Purple Urine Bag Syndrome in a Home-Dwelling Elderly Female with Lumbar Compression Fracture: A Case Report
Abstract
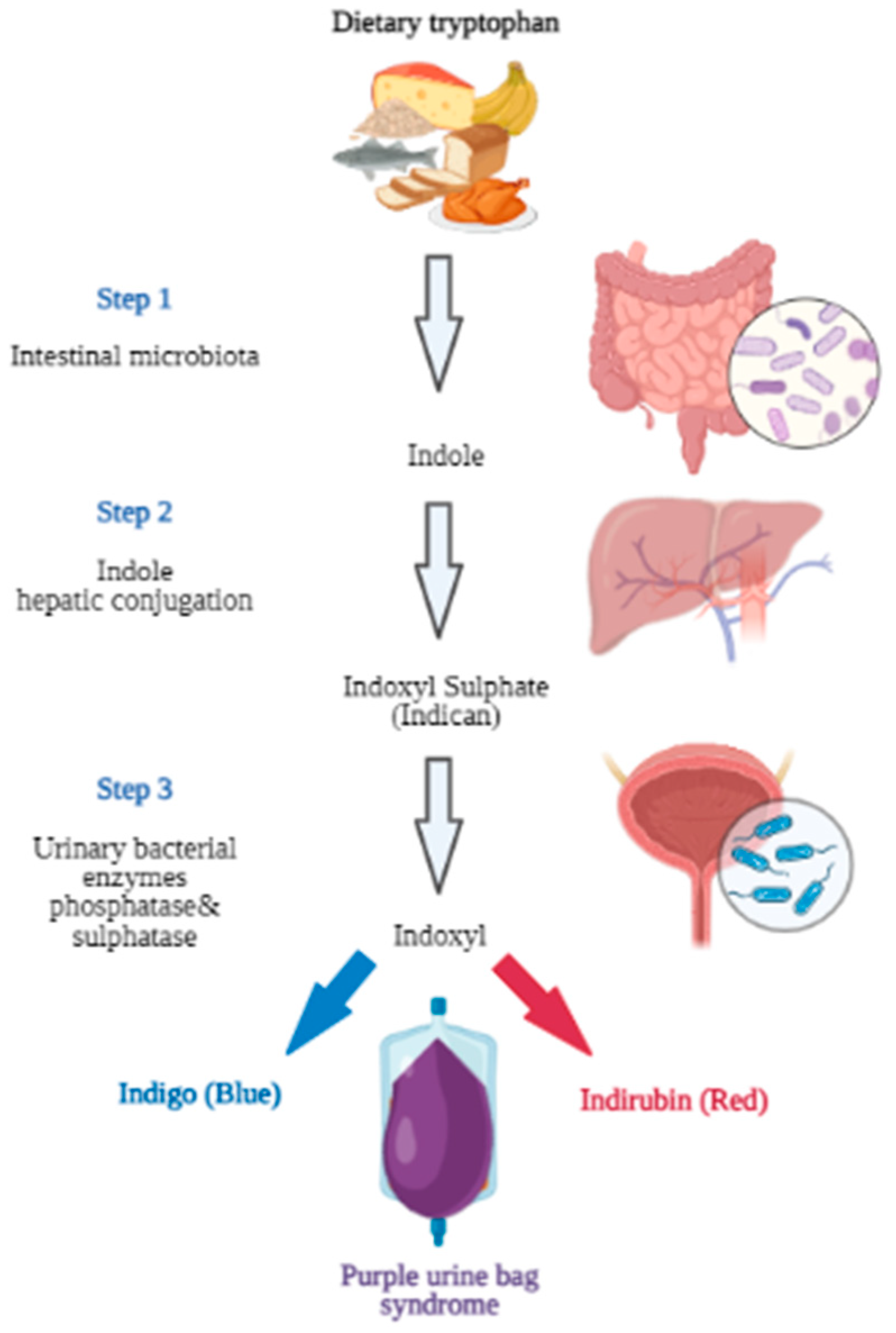
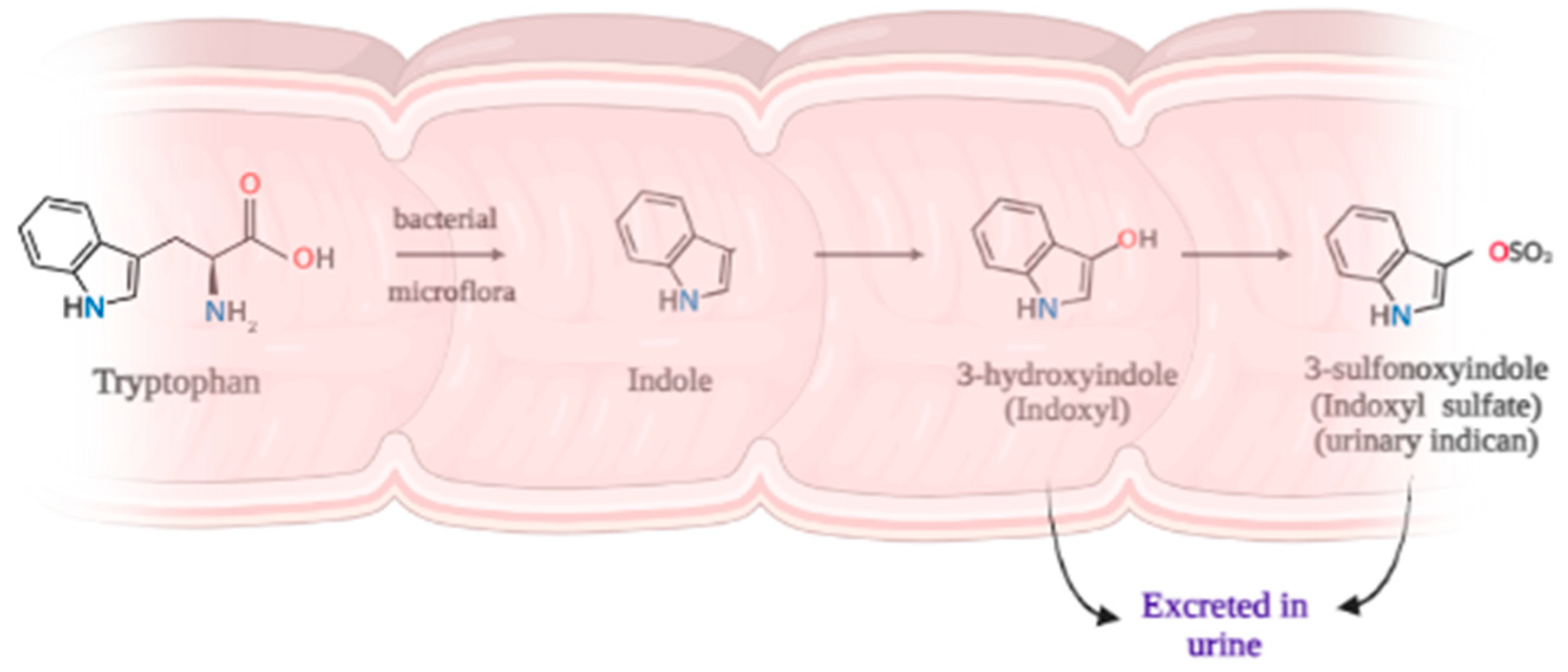
1. Introduction
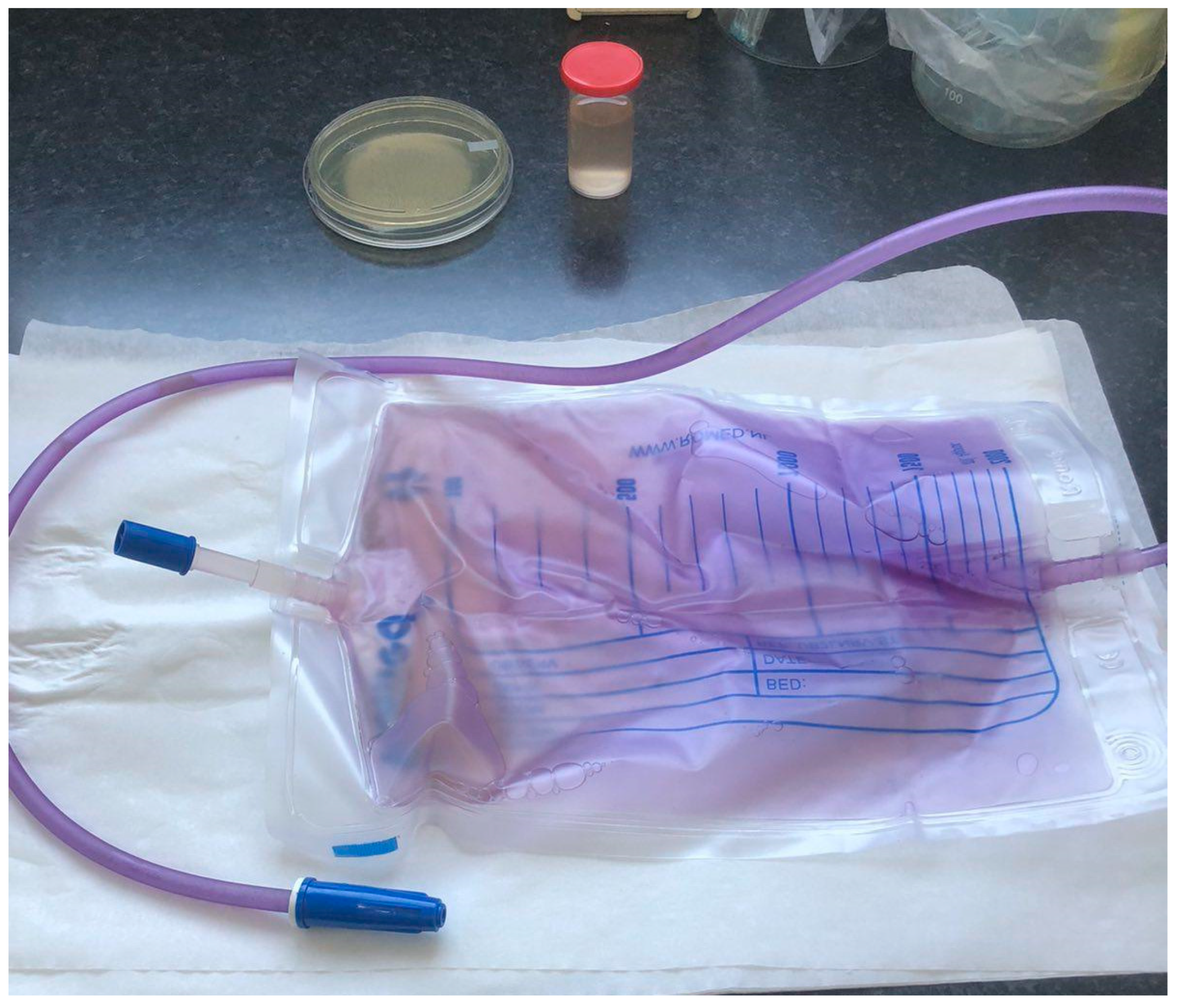
2. Case Presentation
Microbiological Examination
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallejo-Manzur, F.; Mireles-Cabodevila, E.; Varon, J. Purple urine bag syndrome. Am. J. Emerg. Med. 2005, 23, 521–524. [Google Scholar] [CrossRef]
- Kumar, R.; Devi, K.; Kataria, D.; Kumar, J.; Ahmad, I. Purple Urine Bag Syndrome: An Unusual Presentation of Urinary Tract Infection. Cureus 2021, 13, e16319. [Google Scholar] [CrossRef] [PubMed]
- DeRon, N., Jr.; Legan, C. Purple Urine Bag Syndrome: A Rare and Surprising Clinical Presentation. Cureus 2023, 15, e33354. [Google Scholar] [CrossRef] [PubMed]
- Sabanis, N.; Paschou, E.; Papanikolaou, P.; Zagkotsis, G. Purple Urine Bag Syndrome: More Than Eyes Can See. Curr. Urol. 2019, 13, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Su, Y.J. Trends in the epidemiology of purple urine bag syndrome: A systematic review. Biomed. Rep. 2018, 8, 249–256. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; The CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 13.0; EUCAST: Växjö, Sweden, 2023. [Google Scholar]
- Barlow, G.B.; Dickson, J.A.S. Purple urine bags. Lancet 1978, 311, 220–221. [Google Scholar] [CrossRef]
- Campagne, J.; Kurt, S.; Moulinet, T.; Mohamed, S.; Deibener-Kaminsky, J.; Jaussaud, R. Une couleur inhabituelle. Rev. Med. Interne 2021, 42, 61–62. [Google Scholar] [CrossRef]
- Worku, D.A. Purple urine bag syndrome: An unusual but important manifestation of urinary tract infection. Case report and literature review. SAGE Open Med. Case Rep. 2019, 7, 2050313X18823105. [Google Scholar] [CrossRef]
- Boo, W.H. An elderly man with purple urine. Aust. J. Gen. Pract. 2021, 50, 381–382. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Young, K.D. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 2013, 159, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Banoglu, E.; Jha, G.G.; King, R.S. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 235–240. [Google Scholar] [CrossRef]
- Michael, A.F.; Drummond, K.N.; Doeden, D.; Erson, J.A.; Good, R.A. Tryptophan metabolism in man. J. Clin. Investig. 1964, 43, 1730–1746. [Google Scholar] [CrossRef] [PubMed]
- Huć, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Alan, S.L.; Yu, M.B.; Chir, B. The Pathophysiology of Uremia. In Brenner and Rector’s The Kidney; Meyer, T.W., Hostetter, T.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 52, pp. 1790–1804.e6. [Google Scholar]
- Abe, M.; Furuichi, M.; Ishimitsu, T.; Tojo, A. Analysis of purple urine bag syndrome by low vacuum scanning electron microscopy. Med. Mol. Morphol. 2022, 55, 123–130. [Google Scholar] [CrossRef]
- Chong, V.H. Misconception about purple urine bag syndrome. QJM Int. J. Med. 2020, 113, 445. [Google Scholar] [CrossRef]
- Popoola, M.; Hillier, M. Purple Urine Bag Syndrome as the Primary Presenting Feature of a Urinary Tract Infection. Cureus 2022, 14, e23970. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8, 10-1128. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and Urinary Tract Infections. Microbiol. Spectr. 2015, 3, 383–433. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Omid, M.; Arzanlou, M.; Amani, M.; Shokri Al-Hashem, S.K.; Amir Mozafari, N.; Peeri Doghaheh, H. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol. Lett. 2015, 362, fnv049. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Huang, Z.; Yang, T.; Wang, G.; Li, P.; Yang, P.; Li, P.; Yang, B.; Li, J. Pathogenesis of Proteus mirabilis in Catheter-Associated Urinary Tract Infections. Urol. Int. 2021, 105, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hung, E.W.; Darouiche, R.O.; Trautner, B.W. Proteus bacteriuria is associated with significant morbidity in spinal cord injury. Spinal Cord. 2007, 45, 616–620. [Google Scholar] [CrossRef][Green Version]
- Stover, S.L.; Lloyd, L.K.; Waites, K.B.; Jackson, A.B. Urinary tract infection in spinal cord injury. Arch. Phys. Med. Rehabil. 1989, 70, 47–54. [Google Scholar] [CrossRef]
- DeVivo, M.J.; Fine, P.R.; Cutter, G.R.; Maetz, H.M. The risk of renal calculi in spinal cord injury patients. J. Urol. 1984, 131, 857–860. [Google Scholar] [CrossRef]
- Tul Llah, S.; Khan, S.; Dave, A.; Morrison, A.J.; Jain, S.; Hermanns, D. A Case of Purple Urine Bag Syndrome in a Spastic Partial Quadriplegic Male. Cureus 2016, 8, e552. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, A.; Thami, G.; Agrawal, N. Purple urine bag syndrome: A simple and rare spot diagnosis in Uroscopic rainbow. Urol. Case Rep. 2020, 35, 101533. [Google Scholar] [CrossRef]
- Llenas-García, J.; García-López, M.; Pérez-Bernabeu, A.; Cepeda, J.M.; Wikman-Jorgensen, P. Purple urine bag syndrome: A systematic review with meta-analysis. Eur. Geriatr. Med. 2017, 8, 221–227. [Google Scholar] [CrossRef]
- Fusco, A.; Coretti, L.; Savio, V.; Buommino, E.; Lembo, F.; Donnarumma, G. Biofilm Formation and Immunomodulatory Activity of Proteus mirabilis Clinically Isolated Strains. Int. J. Mol. Sci. 2017, 18, 414. [Google Scholar] [CrossRef]
- Rooney, H.; Mokool, L.; Ramsay, A.; Nalagatla, S. Purple urine bag syndrome: A truly harmless sign? Scott. Med. J. 2018, 63, 99–101. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Waheed, M.A.; Shah, S.; Muhammad Shah, S.Y.; Mumtaz, H. Purple urine bag syndrome: A case report. Int. J. Surg. Case Rep. 2022, 99, 107721. [Google Scholar] [CrossRef]
- Amer, M.A.; Ramadan, M.A.; Attia, A.S.; Wasfi, R. Silicone Foley catheters impregnated with microbial indole derivatives inhibit crystalline biofilm formation by Proteus mirabilis. Front. Cell. Infect. Microbiol. 2022, 12, 1010625. [Google Scholar] [CrossRef]
- Kanti, S.P.Y.; Csóka, I.; Jójárt-Laczkovich, O.; Adalbert, L. Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines 2022, 10, 2580. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Mukai, T. Purple urine bags reflecting an aging society. Clin. Case Rep. 2021, 9, e04295. [Google Scholar] [CrossRef] [PubMed]
- Alex, R.; Manjunath, K.; Srinivasan, R.; Basu, G. Purple urine bag syndrome: Time for awareness. J. Fam. Med. Prim. Care 2015, 4, 130–131. [Google Scholar] [CrossRef]

| Year | Number of Microbiological Urine Analyses 1 | Number of P. mirabilis Detected | Incidence % |
|---|---|---|---|
| 2023 | 21,558 | 410 | 1.90 |
| 2022 | 41,544 | 830 | 2.00 |
| 2021 | 34,730 | 658 | 1.89 |
| 2020 | 27,049 | 490 | 1.81 |
| Total | 124,881 | 2388 | 1.91 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, M.B.; Medić, D.D.; Velicki, R.S.; Jovanović Galović, A.I. Purple Urine Bag Syndrome in a Home-Dwelling Elderly Female with Lumbar Compression Fracture: A Case Report. Healthcare 2023, 11, 2251. https://doi.org/10.3390/healthcare11162251
Popović MB, Medić DD, Velicki RS, Jovanović Galović AI. Purple Urine Bag Syndrome in a Home-Dwelling Elderly Female with Lumbar Compression Fracture: A Case Report. Healthcare. 2023; 11(16):2251. https://doi.org/10.3390/healthcare11162251
Chicago/Turabian StylePopović, Milka B., Deana D. Medić, Radmila S. Velicki, and Aleksandra I. Jovanović Galović. 2023. "Purple Urine Bag Syndrome in a Home-Dwelling Elderly Female with Lumbar Compression Fracture: A Case Report" Healthcare 11, no. 16: 2251. https://doi.org/10.3390/healthcare11162251
APA StylePopović, M. B., Medić, D. D., Velicki, R. S., & Jovanović Galović, A. I. (2023). Purple Urine Bag Syndrome in a Home-Dwelling Elderly Female with Lumbar Compression Fracture: A Case Report. Healthcare, 11(16), 2251. https://doi.org/10.3390/healthcare11162251