The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
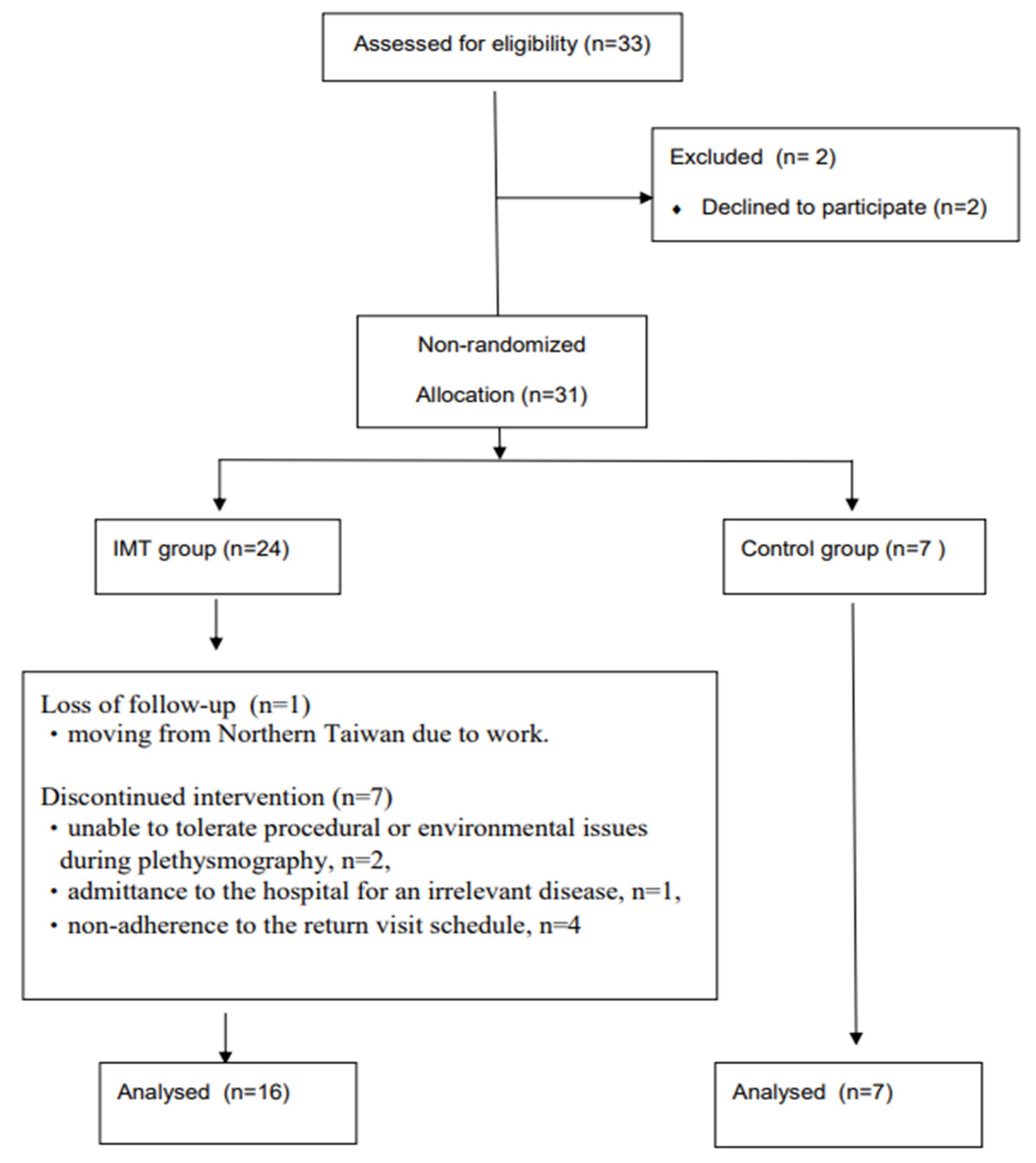
2.2. Participants
2.3. Procedures
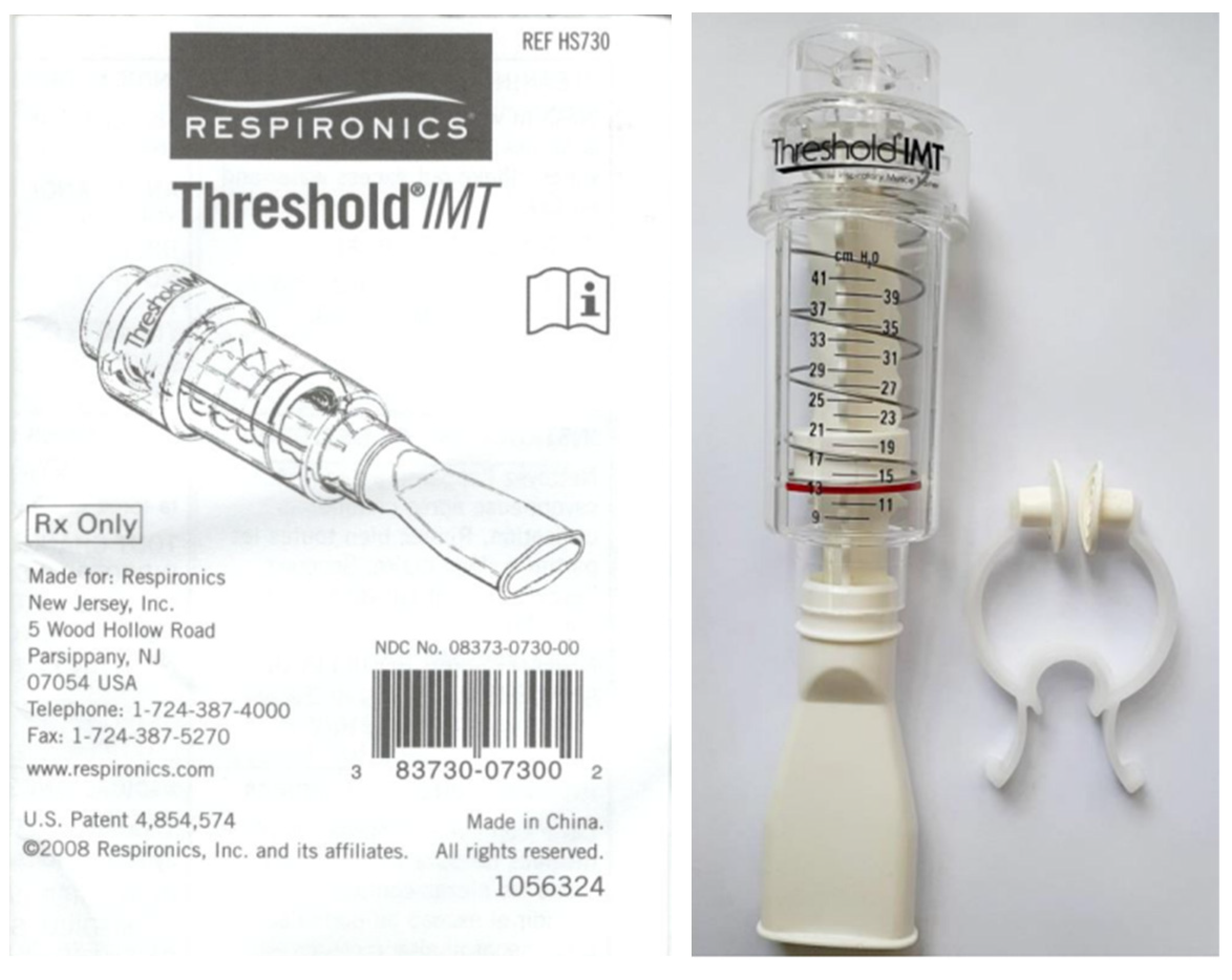
2.3.1. The IMT Device and Prescribed Training
2.3.2. Clinical Disease Symptom Severity Evaluations
The Modified British Medical Research Council (mMRC) Dyspnea Scale
The COPD Assessment Test (CAT) Score
2.3.3. Measurement of Lung Function, Pulmonary Physiology, and Exercise Tests
Total-Body Plethysmography
Manovacuometry
The 6 min Walking Test
2.4. Statistical Analysis
3. Results
3.1. Participant Demographics
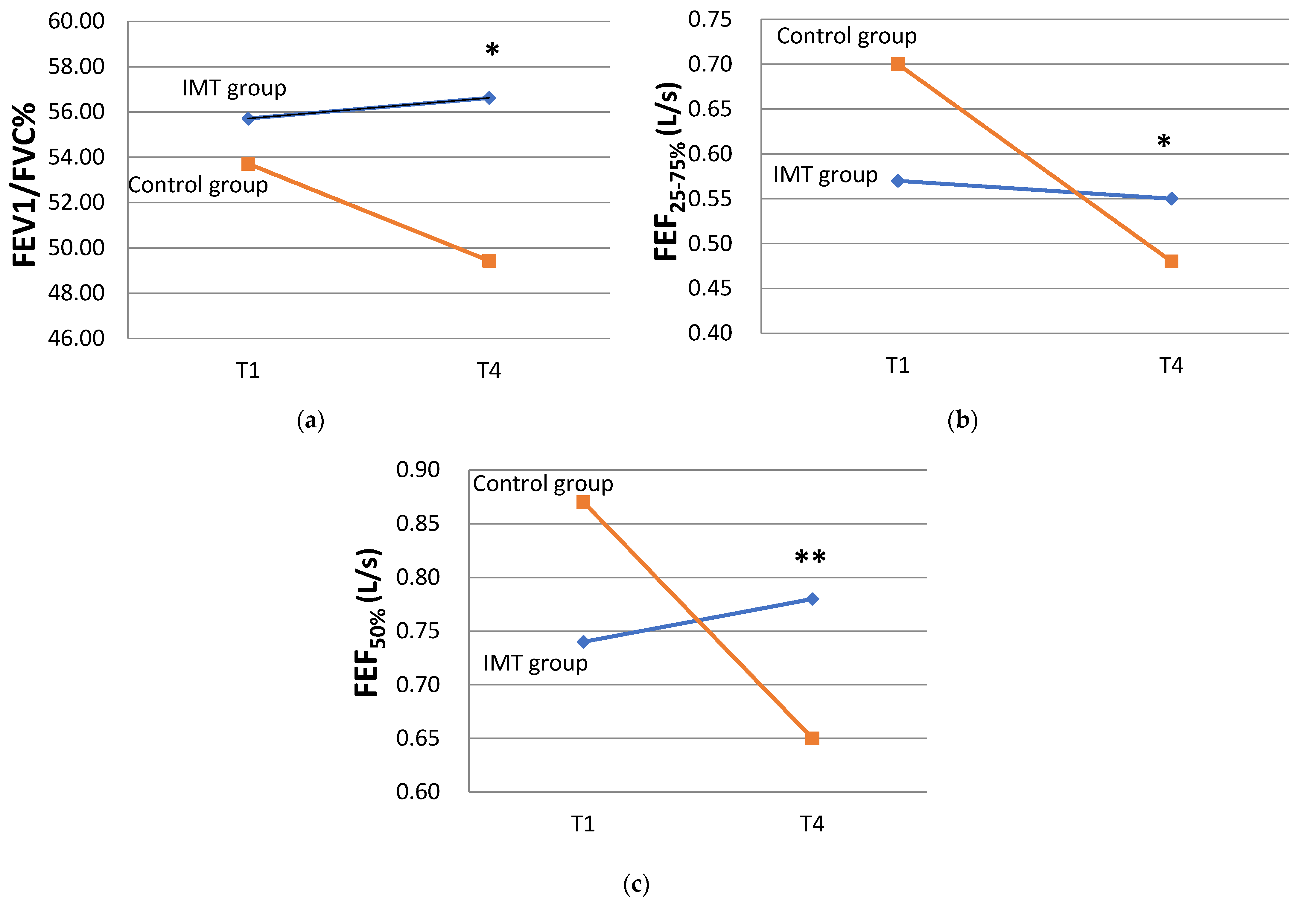
3.2. Effects of IMT on Lung Function in COPD Participants
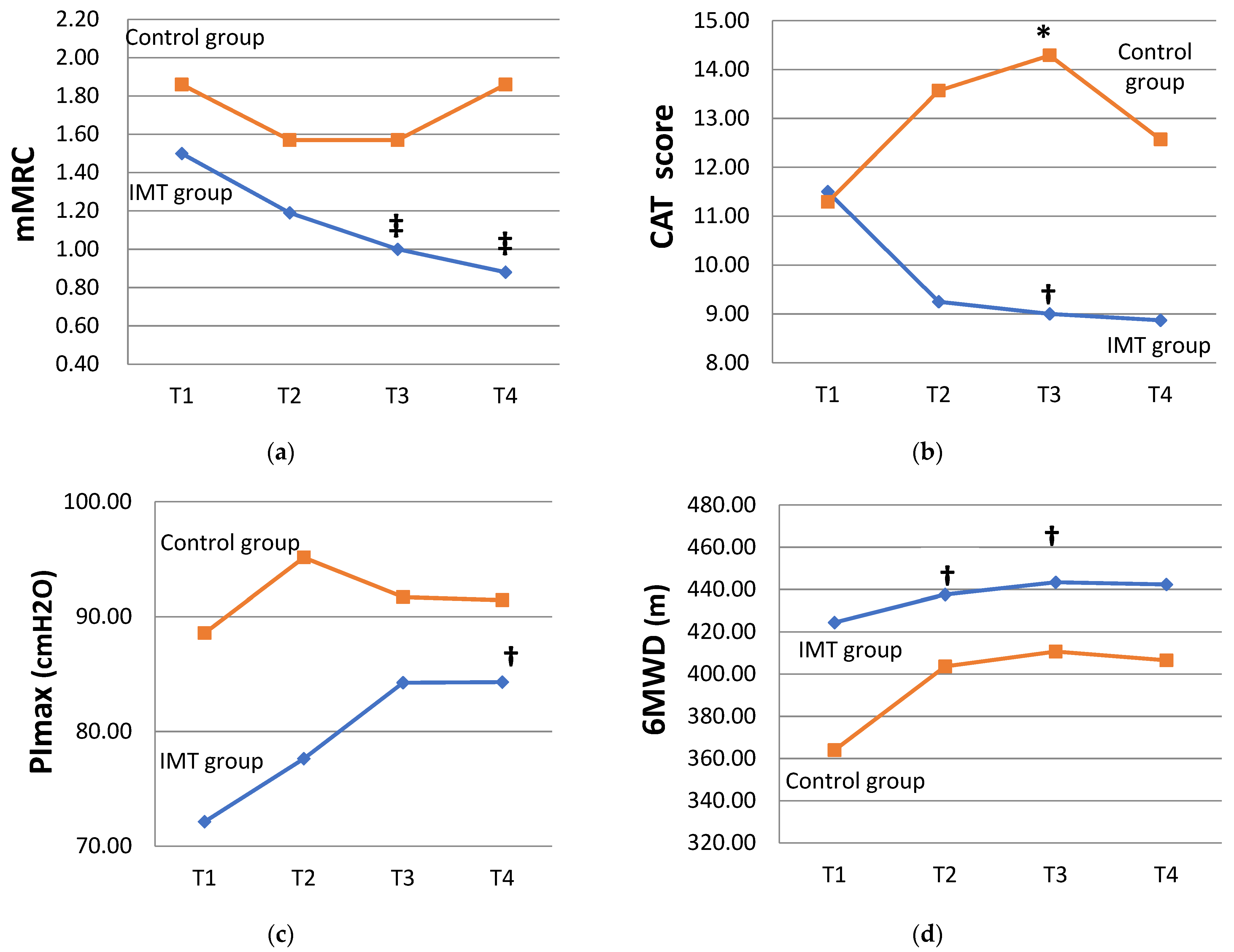
3.3. Effects of IMT on Disease-Associated Symptoms in COPD Participants
4. Discussion
4.1. Beneficial Effects of IMT on Lung Function and Heart Rate in COPD Participants
4.2. Beneficial Effects of IMT on Disease-Associated Symptoms of COPD Participants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agusti, A.; Beasley, R.; Celli, B.R.; Criner, G.; Halpin, D.; Varela, M.V.; Montes de Oca, M.; Mortimer, K.; Salvi, S.; Vogelmeier, C.; et al. Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professional; Global Initiative for Chronic Obstructive Lung Disease: Deer Park, IL, USA, 2023. [Google Scholar]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuch, Y.; Jenkins, C.; Rodriguez-Roisin, R.; Well, C.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.M.; Sciurba, F.C. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef]
- Usmani, O.S.; Barnes, P.J. Assessing and treating small airways disease in asthma and chronic obstructive pulmonary disease. Ann. Med. 2012, 44, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, V.; Barisione, G.; Crimi, E. Pulmonary physiology: Future directions for lung function testing in COPD. Respirology 2015, 20, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Criée, C.P.; Sorichter, S.; Smith, H.J.; Kardos, P.; Merget, R.; Heise, D.; Berdel, D.; Köhler, D.; Magnussen, H.; Marek, W.; et al. A Body plethysmography—Its principles and clinical use. Respir. Med. 2011, 105, 959–971. [Google Scholar] [CrossRef]
- Agustí, A.; Vogelmeier, C.; Faner, R. COPD 2020: Changes and challenges. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L879–L883. [Google Scholar] [CrossRef]
- Basso-Vanelli, R.P.; Di Lorenzo, V.A.P.; Labadessa, I.G.; Regueiro, E.M.; Jamami, M.; Gomes, E.L.; Costa, D. Effects of Inspiratory Muscle Training and Calisthenics-and-Breathing Exercises in COPD With and Without Respiratory Muscle Weakness. Respir. Care 2016, 61, 50–60. [Google Scholar] [CrossRef]
- Petrovic, M.; Reiter, M.; Zipko, H.; Pohl, W.; Wanke, T. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2012, 7, 797–805. [Google Scholar] [CrossRef]
- Crowe, J.; Reid, W.D.; Geddes, E.L.; O’Brien, K.; Brooks, D. Inspiratory muscle training compared with other rehabilitation interventions in adults with chronic obstructive pulmonary disease: A systematic literature review and meta-analysis. COPD 2005, 2, 319–329. [Google Scholar] [CrossRef]
- Gosselink, R.; Troosters, T.; Decramer, M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am. J. Respir. Crit. Care Med. 1996, 153, 976–980. [Google Scholar] [CrossRef]
- Sahin, H.; Naz, I.; Varol, Y.; Aksel, N.; Tuksavul, F.; Ozsoz, A. Is a pulmonary rehabilitation program effective in COPD patients with chronic hypercapnic failure? Expert Rev. Respir. Med. 2016, 10, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; American College of Sports Medicine; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.K. The role of inspiratory muscle function and training in the genesis of dyspnoea in asthma and COPD. Prim. Care Respir. J. 2005, 14, 186–194. [Google Scholar] [CrossRef]
- Shoemaker, M.; Donker, S.; Lapoe, A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: The state of the evidence. Cardiopulm. Phys. Ther. J. 2009, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Gandullo, E.; Hidalgo-Molina, A.; Montoro-Ballesteros, F.; Morales-González, M.; Muñoz-Ramírez, I.; Arnedillo-Muñoz, A. Inspiratory Muscle Training in Patients with Chronic Obstructive Pulmonary Disease (COPD) as Part of a Respiratory Rehabilitation Program Implementation of Mechanical Devices: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5564. [Google Scholar] [CrossRef]
- Padula, C.A.; Yeaw, E. Inspiratory muscle training: Integrative review. Res. Theory Nurs. Pract. 2006, 20, 291–304. [Google Scholar] [CrossRef]
- Figueiredo, R.I.N.; Azambuja, A.M.; Cureau, F.V.; Sbruzzi, G. Inspiratory Muscle Training in COPD. Respir. Care 2020, 65, 1189–1201. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Statistics & Publications. Taiwan: Ministry of Health and Welfare. 2022. Available online: https://dep.mohw.gov.tw/DOS/lp-5069-113-xCat-y110.html (accessed on 30 January 2023).
- Cheng, S.; Chan, M.C.; Wang, C.C.; Lin, C.H.; Wang, H.C.; Hsu, J.Y.; Hang, L.W.; Chang, C.J.; Perng, D.W.; Yu, C.J. COPD in Taiwan: A National Epidemiology Survey. Int. J. Chron. Obstruct. Pulm. Dis. 2015, 10, 2459–2467. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Statistics & Publications Taiwan: Ministry of Health and Welfare. Available online: https://dep.mohw.gov.tw/DOS/lp-4268-113.html (accessed on 30 July 2019).
- Fu, S.N.; Yu, W.C.; Wong, C.K.; Lam, C.H. Prevalence of undiagnosed airflow obstruction among people with a history of smoking in a primary care setting. Int. J. Chron. Obstruct. Pulm. Dis. 2016, 11, 2391–2399. [Google Scholar] [CrossRef][Green Version]
- Jenkins, S.C. 6-Minute walk test in patients with COPD: Clinical applications in pulmonary rehabilitation. Physiotherapy 2007, 93, 175–182. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Arena, R.; Riebe, D.; Thompson, P.D. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; American College of Sports Medicine; Wolters Kluwer Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Kim, S.; Oh, J.; Kim, Y.I.; Ban, H.J.; Kwon, Y.S.; Oh, I.J.; Kim, K.S.; Kim, Y.C.; Lim, S.C. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: A cross-sectional analyses. BMC Pulm. Med. 2013, 3, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.Y.; Lin, J.R.; Lin, M.S.; Chen, W.; Chen, Y.J.; Yan, Y.H. The modified Medical Research Council dyspnoea scale is a good indicator of health-related quality of life in patients with chronic obstructive pulmonary disease. Singapore Med. J. 2013, 54, 321–327. [Google Scholar] [CrossRef]
- Jones, P.W.; Adamek, L.; Nadeau, G.; Banik, N. Comparisons of health status scores with MRC grades in COPD: Implications for the GOLD 2011 classification. Eur. Respir. J. 2013, 42, 647–654. [Google Scholar] [CrossRef]
- Companies GSK. COPD Assessment Test. 2009. Available online: https://www.catestonline.org/patient-site-test-page-chinese-taiwan.html (accessed on 26 June 2017).
- Jones, P.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.-H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Kacmarek, R.M.; Stoller, J.K.; Heuer, A.J. Egan’s Fundamentals of Respiratory Care; Elsevier: Toronto, ON, Canada, 2017. [Google Scholar]
- Holland, A.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Mehani, S.H.M. Comparative study of two different respiratory training protocols in elderly patients with chronic obstructive pulmonary disease. Clin. Interv. Aging 2017, 12, 1705–1715. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Stoller, J.K. Assessing small airway disease in GLI versus NHANES III based spirometry using area under the expiratory flow-volume curve. BMJ Open Respir. Res. 2019, 6, e000511. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Feng, Y.; Liang, B. The measurement of lung volumes using body plethysmography and helium dilution methods in COPD patients: A correlation and diagnosis analysis. Sci. Rep. 2016, 6, 37550. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T. Resting heart rate and relation to disease and longevity: Past, present and future. Scand. J. Clin. Lab. Investig. 2019, 79, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nauman, J.; Janszky, I.; Vatten, L.J.; Wisløff, U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA 2011, 306, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Fleck, S.J.; Deschenes, M.R. Exercise Physiology: Integrated from Theory to Practical Applications; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2012. [Google Scholar]
- O’Donnell, D.E.; Milne, K.M.; James, M.D.; de Torres, J.P.; Neder, J.A. Dyspnea in COPD: New Mechanistic Insights and Management Implications. Adv. Ther. 2020, 37, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; Folli, A.; Curci, C.; D’Abrosca, F.; Moalli, S.; Mezian, K.; de Sire, A.; Invernizzi, M. Osteosarcopenia in Patients with Chronic Obstructive Pulmonary Diseases: Which Pathophysiologic Implications for Rehabilitation? Int. J. Environ. Res. Public Health 2022, 19, 14314. [Google Scholar] [CrossRef] [PubMed]
| Participant Demographics | Total Cohort (n = 23) N (%) or Mean ± SD | Study Groups | p Value | |
|---|---|---|---|---|
| IMT (n = 16) N (%) or Mean ± SD | Control (n = 7) N (%) or Mean ± SD | |||
| Gender | 0.273 a | |||
| Male | 19 (82.6) | 12 (75.0) | 7 (100.0) | |
| Female | 4 (17.4) | 4 (25.0) | 0 (0.0) | |
| Age (years) | 68.29 ± 10.87 | 67.25 ± 1.69 | 70.57 ± 12.66 | 0.535 |
| BMI | 23.54 ± 4.79 | 23.10 ± 4.26 | 24.55 ± 6.10 | 0.720 |
| Smoking status | 0.087 a | |||
| Never smoker | 10 (43.5) | 9 (56.2) | 1 (14.3) | |
| Former smoker | 9 (39.1) | 4 (25.0) | 5 (71.4) | |
| Current smoker | 4 (17.4) | 3 (18.8) | 1 (14.3) | |
| Severity of airflow limitation | 0.202 a | |||
| Moderate | 15 (65.2) | 12 (75.0) | 3 (42.9) | |
| Severe | 7 (30.4) | 3 (18.8) | 4 (57.1) | |
| Extremely severe | 1 (4.3) | 1 (6.3) | 0 (0.0) | |
| Medication | ||||
| LAMA | 1 (4.3) | 1 (6.3) | 0 (0.0) | 1.000 a |
| LABA | 3 (13.0) | 2 (12.5) | 1 (14.3) | 1.000 a |
| LAMA/LABA | 6 (26.1) | 3 (18.8) | 3 (42.9) | 0.318 a |
| ICS/LABA | 6 (26.1) | 3 (18.8) | 3 (42.9) | 0.318 a |
| ICS/LAMA/LABA | 6 (26.1) | 6 (37.5) | 0 (0.0) | 0.124 a |
| Theophylline | 4 (17.4) | 4 (25.0) | 0 (0.0) | 0.273 a |
| OCS | 4 (17.4) | 4 (25.0) | 0 (0.0) | 0.273 a |
| Number of AEs in the previous year | 1.61 ± 1.95 | 2.00 ± 2.03 | 0.71 ± 1.50 | 0.154 |
| ≥2 AEs in the previous year | 9 (39.1) | 8 (50.0) | 1 (14.3) | 0.176 a |
| Variable | Time | Study Group | |||
|---|---|---|---|---|---|
| IMT (n = 16) Mean ± SD | p Value | Control (n = 7) Mean ± SD | p Value | ||
| TLC (L) | T1 | 4.53 ± 1.01 | 6.48 ± 1.28 | ||
| T4 | 4.90 ± 0.84 | 0.152 | 6.38 ± 1.11 | 0.612 | |
| RV (L) | T1 | 2.15 ± 0.87 | 4.02 ± 1.28 | ||
| T4 | 2.18 ± 1.02 | 0.917 | 3.19 ± 1.69 | 0.553 | |
| RV/TLC% | T1 | 52.86 ± 11.06 | 61.00 ± 9.36 | ||
| T4 | 49.77 ± 16.06 | 0.421 | 57.71 ± 10.09 | 0.916 | |
| FRC (L) | T1 | 3.07 ± 1.06 | 4.66 ± 1.15 | ||
| T4 | 3.40 ± 0.85 | 0.064 | 4.60 ± 1.19 | 0.866 | |
| VC (L) | T1 | 2.09 ± 0.47 | 2.47 ± 0.55 | ||
| T4 | 2.42 ± 0.73 | 0.011 * | 2.63 ± 0.54 | 0.063 | |
| VT (L) | T1 | 0.73 ± 0.25 | 0.72 ± 0.17 | ||
| T4 | 0.74 ± 0.20 | 0.484 | 0.81 ± 0.29 | 0.233 | |
| ERV (L) | T1 | 0.56 ± 0.38 | 0.48 ± 0.28 | ||
| T4 | 0.90 ± 0.41 | 0.012 * | 0.62 ± 0.36 | 0.345 | |
| DLCO | T1 | 9.80 ± 4.48 | 9.06 ± 4.11 | ||
| (mL/mmHg/min) | T4 | 9.98 ± 4.63 | 0.248 | 8.93 ± 3.66 | 0.933 |
| DLCO/VA | T1 | 3.45 ± 1.16 | 2.42 ± 0.80 | ||
| (mL/mmHg/min/L) | T4 | 3.43 ± 1.20 | 1.000 | 2.24 ± 0.80 | 0.176 |
| VA (L) | T1 | 2.90 ± 0.83 | 3.67 ± 1.25 | ||
| T4 | 2.91 ± 0.70 | 0.133 | 4.02 ± 1.01 | 0.398 | |
| FVC (L) | T1 | 2.06 ± 0.50 | 2.32 ± 0.37 | ||
| T4 | 2.10 ± 0.49 | 0.506 | 2.58 ± 0.61 | 0.063 | |
| FEV1 (L) | T1 | 1.16 ± 0.39 | 1.29 ± 0.37 | ||
| T4 | 1.18 ± 0.36 | 0.442 | 1.26 ± 0.40 | 0.553 | |
| FEV1/FVC% | T1 | 55.71 ± 9.99 | 53.71 ± 11.70 | ||
| T4 | 56.62 ± 9.35 | 0.916 | 49.43 ± 12.86 | 0.061 | |
| FEF25–75% (L/s) | T1 | 0.57 ± 0.32 | 0.70 ± 0.33 | ||
| T4 | 0.55 ± 0.22 | 0.889 | 0.48 ± 0.22 | 0.018 * | |
| FEF50% (L/s) | T1 | 0.74 ± 0.48 | 0.87 ± 0.51 | ||
| T4 | 0.78 ± 0.45 | 0.552 | 0.65 ± 0.35 | 0.018 * | |
| PEF (L/s) | T1 | 3.62 ± 1.47 | 3.95 ± 0.41 | ||
| T4 | 3.60 ± 1.27 | 0.917 | 3.81 ± 0.94 | 0.499 | |
| mMRC (grade) | T1 | 1.50 ± 0.63 | 1.86 ± 0.90 | ||
| T4 | 0.88 ± 0.62 | 0.008 * | 1.86 ± 1.22 | 1.000 | |
| CAT (score) | T1 | 11.50 ± 6.16 | 11.29 ± 9.01 | ||
| T4 | 8.88 ± 6.54 | 0.064 | 12.57 ± 10.47 | 0.395 | |
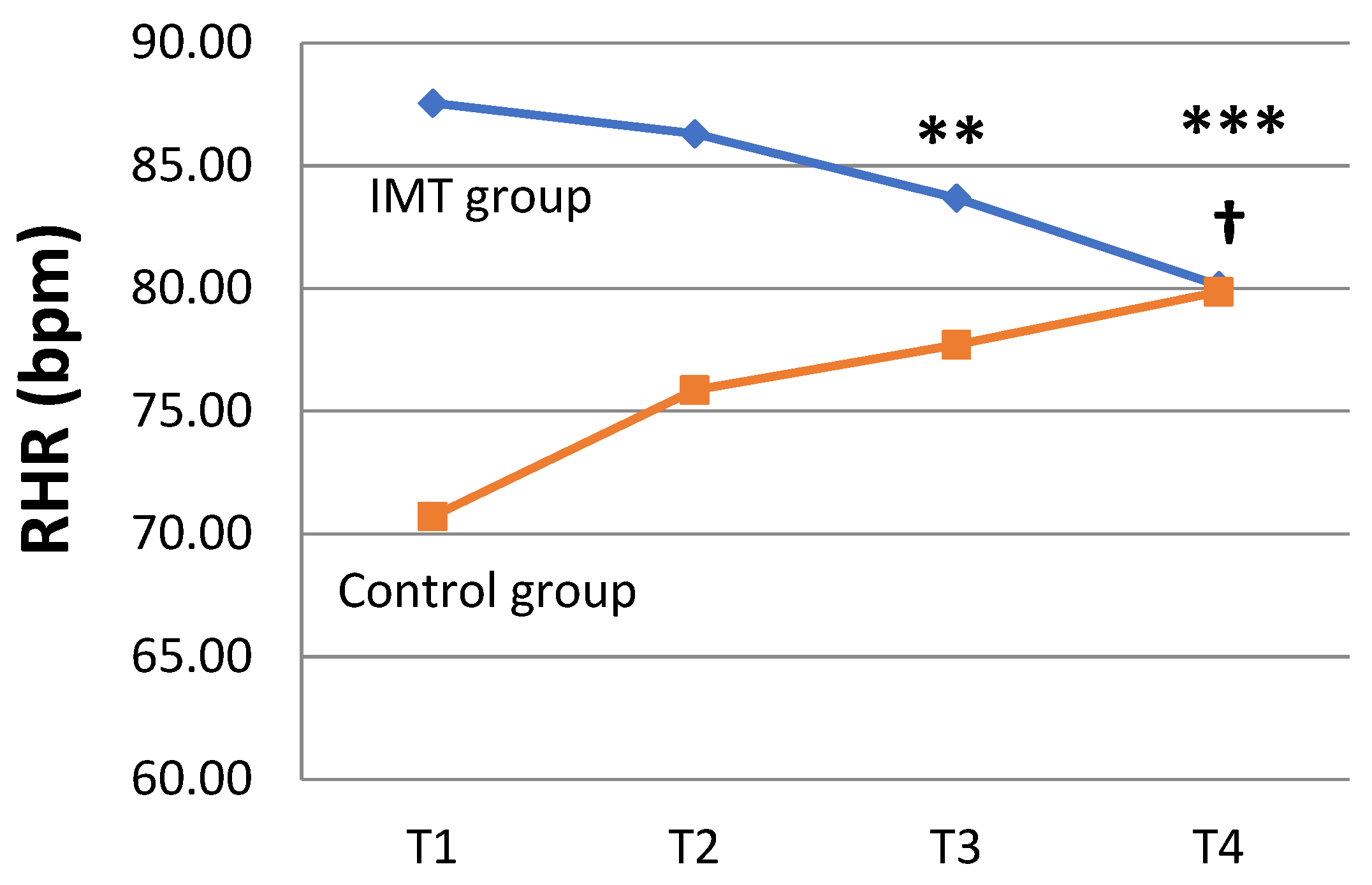
| HR (bpm) | T1 | 87.56 ± 13.04 | 70.71 ± 12.83 | ||
| T4 | 80.13 ± 10.23 | 0.011 * | 79.86 ± 10.42 | 0.028 * | |
| PImax (cmH2O) | T1 | 72.13 ± 26.75 | 88.57 ± 26.07 | ||
| T4 | 84.31 ± 23.72 | 0.011 * | 91.43 ± 27.46 | 1.000 | |
| 6MWD (m) | T1 | 424.44 ± 66.40 | 364.00 ± 130.90 | ||
| T4 | 442.38 ± 68.01 | 0.070 | 406.57 ± 82.43 | 0.204 | |
| Study Variable | Beta Coefficient (B) | 95% Wald Confidence Interval | p-Value | |||
|---|---|---|---|---|---|---|
| FEV1/FVC% | Intercept | 53.71 | 45.69 | to | 61.74 | 0.000 ǂ |
| Study group (IMT vs. control) | 2.00 | −7.48 | to | 11.48 | 0.679 | |
| Time point | ||||||
| T4 vs. T1 | −4.29 | −7.24 | to | −1.33 | 0.004 ƚ | |
| Study group × time point | ||||||
| IMT × (T4–T1) | 5.21 | 0.46 | to | 9.96 | 0.032 * | |
| FEF25–75% | Intercept | 0.70 | 0.47 | to | 0.92 | 0.000 ǂ |
| (L/s) | Study group (IMT vs. control) | −0.13 | −0.41 | to | 0.15 | 0.370 |
| Time point | ||||||
| T4 vs. T1 | −0.22 | −0.32 | to | −0.12 | 0.000 ǂ | |
| Study group × time point | ||||||
| IMT × (T4–T1) | 0.20 | 0.04 | to | 0.35 | 0.012 * | |
| FEF50% | Intercept | 0.87 | 0.52 | to | 1.22 | 0.000 ǂ |
| (L/s) | Study group (IMT vs. control) | −0.13 | −0.56 | to | 0.29 | 0.546 |
| Time point | ||||||
| T4 vs. T1 | −0.22 | −0.36 | to | −0.09 | 0.000 ǂ | |
| Study group × time point | ||||||
| IMT × (T4–T1) | 0.26 | 0.08 | to | 0.43 | 0.004 ƚ | |
| mMRC | Intercept | 1.86 | 1.24 | to | 2.47 | 0.000 ǂ |
| (grade) | Study group (IMT vs. control) | −0.36 | −1.04 | to | 0.33 | 0.308 |
| Time point | ||||||
| T2 vs. T1 | −0.29 | −1.23 | to | 0.66 | 0.554 | |
| T3 vs. T1 | −0.29 | −0.94 | to | 0.37 | 0.391 | |
| T4 vs. T1 | −3.37 × 10−17 | −0.56 | to | 0.56 | 1.000 | |
| Study group × time point | ||||||
| IMT × (T2–T1) | −0.03 | −1.02 | to | 0.96 | 0.958 | |
| IMT × (T3–T1) | −0.21 | −0.91 | to | 0.48 | 0.547 | |
| IMT × (T4–T1) | −0.63 | −1.28 | to | 0.03 | 0.062 | |
| CAT | Intercept | 11.29 | 5.10 | to | 17.47 | 0.000 ǂ |
| (score) | Study group (IMT vs. control) | 0.21 | -6.62 | to | 7.05 | 0.951 |
| Time point | ||||||
| T2 vs. T1 | 2.29 | -1.94 | to | 6.51 | 0.289 | |
| T3 vs. T1 | 3.00 | -1.37 | to | 7.37 | 0.179 | |
| T4 vs. T1 | 1.29 | -3.69 | to | 6.26 | 0.612 | |
| Study group × time point | ||||||
| IMT × (T2–T1) | −4.54 | −9.47 | to | 1.61 | 0.071 | |
| IMT × (T3–T1) | −5.50 | −10.31 | to | −0.695 | 0.025 * | |
| IMT × (T4–T1) | −3.91 | −9.42 | to | 1.61 | 0.165 | |
| RHR (bpm) | Intercept | 70.71 | 61.92 | to | 79.51 | 0.000 ǂ |
| Study group (IMT vs. control) | 16.85 | 6.09 | to | 27.60 | 0.002 ƚ | |
| Time point | ||||||
| T2 vs. T1 | 5.14 | −0.57 | to | 10.34 | 0.053 | |
| T3 vs. T1 | 7.00 | 2.37 | to | 11.64 | 0.003 ƚ | |
| T4 vs. T1 | 9.14 | 3.82 | to | 14.46 | 0.001 ƚ | |
| Study group × time point | ||||||
| IMT × (T2–T1) | −6.39 | −13.29 | to | 0.51 | 0.069 | |
| IMT × (T3–T1) | −10.88 | −18.22 | to | −3.53 | 0.004 ƚ | |
| IMT × (T4–T1) | −16.58 | −23.51 | to | −9.65 | 0.000 ǂ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.; Lin, H.-C.; Liu, H.-E.; Han, C.-Y.; Chang, P.-J. The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease. Healthcare 2023, 11, 2310. https://doi.org/10.3390/healthcare11162310
Chang W, Lin H-C, Liu H-E, Han C-Y, Chang P-J. The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease. Healthcare. 2023; 11(16):2310. https://doi.org/10.3390/healthcare11162310
Chicago/Turabian StyleChang, Wen, Horng-Chyuan Lin, Hsueh-Erh Liu, Chin-Yen Han, and Po-Jui Chang. 2023. "The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease" Healthcare 11, no. 16: 2310. https://doi.org/10.3390/healthcare11162310
APA StyleChang, W., Lin, H.-C., Liu, H.-E., Han, C.-Y., & Chang, P.-J. (2023). The Effectiveness of Home-Based Inspiratory Muscle Training on Small Airway Function and Disease-Associated Symptoms in Patients with Chronic Obstructive Pulmonary Disease. Healthcare, 11(16), 2310. https://doi.org/10.3390/healthcare11162310






