Effectiveness of Robot Interventions for Cognitive and Psychological Outcomes among Older Adults with Cognitive Impairment: A Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Design
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
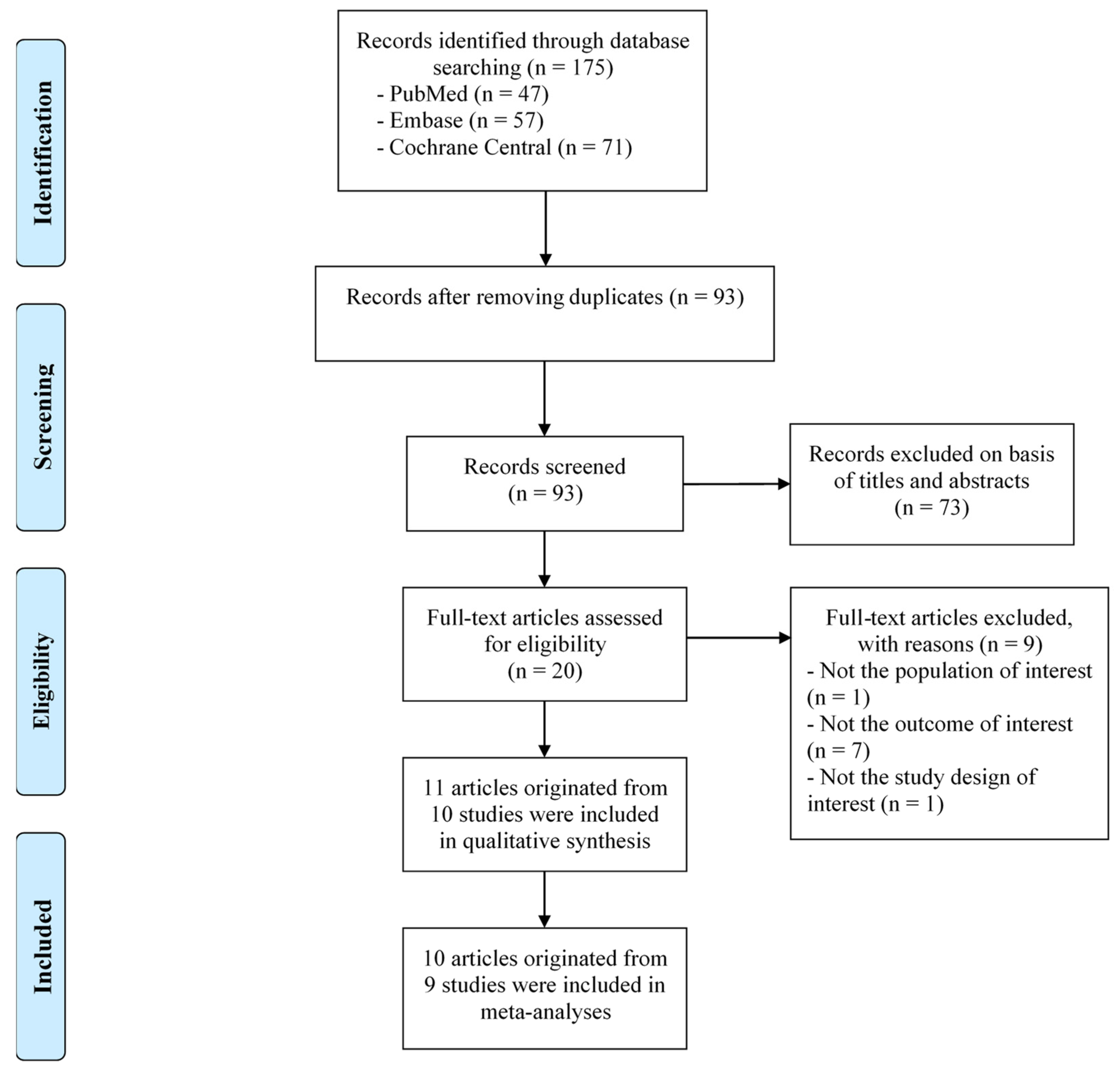
2.4. Selection Process
2.5. Data Extraction
2.6. Risk-of-Bias Assessment
2.7. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Included Studies
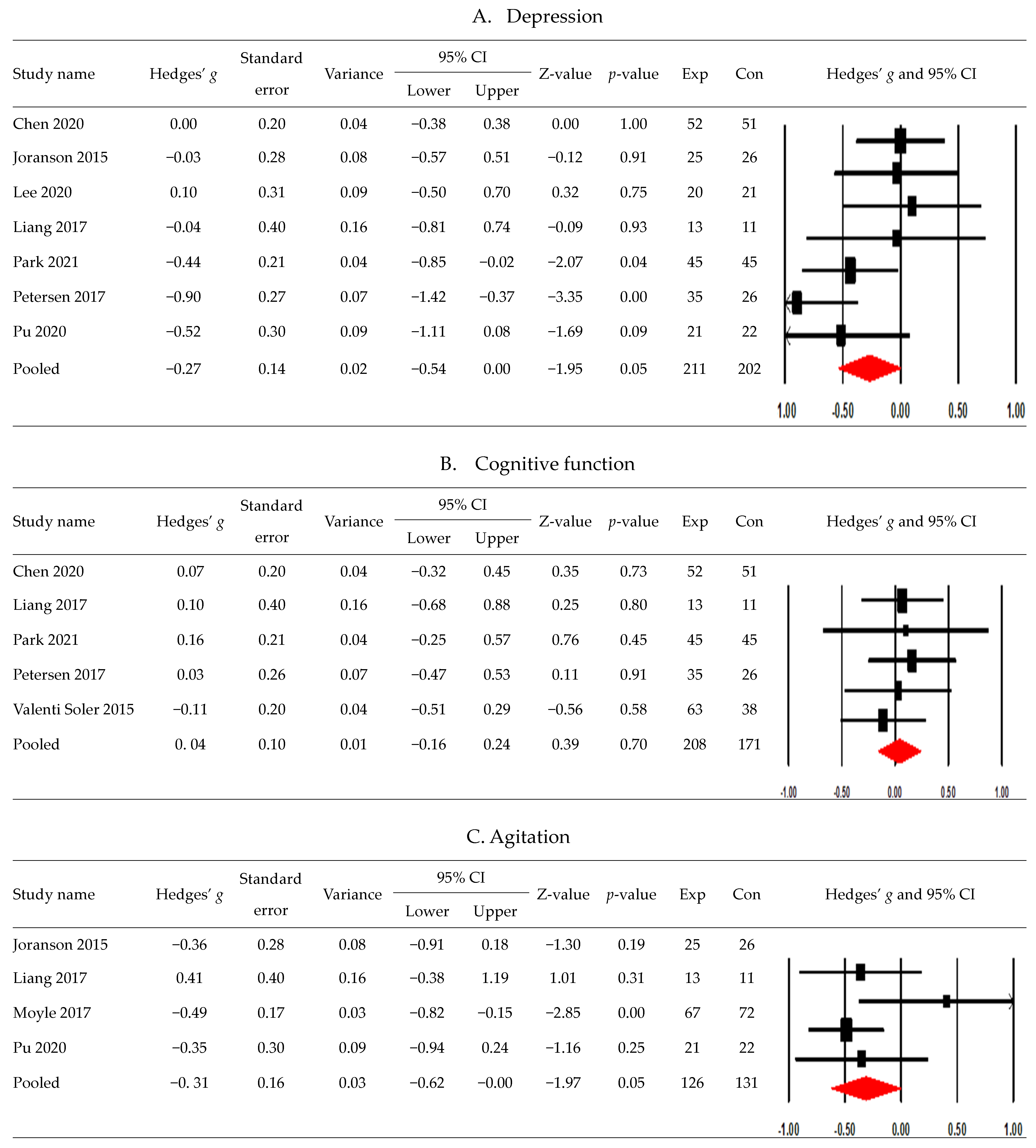
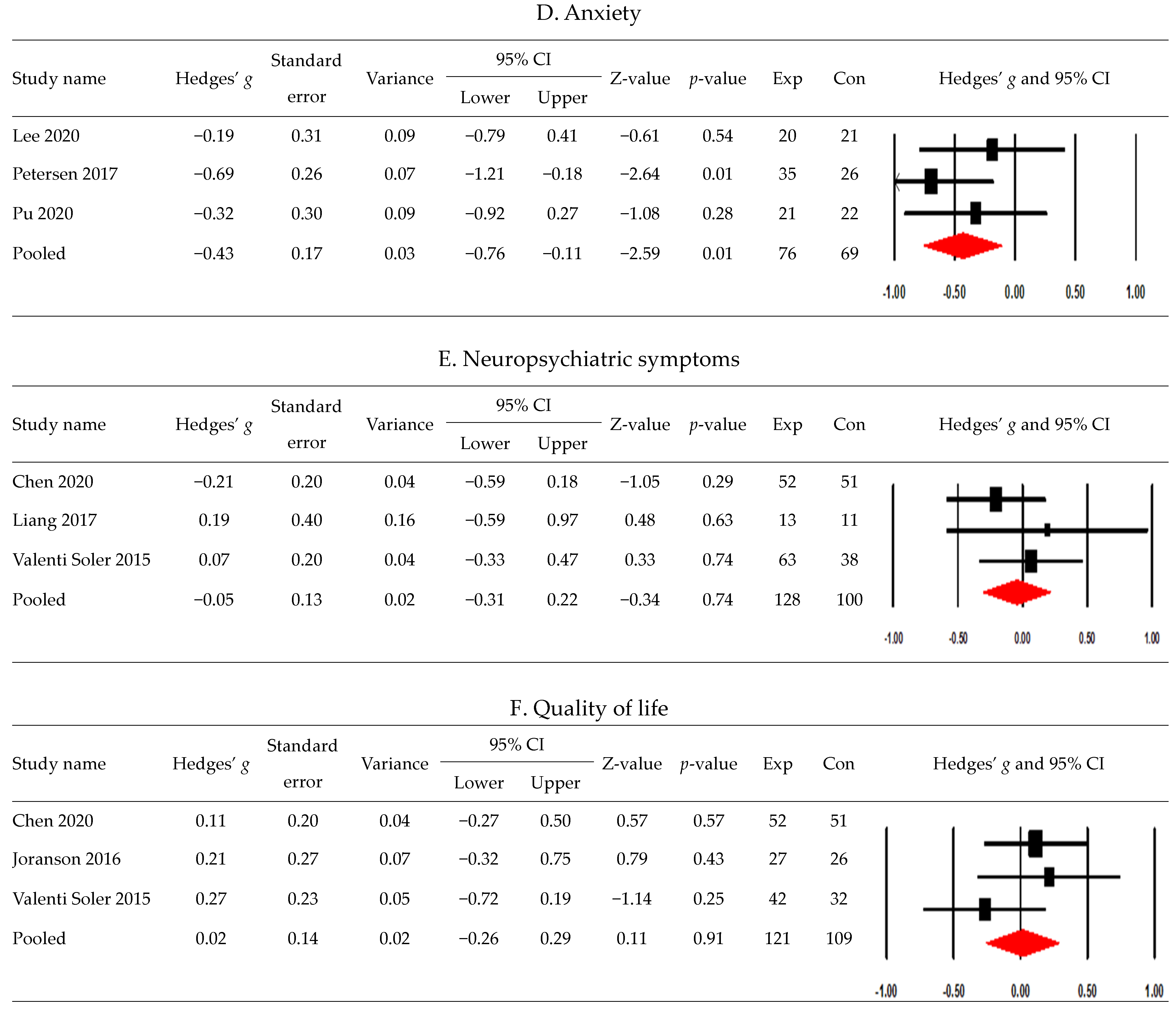
3.4. Postintervention Effects
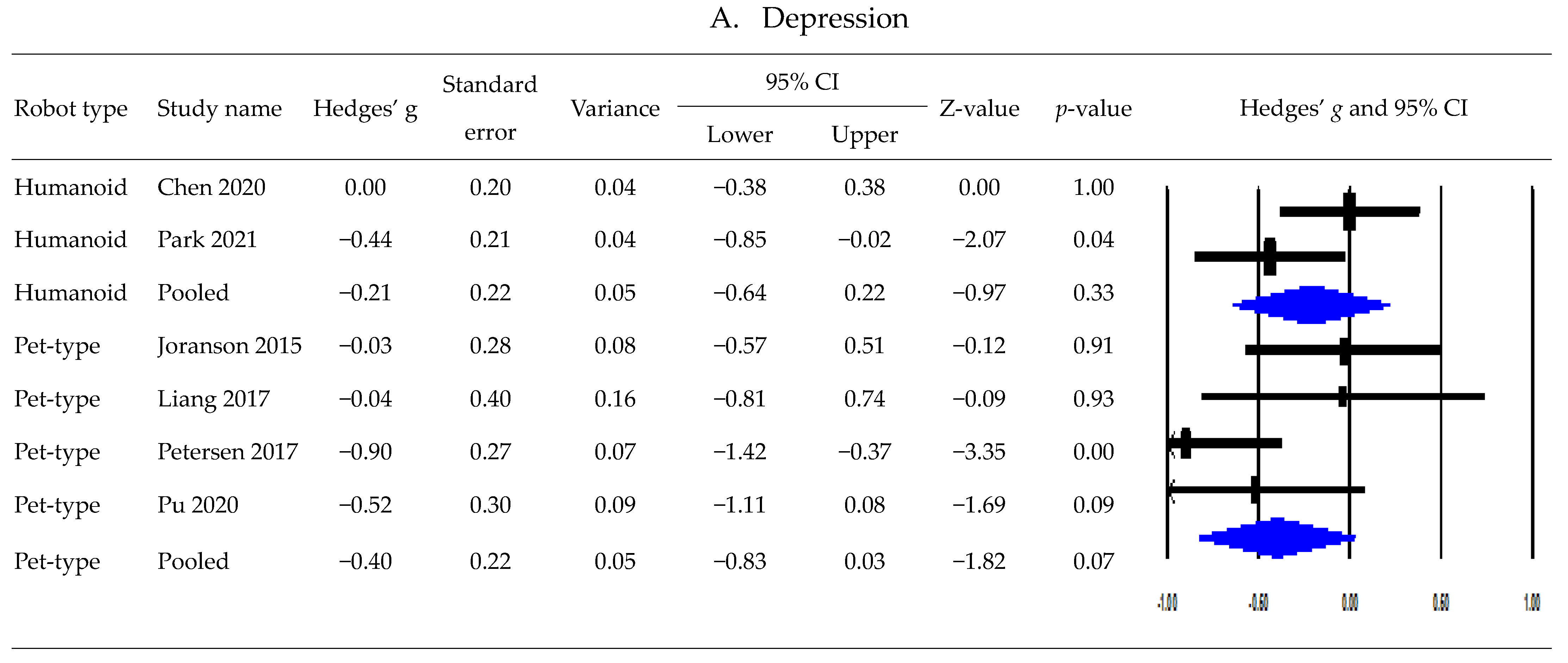
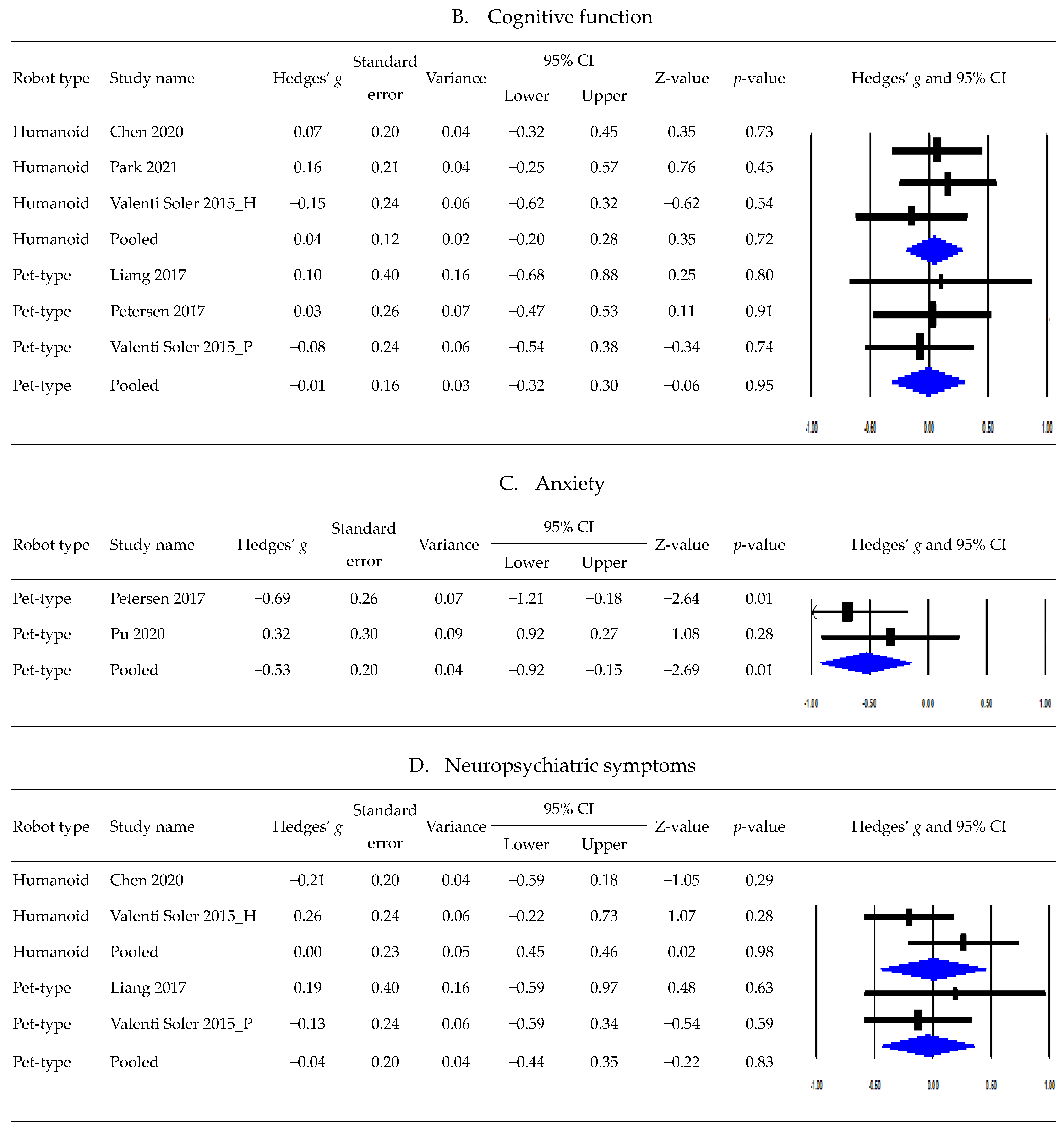
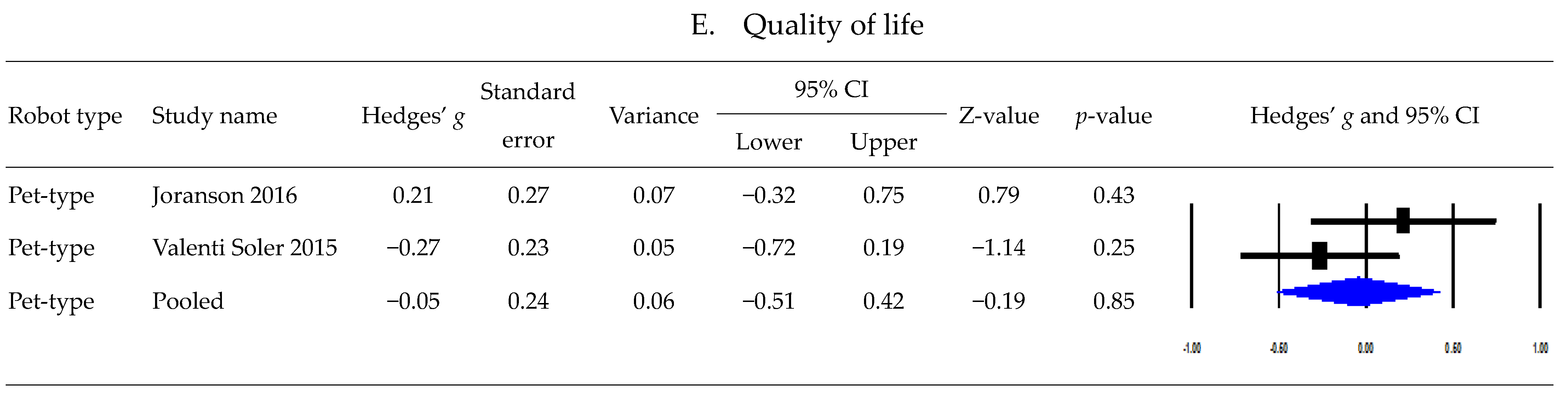
3.5. Postintervention Effects Based on Robot Type
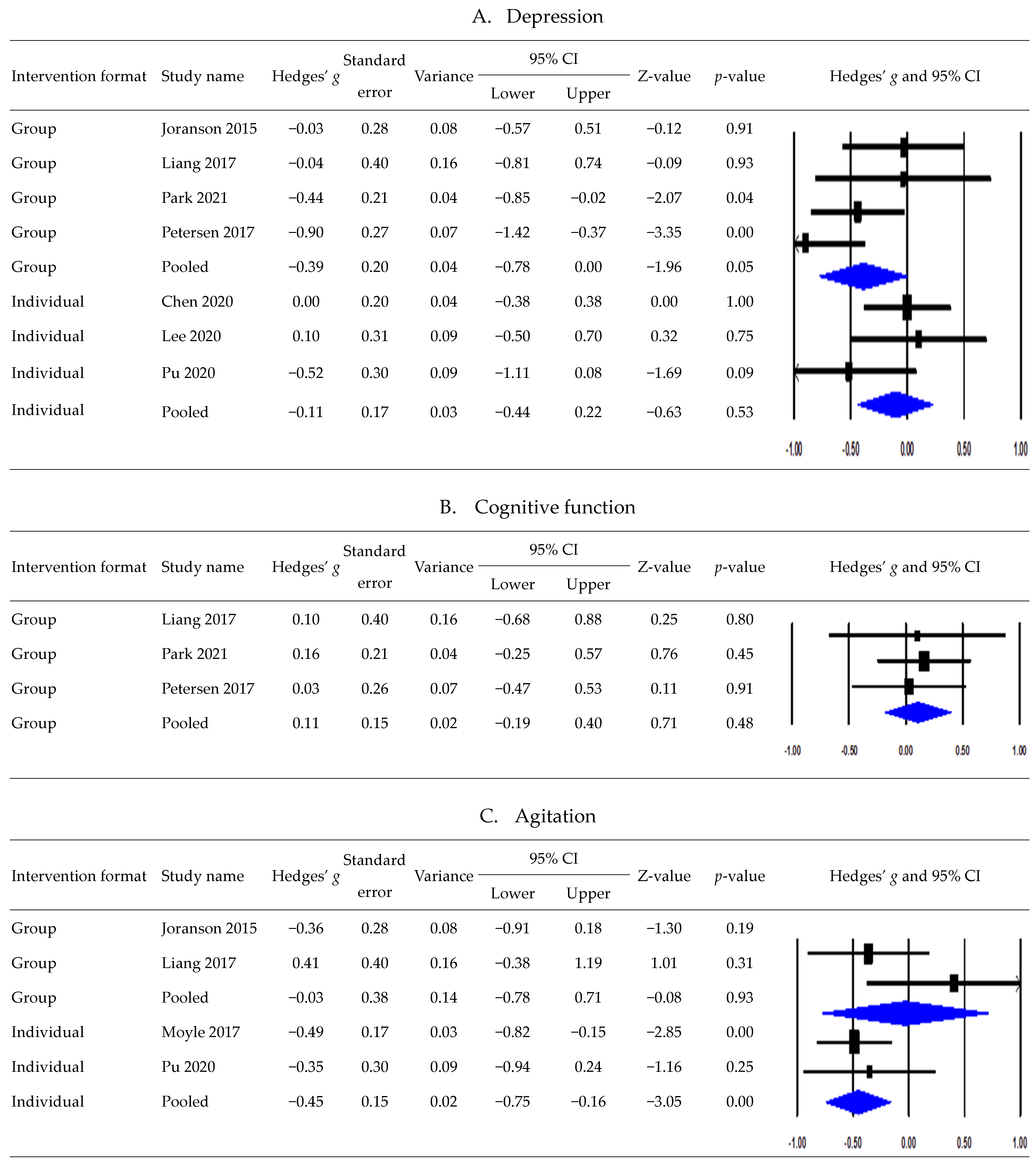
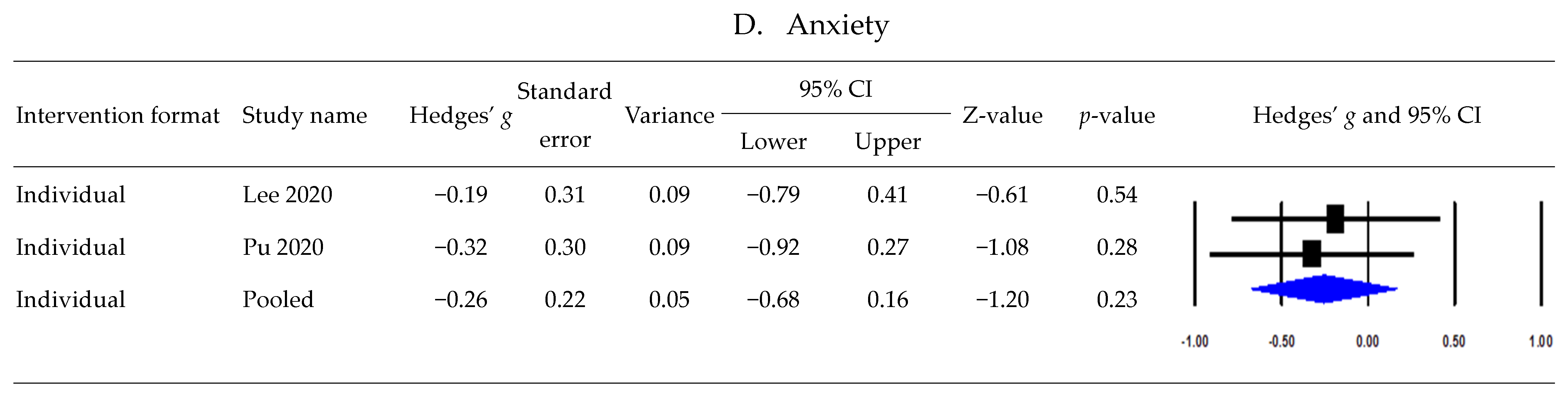
3.6. Postintervention Effects According to Intervention Formats
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Global Dementia Cases Forecasted to Triple by 2050. Alzheimer’s Assoc Medea Line. Available online: https://www.alz.org/aaic/releases_2021/global-prevalence.asp (accessed on 27 July 2021).
- Shon, C.; Yoon, H. Health-economic burden of dementia in South Korea. BMC Geriatr. 2021, 21, 549. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics. 11th Revision. Available online: https://icd.who.int/browse11/l-m/en (accessed on 27 July 2021).
- Majer, R.; Simon, V.; Csiba, L.; Kardos, L.; Frecska, E.; Hortobágyi, T. Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes. Open Med. 2019, 14, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.Y.; Lee, B. Prevalence of behavioral and psychological symptoms of dementia in community-dwelling dementia patients: A systematic review. Front. Psychiatry 2021, 12, 741059. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chen, T.F.; Chiu, M.J. From mild cognitive impairment to subjective cognitive decline: Conceptual and methodological evolution. Neuropsychiatr. Dis. Treat. 2017, 13, 491–498. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2019, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Velayudhan, L. Neuropsychiatric symptoms in mild cognitive impairment: A literature review. Dement. Geriatr. Cogn. Disord. 2020, 49, 146–155. [Google Scholar] [CrossRef]
- Kasper, S.; Bancher, C.; Eckert, A.; Förstl, H.; Frölich, L.; Hort, J.; Palomo, M.S.M. Management of mild cognitive impairment (MCI): The need for national and international guidelines. World J. Biol. Psychiatry 2020, 21, 579–594. [Google Scholar] [CrossRef]
- Vandemeulebroucke, T.; Dzi, K.; Gastmans, C. Older adults’ experiences with and perceptions of the use of socially assistive robots in aged care: A systematic review of quantitative evidence. Arch. Gerontol. Geriatr. 2021, 95, 104399. [Google Scholar] [CrossRef]
- Hung, L.; Liu, C.; Woldum, E.; Au-Yeung, A.; Berndt, A.; Wallsworth, C.; Horne, N.; Gregorio, M.; Mann, J.; Chaudhury, H. The benefits of and barriers to using a social robot PARO in care settings: A scoping review. BMC Geriatr. 2019, 19, 232. [Google Scholar] [CrossRef]
- Abdi, J.; Al-Hindawi, A.; Ng, T.; Vizcaychipi, M.P. Scoping review on the use of socially assistive robot technology in elderly care. BMJ Open 2018, 8, e018815. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ishii, A.; Yamano, E.; Ogikubo, H.; Okazaki, M.; Kamimura, K.; Konishi, Y.; Emoto, S.; Watanabe, Y. Effect of a human-type communication robot on cognitive function in elderly women living alone. Med. Sci. Monit. 2012, 18, CR550–CR557. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, B.R.; Kim, H.; Kim, S.H.; Chun, M.Y.; Park, H.K.; Park, K.D.; Jeong, J.H.; Kim, G.H. Four-week, home-based, robot cognitive intervention for patients with mild cognitive impairment: A pilot randomized controlled trial. Dement. Neurocognitive Disord. 2020, 19, 96–107. [Google Scholar] [CrossRef]
- Sather, R.; Soufineyestani, M.; Khan, A.; Imtiaz, N. Use of humanoid robot in dementia care: A literature review. J. Aging Sci. 2021, 9, 249. [Google Scholar]
- Ong, Y.C.; Tang, A.; Tam, W. Effectiveness of robot therapy in the management of behavioural and psychological symptoms for individuals with dementia: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 140, 381–394. [Google Scholar] [CrossRef]
- Saragih, I.D.; Tonapa, S.I.; Sun, T.L.; Chia-Ju, L.; Lee, B.O. Effects of robotic care interventions for dementia care: A systematic review and meta-analysis randomised controlled trials. J. Clin. Nurs. 2021, 30, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.C.; Lan, S.H.; Hsieh, Y.P.; Lin, L.Y.; Lan, S.J.; Chen, J.C. Effectiveness of companion robot care for dementia: A systematic review and meta-analysis. Innov. Aging. 2021, 5, igab013. [Google Scholar] [CrossRef]
- Liang, A.; Piroth, I.; Robinson, H.; MacDonald, B.; Fisher, M.; Nater, U.M.; Skoluda, N.; Broadbent, E. A pilot randomized trial of a companion robot for people with dementia living in the community. J. Am. Med. Dir. Assoc. 2017, 18, 871–878. [Google Scholar] [CrossRef]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Cochrane, AB, Canada, 2021; Version 6.2. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Featherstone, R.; Littlewood, A.; Marshall, C.; Metzendorf, M.I.; Noel-Storr, A.; Paynter, R.; Rader, T.; et al. Chapter 4: Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Cochrane, AB, Canada, 2022. [Google Scholar]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Eldridge, S.; Campbell, M.; Campbell, M.; Dahota, A.; Giraudeau, B.; Higgins, J.; Reeves, B.; Siegfried, N. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2) Additional Considerations for Cluster-Randomized Trials (RoB 2 CRT). 2021. Available online: https://www.unisa.edu.au/contentassets/72bf75606a2b4abcaf7f17404af374ad/rob2-0_cluster_parallel_guidance.pdf (accessed on 11 August 2023).
- Deeks, J.J.; Higgins JP, T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Cochrane, AB, Canada, 2022. [Google Scholar]
- Otake-Matsuura, M.; Tokunaga, S.; Watanabe, K.; Abe, M.S.; Sekiguchi, T.; Sugimoto, H.; Kishimoto, T.; Kudo, T. Cognitive intervention through photo-integrated conversation moderated by robots (PICMOR) program: A randomized controlled trial. Front Robot AI 2021, 8, 633076. [Google Scholar] [CrossRef] [PubMed]
- Jøranson, N.; Olsen, C.; Calogiuri, G.; Ihlebæk, C.; Pedersen, I. Effects on sleep from group activity with a robotic seal for nursing home residents with dementia: A cluster randomized controlled trial. Int. Psychogeriatr. 2021, 33, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Lou, V.W.; Tan, K.C.; Wai, M.Y.; Chan, L.L. Changes in technology acceptance among older people with dementia: The role of social robot engagement. Int. J. Med. Inform. 2020, 141, 104241. [Google Scholar] [CrossRef] [PubMed]
- Mervin, M.C.; Moyle, W.; Jones, C.; Murfield, J.; Draper, B.; Beattie, E.; Shum, D.H.K.; O’Dwyer, S.; Thalib, L. The cost-effectiveness of using PARO, a therapeutic robotic seal, to reduce agitation and medication use in dementia: Findings from a cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 2018, 19, 619–622.e1. [Google Scholar] [CrossRef] [PubMed]
- Moyle, W.; Jones, C.; Murfield, J.; Thalib, L.; Beattie, E.; Shum, D.; O’Dwyer, S.; Mervin, M.C.; Draper, B. Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: A cluster-randomised controlled trial. Maturitas 2018, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Moyle, W.; Jones, C.; Todorovic, M. The effect of a social robot intervention on sleep and motor activity of people living with dementia and chronic pain: A pilot randomized controlled trial. Maturitas 2021, 144, 16–22. [Google Scholar] [CrossRef]
- Thodberg, K.; Sørensen, L.U.; Videbech, P.B.; Poulsen, P.H.; Houbak, B.; Damgaard, V.; Keseler, I.; Edwards, D.; Christensen, J.W. Behavioral responses of nursing home residents to visits from a person with a dog, a robot seal or a toy cat. Anthrozoos 2016, 29, 107–121. [Google Scholar] [CrossRef]
- Werner, C.; Moustris, G.P.; Tzafestas, C.S.; Hauer, K. User-oriented evaluation of a robotic rollator that provides navigation assistance in frail older adults with and without cognitive impairment. Gerontology 2018, 64, 278–290. [Google Scholar] [CrossRef]
- Jones, C.; Moyle, W.; Murfield, J.; Draper, B.; Shum, D.; Beattie, E.; Thalib, L. Does cognitive impairment and agitation in dementia influence intervention effectiveness? Findings from a cluster-randomized-controlled trial with the therapeutic robot, PARO. J. Am. Med. Dir. Assoc. 2018, 19, 623–626. [Google Scholar] [CrossRef]
- Jøranson, N.; Pedersen, I.; Rokstad, A.M.; Ihlebæk, C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: A cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 867–873. [Google Scholar] [CrossRef]
- Jøranson, N.; Pedersen, I.; Rokstad, A.M.; Ihlebaek, C. Change in quality of life in older people with dementia participating in Paro-activity: A cluster-randomized controlled trial. J. Adv. Nurs. 2016, 72, 3020–3033. [Google Scholar] [CrossRef]
- Moyle, W.; Jones, C.J.; Murfield, J.E.; Thalib, L.; Beattie, E.R.A.; Shum, D.K.H.; O’Dwyer, S.T.; Mervin, M.C.; Draper, B.M. Use of a robotic seal as a therapeutic tool to improve dementia symptoms: A cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 2017, 18, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Park, E.A.; Jung, A.R.; Lee, K.A. The humanoid robot sil-bot in a cognitive training program for community-dwelling elderly people with mild cognitive impairment during the COVID-19 pandemic: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 8198. [Google Scholar] [CrossRef] [PubMed]
- Thodberg, K.; Sørensen, L.U.; Christensen, J.W.; Poulsen, P.H.; Houbak, B.; Damgaard, V.; Keseler, I.; Edwards, D.; Videbech, P.B. Therapeutic effects of dog visits in nursing homes for the elderly. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2016, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Valentí Soler, M.; Agüera-Ortiz, L.; Olazarán Rodríguez, J.; Mendoza Rebolledo, C.; Pérez Muñoz, A.; Rodríguez Pérez, I.; Martínez Martín, P. Social robots in advanced dementia. Front. Aging Neurosci. 2015, 7, 133. [Google Scholar] [CrossRef]
- Chen, K.; Lou, V.W.; Tan, K.C.; Wai, M.Y.; Chan, L.L. Effects of a humanoid companion robot on dementia symptoms and caregiver distress for residents in long-term care. J. Am. Med. Dir. Assoc. 2020, 21, 1724–1728.e3. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.; Houston, S.; Qin, H.; Tague, C.; Studley, J. The utilization of robotic pets in dementia care. J. Alzheimers Dis. 2017, 55, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Moyle, W.; Jones, C.; Todorovic, M. The effect of using PARO for people living with dementia and chronic pain: A pilot randomized controlled trial. J. Am. Med. Dir. Assoc. 2020, 21, 1079–1085. [Google Scholar] [CrossRef]
- Leng, M.; Zhao, Y.; Wang, Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: A systematic review and Bayesian network meta-analysis. Int. J. Nurs. Stud. 2020, 102, 103489. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, A.Y.; Liu, T.; Choy JC, P.; Ma MS, L.; Wong, G.; Lum, T. Degree of personalisation in tailored activities and its effect on behavioural and psychological symptoms and quality of life among people with dementia: A systematic review and meta-analysis. BMJ Open 2021, 11, e048917. [Google Scholar] [CrossRef]
- Couch, E.; Lawrence, V.; Co, M.; Prina, M. Outcomes tested in non-pharmacological interventions in mild cognitive impairment and mild dementia: A scoping review. BMJ Open 2020, 10, e035980. [Google Scholar] [CrossRef] [PubMed]
| Author/Country | Study Design | Participants’ Characteristics (Sample Size, Settings and Cognitive Status, Age Range, Mean or Median Age, and Sex) | Intervention Characteristics (Intervention Method, Duration, Frequency, and Time/Session) | Comparison | Outcomes Related to Cognitive and Psychological Status (Measurement Tools) | Measurement Assessment Time Points |
|---|---|---|---|---|---|---|
| Chen et al. [42]/Hong Kong | RCT with ABAB withdrawal design | 103 (E: 52, C: 51); long-term care facility residents with dementia; aged 67–108 years; mean age, 87.2 years; 79.6% were women | Individual, unfacilitated intervention with a humanoid robot (Kabochan); usual care for 8 weeks (baseline phase), then intervention for 8 weeks (intervention phase), then usual care for 8 weeks (intervention-withdrawal phase), and then intervention for 8 weeks (intervention-reintroduction phase) | Usual care | Cognitive function (Montreal Cognitive Assessment 5 min Protocol) Depression (Geriatric Depression Scale) Neuropsychiatric symptoms (Neuropsychiatric Inventory Questionnaire) Quality of life (Quality of Life in Alzheimer’s Disease Scale) | Week 1 (baseline), week 8 (baseline), week 16 (post-first intervention), week 24 (withdrawal), and week 32 (post-second intervention) |
| Jøranson et al. [36]/Norway Jøranson et al. [37]/Norway | CRT | 53 (E: 27, C: 26); residents with mild to severe dementia in nursing home; aged 62–95 years; mean age, 84.0 years; 67% were women | Group activity with the seal robot (PARO) delivered in nursing homes; for 12 weeks, twice a week; each session lasted 30 min | Treatment as usual | Agitation (Brief Agitation Rating Scale) Depression (Cornell Scale for Depression in Dementia) Quality of life (Quality of Life in Late-Stage Dementia Scale) | Baseline, postintervention, and 3 months after the end of intervention (follow-up) |
| Lee et al. [15]/Korea | RCT | 41 (E: 20, C: 21); MCI patients recruited from a hospital; aged 60 years or older; mean age, 74.0 years; 39.1% were women | Home-based cognitive intervention with a personal robot (Bomy); for 4 weeks, 5 days a week; each session lasted 60 min | No intervention | Anxiety (Geriatric Anxiety Inventory) Depression (Geriatric Depression Scale-Short Form) Spatial working memory, paired-associates learning, rapid visual information processing (Cambridge Neuropsychological Test Automated Battery) | Baseline and postintervention |
| Liang et al. [20]/New Zealand | RCT | 24 (E: 13, C: 11); participants with dementia who attended dementia daycare centers; aged 67–98 years; mean age, 83.8 years; 64% were women | Group activity with a seal robot (PARO) at daycare centers and interactions with the robot at home; for 6 weeks, 2–3 times a week; each session lasted 30 min | Standard care | Agitation (Cohen–Mansfield Agitation Inventory-Short Form) Cognitive function (Addenbrooke’s Cognitive Examination) Depression (Cornell Scale for Depression in Dementia) Neuropsychiatric symptoms (Neuropsychiatric Inventory Brief Questionnaire Form) | Baseline, 6 weeks (postintervention), and 12 weeks (follow-up) |
| Moyle et al. [38]/Australia | CRT | 415 (E: 138, C1: 140, C2: 137); long-term care facility residents with dementia; aged 60 years or older; mean age, 85.0 years; 75.7% were women | Individual, unfacilitated sessions with a robotic seal (PARO); for 10 weeks, thrice a week; each session lasted 15 min | C1: nonrobotic plush toy, C2: usual care | Agitation (Cohen–Mansfield Agitation Inventory-Short Form) Mood states (Coded video observations) | Baseline, week 1, week 5, week 10 (postintervention), and week 15 (follow-up) |
| Park et al. [39]/Korea | RCT | 135 (E: 45, C1: 45, C2: 45); community-dwelling older adults with MCI or subjective memory complaints recruited from a dementia center; aged 60 years or older; mean age, 75.9 years; 72.6% were women | Robot-assisted cognitive training group program (humanoid robot, Sil-bot) delivered in a dementia center; for 6 weeks, twice a week; each session lasted 60 min | C1: traditional cognitive training, C2: no intervention | Cognitive function (Mini-Mental State Examination-Dementia Screening) Depression (Geriatric Depression Scale-Short Form) Neuropsychological assessment (Consortium to Establish a Registry for Alzheimer’s Disease) Subjective memory complaints (Subjective Memory Complaint Questionnaire) | Baseline and postintervention |
| Peterson et al. [43]/USA | RCT | 61 (E: 35, C: 26); long-term care facility residents with mild to moderate dementia; aged 65 years or older; mean age, 83.4 years; 77.0% were women | Group sessions with a robotic pet (PARO); for 3 months, thrice a week; each session lasted 20 min | Standard care | Anxiety (Rating Anxiety in Dementia) Cognitive function (Global Deterioration Scale) Depression (Cornell Scale for Depression in Dementia) | Baseline and postintervention |
| Pu et al. [44]/Australia | RCT | 43 (E: 21, C: 22); long-term care facility residents with chronic pain and dementia; aged 65–97 years; mean age, 86.0 years; 69.8% were women | Individual, unfacilitated sessions with a robotic seal (PARO); for 6 weeks, 5 days a week; each session lasted 30 min | Usual care | Agitation (Cohen–Mansfield Agitation Inventory-Short Form) Anxiety (Rating Anxiety in Dementia) Depression (Cornell Scale for Depression in Dementia) | Baseline and postintervention |
| Thodberg et al. [33]/Denmark | RCT | 100 (E1: 35, E2: 35, C: 30); nursing home residents with dementia; age 79–90 years; median age, 85.5 years; 69% were women | E1: Visits from a person accompanied by a dog, E2: visits from a person accompanied by a robot seal (PARO); for 6 weeks; biweekly; each visit lasted 10 min | Soft toy cat | Cognitive function (Mini-Mental State Examination) Dementia symptoms (Gottfries–Bråne–Steen scale) Depression (Geriatric Depression Scale) Symptoms of delirium (Confusion Assessment Method) | The week before and after the visit period |
| Valentí Soler et al. [41]/Spain | RCT | Phase 1: 101 (E1: 30, E2: 33, C: 38) Phase 2: 110 (E1: 42, E2: 36, C: 32); nursing home residents with dementia; aged 58–100 years (Phase 1), aged 59–101 years (Phase 2); mean age, 84.7 years (Phases 1 and 2); 88% were women in Phase 1; 90% were women in Phase 2 | Phase 1: E1: humanoid robot (NAO), E2: pet robot (PARO) Phase 2: E1: pet robot (PARO), E2: real animal (dog); for 3 months; 2 days a week; each session lasted 30–40 min | Standard care | Apathy (Apathy Scale for Institutionalized Patients with Dementia Nursing Home version) Cognitive function (Mini-Mental State Examination, Severe Mini-Mental State Examination) Neuropsychiatric symptoms (Neuropsychiatric Inventory) Quality of life (Quality of Life in Late-Stage Dementia Scale) | Baseline and postintervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, D.; Shim, M.-S. Effectiveness of Robot Interventions for Cognitive and Psychological Outcomes among Older Adults with Cognitive Impairment: A Meta-Analysis. Healthcare 2023, 11, 2341. https://doi.org/10.3390/healthcare11162341
Noh D, Shim M-S. Effectiveness of Robot Interventions for Cognitive and Psychological Outcomes among Older Adults with Cognitive Impairment: A Meta-Analysis. Healthcare. 2023; 11(16):2341. https://doi.org/10.3390/healthcare11162341
Chicago/Turabian StyleNoh, Dabok, and Mi-So Shim. 2023. "Effectiveness of Robot Interventions for Cognitive and Psychological Outcomes among Older Adults with Cognitive Impairment: A Meta-Analysis" Healthcare 11, no. 16: 2341. https://doi.org/10.3390/healthcare11162341
APA StyleNoh, D., & Shim, M.-S. (2023). Effectiveness of Robot Interventions for Cognitive and Psychological Outcomes among Older Adults with Cognitive Impairment: A Meta-Analysis. Healthcare, 11(16), 2341. https://doi.org/10.3390/healthcare11162341






