Sastre, R., Hernández-González, V., Legaz-Arrese, A., Corbi, F., & Reverter-Masia, J. (2023). Cardiac Troponin Release after Exercise in Healthy Young Athletes: A Systematic Review. Healthcare, 11(16), 2342. https://doi.org/10.3390/healthcare11162342








