Effect of Antithrombin III Administration on the Prognosis of Severe Trauma Patients with Disseminated Intravascular Coagulation
Abstract
1. Introduction
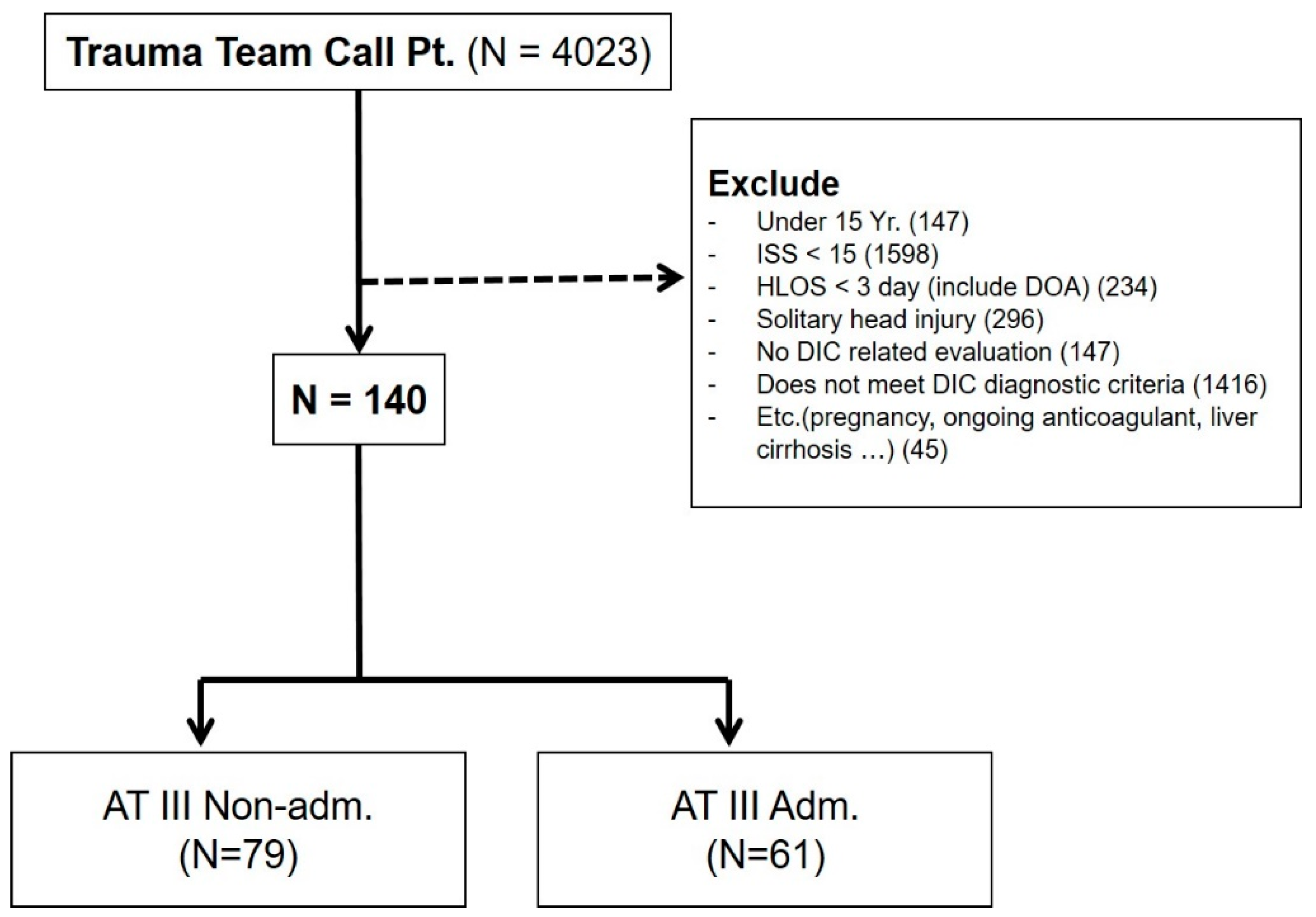
2. Methods
2.1. Patient Enrollment and Data Collection
2.2. Clinical Variables
2.3. Statistical Analysis
3. Results
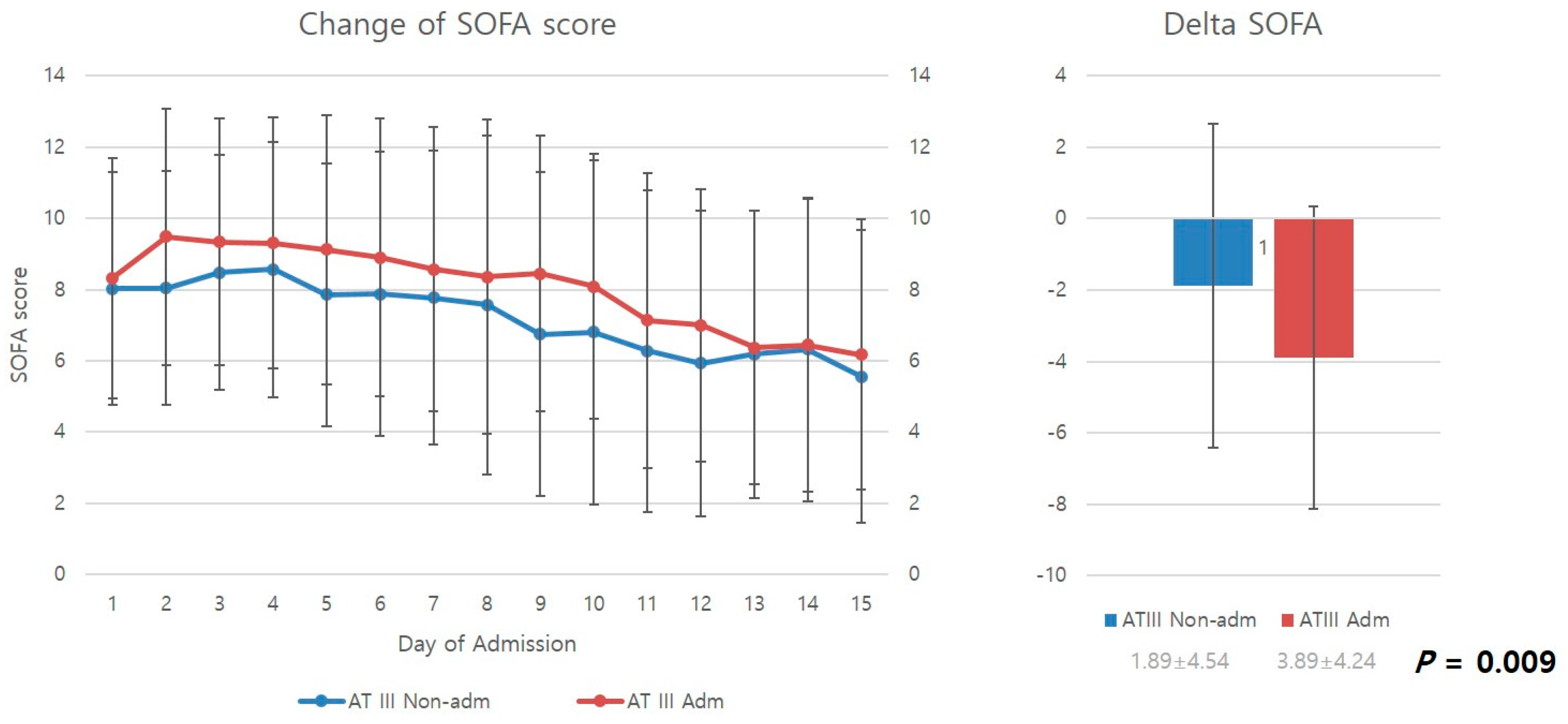
3.1. Comparison of Change in SOFA Score between the Two Groups
3.2. Comparison of Transfusion Requirements between the Two Groups
3.3. Comparison of Organ Support Requirements between the Two Groups
3.4. Comparison of Clinical Outcomes between the Two Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Cohen, M.J.; Davenport, R.A. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr. Opin. Crit. Care 2007, 13, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Nanzaki, S.; Kemmotsu, O. Disseminated intravascular coagulation and sustained systemic inflammatory response syndrome predict organ dysfunctions after trauma: Application of clinical decision analysis. Ann. Surg. 1999, 229, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R.; Lawson, J.H. The coagulopathy of trauma versus disseminated intravascular coagulation. J. Trauma 2006, 60 (Suppl. S6), S12–S19. [Google Scholar] [CrossRef]
- Gando, S.; Sawamura, A.; Hayakawa, M. Trauma, shock, and disseminated intravascular coagulation: Lessons from the classical literature. Ann. Surg. 2011, 254, 10–19. [Google Scholar] [CrossRef]
- Gando, S.; Wada, H.; Thachil, J. Scientific, thrombosis SCoDotISo, haemostasis. Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS). J. Thromb. Haemost. 2013, 11, 826–835. [Google Scholar] [CrossRef]
- Oshiro, A.; Yanagida, Y.; Gando, S.; Henzan, N.; Takahashi, I.; Makise, H. Hemostasis during the early stages of trauma: Comparison with disseminated intravascular coagulation. Crit. Care 2014, 18, R61. [Google Scholar] [CrossRef]
- Spahn, D.R.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Gordini, G.; Stahel, P.F.; Hunt, B.J.; Komadina, R.; Neugebauer, E.; et al. Management of bleeding following major trauma: A European guideline. Crit. Care 2007, 11, R17. [Google Scholar] [CrossRef]
- Fröhlich, M.; Lefering, R.; Probst, C.; Paffrath, T.; Schneider, M.M.; Maegele, M.; Sakka, S.G.; Bouillon, B.; Wafaisade, A. Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. J. Trauma Acute Care Surg. 2014, 76, 921–927, discussion 927. [Google Scholar] [CrossRef]
- Nast-Kolb, D.; Aufmkolk, M.; Rucholtz, S.; Obertacke, U.; Waydhas, C. Multiple organ failure still a major cause of morbidity but not mortality in blunt multiple trauma. J. Trauma Acute Care Surg. 2001, 51, 835–842. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- de Grooth, H.J.; Geenen, I.L.; Girbes, A.R.; Vincent, J.L.; Parienti, J.J.; Oudemans-van Straaten, H.M. SOFA and mortality endpoints in randomized controlled trials: A systematic review and meta-regression analysis. Crit. Care 2017, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Arakawa, M.; Mochizuki, K.; Nishida, O.; Wada, H.; Levy, J.H. Usefulness of measuring changes in SOFA score for the prediction of 28-day mortality in patients with sepsis-associated disseminated intravascular coagulation. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029618824044. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Yamakawa, K.; Ogura, H.; Yuhara, H.; Fujimi, S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: A meta-analysis of randomized controlled trials. J. Thromb. Haemost. 2016, 14, 518–530. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Kaneider, N.C. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul. Fibrinolysis 2006, 17, 521–526. [Google Scholar] [CrossRef]
- Hayakawa, M.; Kudo, D.; Saito, S.; Uchino, S.; Yamakawa, K.; Iizuka, Y.; Sanui, M.; Takimoto, K.; Mayumi, T.; Ono, K.; et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: A multicenter retrospective observational study. Shock 2016, 46, 623–631. [Google Scholar] [CrossRef]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Gando, S.; Otomo, Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit. Care 2015, 19, 72. [Google Scholar] [CrossRef]
- Grottke, O.; Honickel, M.; Braunschweig, T.; Reichel, A.; Schöchl, H.; Rossaint, R. Prothrombin complex concentrate-induced disseminated intravascular coagulation can be prevented by coadministering antithrombin in a porcine trauma model. Anesthesiology 2019, 131, 543–554. [Google Scholar] [CrossRef]
- Waydhas, C.; Nast-Kolb, D.; Gippner-Steppert, C.; Trupka, A.; Pfundstein, C.; Schweiberer, L.; Jochum, M. High-dose antithrombin III treatment of severely injured patients: Results of a prospective study. J. Trauma 1998, 45, 931–940. [Google Scholar] [CrossRef]
- Koami, H.; Sakamoto, Y.; Yamada, K.C.; Matsuda, T.; Nishi, J.; Nakayama, K.; Sakurai, R.; Ohta, M.; Imahase, H.; Yahata, M.; et al. What factor within the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) criteria is most strongly correlated with trauma induced DIC? A retrospective study using thromboelastometry in a single center in Japan. Eur. J. Trauma Emerg. Surg. 2017, 43, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, A.; Hayakawa, M.; Gando, S.; Kubota, N.; Sugano, M.; Wada, T.; Katabami, K.-I. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb. Res. 2009, 124, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Hoffmann, J.N.; Juers, M.; Ostermann, H.; Kienast, J.; Briegel, J.; Strauss, R.; Keinecke, H.-O.; Warren, B.L.; Opal, S.M. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: Efficacy and safety. Crit. Care Med. 2006, 34, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.O.; Park, S.H.; Hong, S.B.; Jang, S. Performance evaluation of five different disseminated intravascular coagulation (DIC) diagnostic criteria for predicting mortality in patients with complicated sepsis. J. Korean Med. Sci. 2016, 31, 1838–1845. [Google Scholar] [CrossRef]
- Gando, S. Disseminated intravascular coagulation in trauma patients. Semin. Thromb. Hemost. 2001, 27, 585–592. [Google Scholar] [CrossRef]

| Score | KSTH | |
|---|---|---|
| Platelets, ×103/L | 0 | >100 |
| 1 | ≤100 | |
| PT, sec | 0 | <3 |
| 1 | ≥3 | |
| aPTT, sec | 0 | <5 |
| 1 | ≥5 | |
| Fibrin-related marker, μg/mL | 0 | No increase |
| 1 | Increase | |
| Fibrinogen, g/L | 0 | >1.5 |
| 1 | ≤1.5 | |
| Total | DIC ≥ 3 |
| AT III Non-Adm. (N = 79) | AT III Adm. (N = 61) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 56.41 ± 16.29 | 58.21 ± 16.48 | 0.518 |
| Gender, N (%) | 0.168 | ||
| Male | 51 (64.6) | 46 (75.4) | |
| Female | 28 (35.4) | 15 (24.6) | |
| BMI, mean ± SD | 24.15 ± 4.86 | 23.36 ± 3.07 | 0.267 |
| Underlying Dz (%) | |||
| HTN | 19 (24.1) | 14 (23) | 0.879 |
| DM | 11 (13.9) | 8 (13.1) | 0.89 |
| CRF | 1(1.3) | 0 (0) | 0.378 |
| CAOD | 0 (0) | 1 (1.6) | 0.253 |
| Cancer | 8 (10.1) | 2 (3.3) | 0.119 |
| AIS, mean ± SD | |||
| Head and neck | 1.72 ± 1.87 | 2.08 ± 2.01 | 0.275 |
| Face | 0.44 ± 0.78 | 0.51 ± 0.91 | 0.649 |
| Chest | 1.87 ± 1.62 | 2.18 ± 1.71 | 0.28 |
| Abdomen | 2.04 ± 1.58 | 1.56 ± 1.56 | 0.075 |
| Extremities | 2.08 ± 1.74 | 1.98 ± 1.69 | 0.753 |
| External | 0.06 ± 0.56 | 0.03 ± 0.18 | 0.684 |
| ISS, mean ± SD | 27.38 ± 12.04 | 28.15 ± 9.33 | 0.681 |
| RTS, mean ± SD | 5.715 ± 1.796 | 6.203 ± 1.806 | 0.115 |
| Intervention (%) | 8 (10.1) | 14 (23) | 0.039 |
| Operation (%) | 70 (88.6) | 53 (86.9) | 0.757 |
| AT III Non-Adm. (N = 79) | AT III Adm. (N = 61) | p-Value | |
|---|---|---|---|
| V/S_worst (within 2 h), mean ± SD | |||
| SBP_worst (mmHg) | 70.9 ± 17.4 | 75.1 ± 23.9 | 0.235 |
| DBP_worst (mmHg) | 38.9 ± 10.9 | 39.6 ± 12.5 | 0.74 |
| PR_worst | 154.0 ± 148.9 | 150.26 ± 133.1 | 0.877 |
| RR_worst | 29.3 ± 11.6 | 38.3 ± 28.8 | 0.012 |
| GCS_worst, mean ± SD | 5.7 ± 4.2 | 5.9 ± 4.1 | 0.729 |
| Lab_initial, mean ± SD | |||
| WBC (109/L) | 14.9 ± 6.8 | 13.8 ± 7.3 | 0.341 |
| Hb (g/dL) | 10.4 ± 2.2 | 10.8 ± 2.5 | 0.301 |
| Plt (109/L) | 171.7 ± 66.9 | 175.3 ± 87.5 | 0.785 |
| Cr (mg/dL) | 1.1 ± 0.8 | 1.0 ± 0.4 | 0.158 |
| T.bil (g/dL) | 0.7 ± 0.7 | 0.7 ± 0.6 | 0.981 |
| aPTT (sec) | 37.3 ± 13.9 | 47.3 ± 67.1 | 0.194 |
| PT(INR) | 1.4 ± 0.3 | 1.4 ± 0.5 | 0.999 |
| pH | 7.283 ± 0.147 | 7.316 ± 0.138 | 0.188 |
| pO2 | 151.6 ± 58.9 | 154.7 ± 65.3 | 0.773 |
| pCO2 | 33.3 ± 10.3 | 31.4 ± 7.1 | 0.212 |
| O2 sat. (%) | 95.3 ± 10.7 | 95.5 ± 12.8 | 0.895 |
| BE (mmol/L) | −10.2 ± 6.0 | −9.0 ± 6.4 | 0.262 |
| Lactate (mmol/L) | 5.6 ± 3.5 | 5.6 ± 3.6 | 0.99 |
| FDP (μg/mL) | 229.3 ± 184.9 | 170.2 ± 137.8 | 0.039 |
| Fibrinogen (mg/dL) | 151.6 ± 103.7 | 168.9 ± 98.3 | 0.32 |
| D-Dimer (ng/mL) | 19,667.1 ± 14,491.7 | 19,708.9 ± 17,012.9 | 0.987 |
| AT III (%) | 63.6 ± 17.7 | 58.5 ± 20.5 | 0.119 |
| AT III Non-Adm. (N = 79) | AT III Adm. (N = 61) | p-Value | |
|---|---|---|---|
| RBC day 1–3, mean ± SD | 1.9 ± 2.0 | 3.0 ± 2.6 | 0.004 |
| RBC day 3–7, mean ± SD | 1.1 ± 2.0 | 1.9 ± 2.2 | 0.025 |
| RBC day 7–14, mean ± SD | 0.9 ± 2.8 | 1.18 ± 2.5 | 0.469 |
| RBC day 14–28, mean ± SD | 0.6 ± 2.2 | 1.2 ± 3.7 | 0.239 |
| FFP day 1–3, mean ± SD | 1.3 ± 1.5 | 1.9 ± 2.1 | 0.053 |
| FFP day 3–7, mean ± SD | 0.6 ± 1.3 | 1.0 ± 1.5 | 0.058 |
| FFP day 7–14, mean ± SD | 0.3 ± 1.8 | 0.6 ± 1.7 | 0.480 |
| FFP day 14–28, mean ± SD | 0.3 ± 1.8 | 0.5 ± 2.7 | 0.519 |
| Plt day 1–3, mean ± SD | 0.9 ± 1.1 | 1.2 ± 1.2 | 0.133 |
| Plt day 3–7, mean ± SD | 0.6 ± 0.9 | 1 ± 1.9 | 0.071 |
| Plt day 7–14, mean ± SD | 0.1 ± 0.3 | 0.4 ± 1.3 | 0.025 |
| Plt day 14–28, mean ± SD | 0.1 ± 0.4 | 0.2 ± 0.7 | 0.339 |
| AT III Non-Adm. (N = 79) | AT III Adm. (N = 61) | p-Value | |
|---|---|---|---|
| Incidence of mechanical ventilation (%) | 66 (88) | 56 (93.3) | 0.297 |
| Duration of mechanical ventilation (day), mean ± SD | 17.5 ± 18.9 | 16.3 ± 12.3 | 0.694 |
| Incidence of renal replacement therapy (%) | 14 (18.7) | 15 (24.6) | 0.402 |
| Duration of renal replacement therapy (day), mean ± SD | 20.5 ± 24.7 | 23.8 ± 16.4 | 0.673 |
| Duration of using iv vasopressor (day), mean ± SD | 5.0 ± 12.8 | 5.2 ± 5.8 | 0.912 |
| AT III Non-Adm. (N = 79) | AT III Adm. (N = 61) | p-Value | |
|---|---|---|---|
| ICU LOS (day), mean ± SD | 23.0 ± 33.6 | 21.4 ± 17.9 | 0.749 |
| H-LOS (day), mean ± SD | 61.8 ± 52.1 | 73.1 ± 71.4 | 0.29 |
| 28-day mortality | 11 (14.7) | 8 (13.1) | 0.795 |
| Overall mortality | 14 (18.7) | 15 (24.6) | 0.402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.S.; Kim, M.J.; Choi, Y.U.; Kim, J.G.; Bae, K.S. Effect of Antithrombin III Administration on the Prognosis of Severe Trauma Patients with Disseminated Intravascular Coagulation. Healthcare 2023, 11, 1476. https://doi.org/10.3390/healthcare11101476
Chung JS, Kim MJ, Choi YU, Kim JG, Bae KS. Effect of Antithrombin III Administration on the Prognosis of Severe Trauma Patients with Disseminated Intravascular Coagulation. Healthcare. 2023; 11(10):1476. https://doi.org/10.3390/healthcare11101476
Chicago/Turabian StyleChung, Jae Sik, Myoung Jun Kim, Young Un Choi, Jun Gi Kim, and Keum Seok Bae. 2023. "Effect of Antithrombin III Administration on the Prognosis of Severe Trauma Patients with Disseminated Intravascular Coagulation" Healthcare 11, no. 10: 1476. https://doi.org/10.3390/healthcare11101476
APA StyleChung, J. S., Kim, M. J., Choi, Y. U., Kim, J. G., & Bae, K. S. (2023). Effect of Antithrombin III Administration on the Prognosis of Severe Trauma Patients with Disseminated Intravascular Coagulation. Healthcare, 11(10), 1476. https://doi.org/10.3390/healthcare11101476





