Influence of Transcranial Direct Current Stimulation and Exercise on Physical Capacity and Gait in Multiple Sclerosis: A Cross-Over Pilot Study
Abstract
1. Introduction
2. Material and Methods
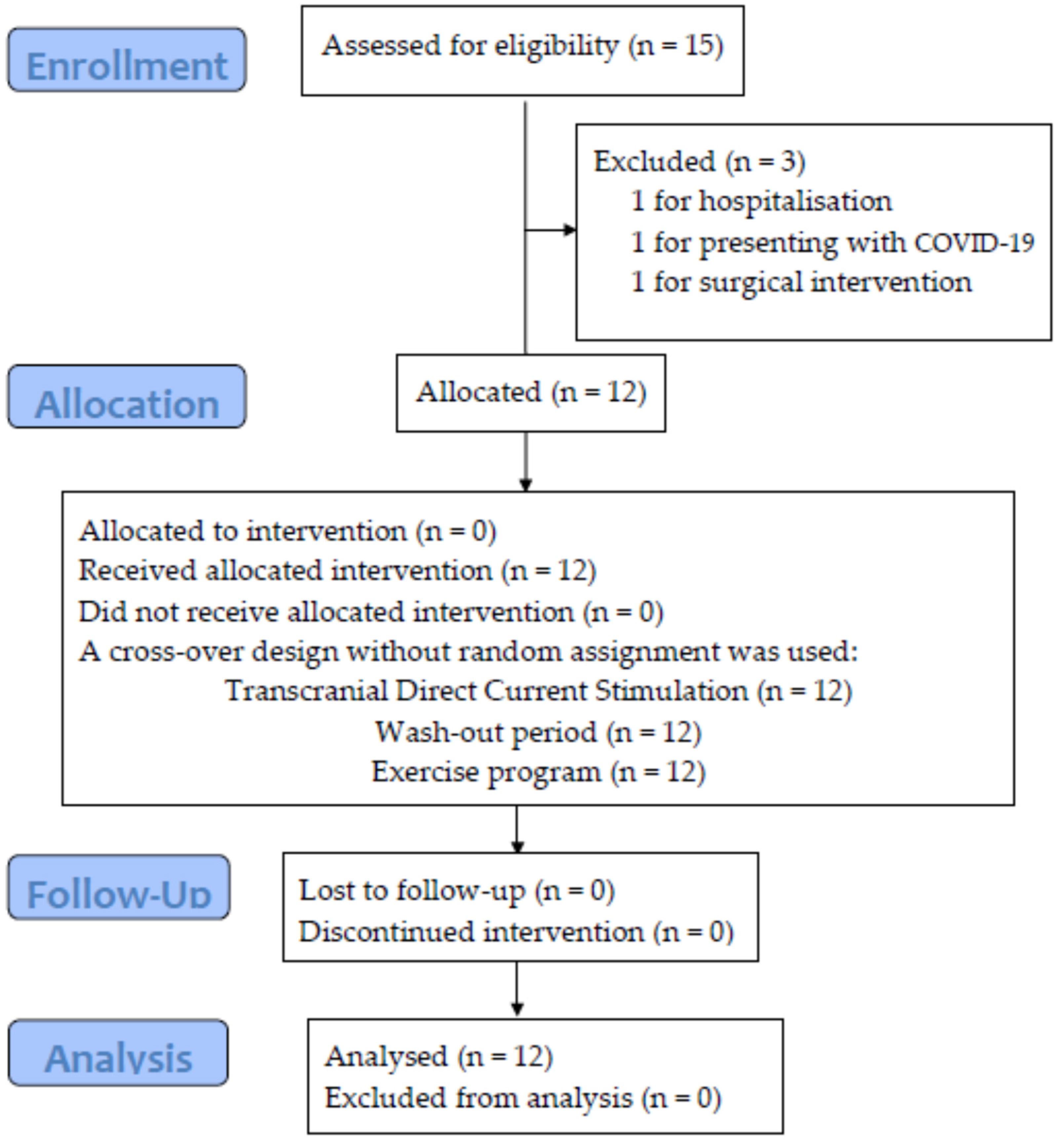
2.1. Design and Participants
2.2. Interventions
2.3. Outcome Measures
2.4. Statistical Analysis
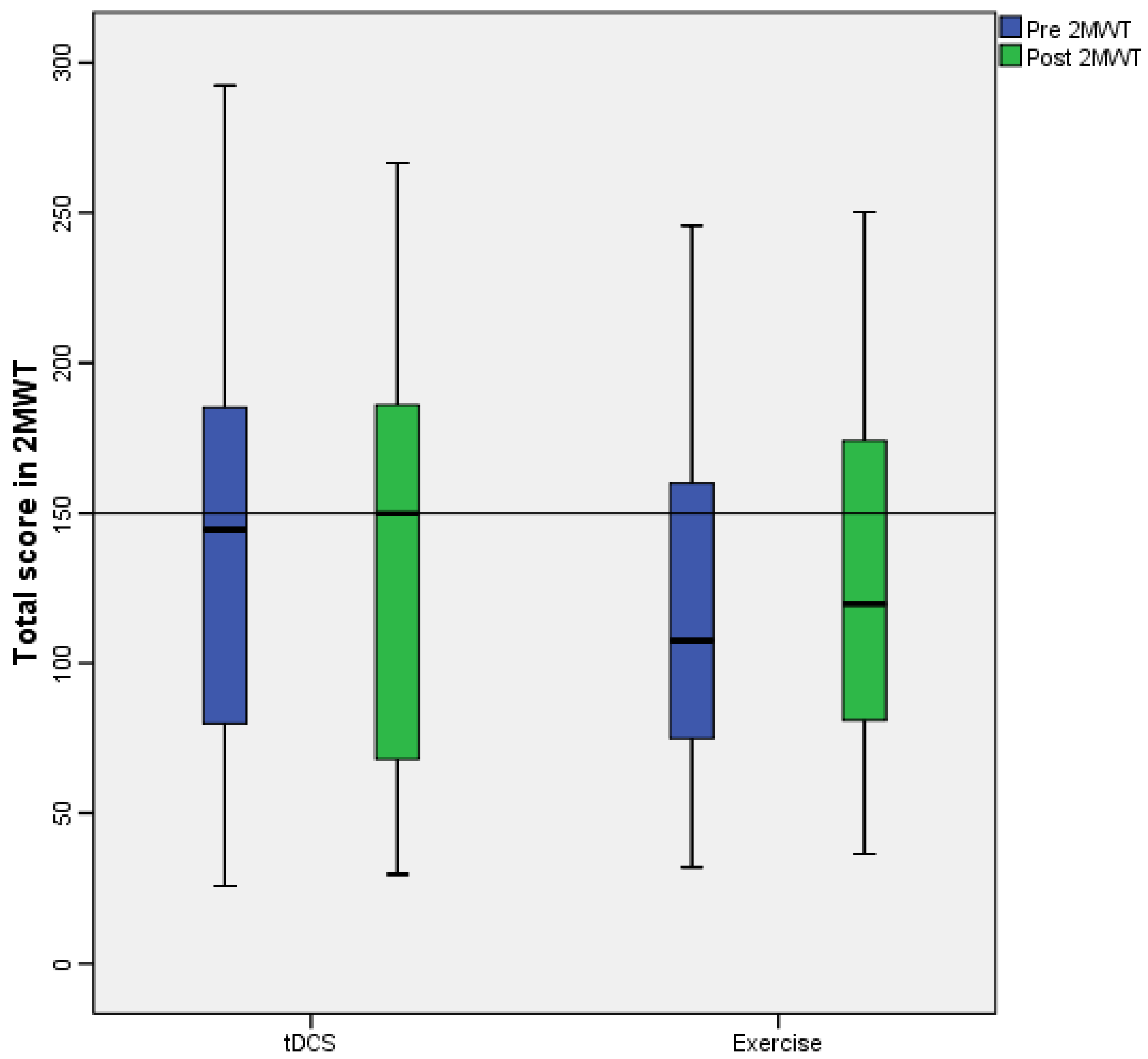
3. Results
4. Discussion
4.1. Practical Application
4.2. Future Line of Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple sclerosis |
| tDCS | Transcranial direct current stimulation |
| M1 | Primary motor cortex |
| DLPFC | Left dorsolateral prefrontal cortex |
| MFIS | Modified fatigue impact scale |
| 6MWT | Six-minute walk test |
| 2MWT | Two-minute walk test |
| IPAQ-SF | International physical activity questionnaire short form |
| EDSS | Expanded disability status scale |
| MID | Minimally important difference |
References
- World Health Organization. Towards a Common Language for Functioning, Disability and Health: ICF. Int. Classif. 2002, 1149, 1–22. [Google Scholar]
- Kerling, A.; Keweloh, K.; Tegtbur, U.; Kück, M.; Grams, L.; Horstmann, H.; Windhagen, A. Physical Capacity and Quality of Life in Patients with Multiple Sclerosis. NeuroRehabilitation 2014, 35, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Gijbels, D.; Romberg, A.; Santoyo, C.; Gebara, B.; De Noordhout, B.M.; Knuts, K.; Béthoux, F.; De Groot, V.; Vaney, C.; et al. Effect of Time of Day on Walking Capacity and Self-Reported Fatigue in Persons with Multiple Sclerosis: A Multi-Center Trial. Mult. Scler. J. 2012, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.; Freeman, J.; Lo, A. Treatment of Progressive Multiple Sclerosis: What Works, What Does Not, and What Is Needed. Lancet Neurol. 2015, 14, 194–207. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, I. Multiple Sclerosis and Physical Exercise: Recommendations for the Application of Resistance-, Endurance- and Combined Training. Mult. Scler. 2008, 14, 35–53. [Google Scholar] [CrossRef]
- Pugliatti, M.; Rosati, G.; Carton, H.; Riise, T.; Drulovic, J.; Vécsei, L.; Milanov, I. The Epidemiology of Multiple Sclerosis in Europe. Eur. J. Neurol. 2006, 13, 700–722. [Google Scholar] [CrossRef]
- Heesen, C.; Böhm, J.; Reich, C.; Kasper, J.; Goebel, M.; Gold, S.M. Patient Perception of Bodily Functions in Multiple Sclerosis: Gait and Visual Function Are the Most Valuable. Mult. Scler. 2008, 14, 988–991. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E. Exercise and Disease Progression in Multiple Sclerosis: Can Exercise Slow down the Progression of Multiple Sclerosis? Ther. Adv. Neurol. Disord. 2012, 5, 81–95. [Google Scholar] [CrossRef]
- Tallner, A.; Waschbisch, A.; Wenny, I.; Schwab, S.; Hentschke, C.; Pfeifer, K.; Mäurer, M. Multiple Sclerosis Relapses Are Not Associated with Exercise. Mult. Scler. J. 2012, 18, 232–235. [Google Scholar] [CrossRef]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a Therapy for Improvement of Walking Ability in Adults with Multiple Sclerosis: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348.e7. [Google Scholar] [CrossRef]
- Motl, R.W.; Arnett, P.A.; Smith, M.M.; Barwick, F.H.; Ahlstrom, B.; Stover, E.J. Worsening of Symptoms Is Associated with Lower Physical Activity Levels in Individuals with Multiple Sclerosis. Mult. Scler. 2008, 14, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Ramari, C.; Hvid, L.G.; de David, A.C.; Dalgas, U. The Importance of Lower-Extremity Muscle Strength for Lower-Limb Functional Capacity in Multiple Sclerosis: Systematic Review. Ann. Phys. Rehabil. Med. 2020, 63, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.F.; Sandroff, B.M.; Motl, R.W. Therapies for Mobility Disability in Persons with Multiple Sclerosis. Expert Rev. Neurother. 2018, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Hiew, S.; Nguemeni, C.; Zeller, D. Efficacy of Transcranial Direct Current Stimulation in People with Multiple Sclerosis: A Review. Eur. J. Neurol. 2022, 29, 648–664. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Oveisgharan, S.; Karimi, Z.; Abdi, S.; Sikaroodi, H. The Use of Brain Stimulation in the Rehabilitation of Walking Disability in Patients with Multiple Sclerosis: A Randomized Double-Blind Clinical Trial Study. Curr. J. Neurol. 2019, 18, 57–63. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Transcranial Direct Current Stimulation (TDCS) to Improve Gait in Multiple Sclerosis: A Timing Window Comparison. Front. Hum. Neurosci. 2019, 13, 420. [Google Scholar] [CrossRef]
- Pilloni, G.; Choi, C.; Shaw, M.T.; Coghe, G.; Krupp, L.; Moffat, M.; Cocco, E.; Pau, M.; Charvet, L. Walking in Multiple Sclerosis Improves with TDCS: A Randomized, Double-Blind, Sham-Controlled Study. Ann. Clin. Transl. Neurol. 2020, 7, 2310–2319. [Google Scholar] [CrossRef]
- Pilloni, G.; Choi, C.; Coghe, G.; Cocco, E.; Krupp, L.B.; Pau, M.; Charvet, L.E. Gait and Functional Mobility in Multiple Sclerosis: Immediate Effects of Transcranial Direct Current Stimulation (TDCS) Paired with Aerobic Exercise. Front. Neurol. 2020, 11, 310. [Google Scholar] [CrossRef]
- Burhan, A.M.; Subramanian, P.; Pallaveshi, L.; Barnes, B.; Montero-Odasso, M. Modulation of the Left Prefrontal Cortex with High Frequency Repetitive Transcranial Magnetic Stimulation Facilitates Gait in Multiple Sclerosis. Case Rep. Neurol. Med. 2015, 2015, 251829. [Google Scholar] [CrossRef]
- Muñoz-Paredes, I.; Herrero, A.J.; Román-Nieto, N.; Peña-Gomez, A.M.; Seco-Calvo, J. Influence of Transcranial Direct Current Stimulation and Exercise on Fatigue and Quality of Life in Multiple Sclerosis. Healthcare 2023, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Paredes, I.; Herrero, A.J.; Llamas-Ramos, R.; Rodríguez-Pérez, V.; Seco-Calvo, J. The Effect of Combining Transcranial Direct Current Stimulation Treatment and an Exercise Program on Fragility in a Population with Multiple Sclerosis: Cross-Over Design Trial. Int. J. Environ. Res. Public Health 2022, 19, 12747. [Google Scholar] [CrossRef] [PubMed]
- Kos, D.; Kerckhofs, E.; Carrea, I.; Verza, R.; Ramos, M.; Jansa, J. Evaluation of the Modified Fatigue Impact Scale in Four Different European Countries. Mult. Scler. 2005, 11, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, S.H.; Han, K.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, S.H.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; et al. Association between Exercise and Risk of Cardiovascular Diseases in Patients with Non-Cystic Fibrosis Bronchiectasis. Respir. Res. 2022, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.; Volz, M.S.; Bikson, M.; Fregni, F. Electrode Positioning and Montage in Transcranial Direct Current Stimulation. J. Vis. Exp. 2011, 23, e2744. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; ISBN 9780880116237. [Google Scholar]
- Goldman, M.D.; Marrie, R.A.; Cohen, J.A. Evaluation of the Six-Minute Walk in Multiple Sclerosis Subjects and Healthy Controls. Mult. Scler. 2008, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Scalzitti, D.A.; Harwood, K.J.; Maring, J.R.; Leach, S.J.; Ruckert, E.A.; Costello, E. Validation of the 2-Minute Walk Test with the 6-Minute Walk Test and Other Functional Measures in Persons with Multiple Sclerosis. Int. J. MS Care 2018, 20, 158–163. [Google Scholar] [CrossRef]
- Gosney, J.L.; Scott, J.A.; Snook, E.M.; Motl, R.W. Physical Activity and Multiple Sclerosis: Validity of Self-Report and Objective Measures. Fam. Community Health 2007, 30, 144–150. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.S.; Maeurer, M.; Dippel, F.W.; Kohlmann, T. Systematic Literature Review and Validity Evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in Patients with Multiple Sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Motl, R.W. Psychometric Properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J. Neurol. Sci. 2013, 331, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Roy-García, I.; Rivas-Ruiz, R.; Pérez-Rodríguez, M.; Palacios-Cruz, L. Correlation: Not All Correlation Entails Causality. Rev. Alerg. Mex. 2019, 66, 354–360. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. Minimal Clinically Important Difference for Change in 6-Minute Walk Test Distance of Adults with Pathology: A Systematic Review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Oosterveer, D.M.; van den Berg, C.; Volker, G.; Wouda, N.C.; Terluin, B.; Hoitsma, E. Determining the Minimal Important Change of the 6-Minute Walking Test in Multiple Sclerosis Patients Using a Predictive Modelling Anchor-Based Method. Mult. Scler. Relat. Disord. 2021, 57, 103438. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Dlugonski, D.; Pilutti, L.A.; Sandroff, B.M.; Motl, R.W. The Reliability, Precision and Clinically Meaningful Change of Walking Assessments in Multiple Sclerosis. Mult. Scler. 2013, 19, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Pilutti, L.A.; Motl, R.W. Does the Six-Minute Walk Test Measure Walking Performance or Physical Fitness in Persons with Multiple Sclerosis? NeuroRehabilitation 2015, 37, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mañago, M.M.; Hebert, J.R.; Kittelson, J.; Schenkman, M. Contributions of Ankle, Knee, Hip, and Trunk Muscle Function to Gait Performance in People with Multiple Sclerosis: A Cross-Sectional Analysis. Phys. Ther. 2018, 98, 595–604. [Google Scholar] [CrossRef]
- Malcay, O.; Grinberg, Y.; Berkowitz, S.; Hershkovitz, L.; Kalron, A. Cognitive-Motor Interference in Multiple Sclerosis: What Happens When the Gait Speed Is Fixed? Gait Posture 2017, 57, 211–216. [Google Scholar] [CrossRef]
- Coghe, G.; Corona, F.; Marongiu, E.; Fenu, G.; Frau, J.; Lorefice, L.; Crisafulli, A.; Galli, M.; Concu, A.; Marrosu, M.G.; et al. Fatigue, as Measured Using the Modified Fatigue Impact Scale, Is a Predictor of Processing Speed Improvement Induced by Exercise in Patients with Multiple Sclerosis: Data from a Randomized Controlled Trial. J. Neurol. 2018, 265, 1328–1333. [Google Scholar] [CrossRef]
- Conklyn, D.; Stough, D.; Novak, E.; Paczak, S.; Chemali, K.; Bethoux, F. A Home-Based Walking Program Using Rhythmic Auditory Stimulation Improves Gait Performance in Patients with Multiple Sclerosis: A Pilot Study. Neurorehabil. Neural Repair 2010, 24, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Fimland, M.S.; Helgerud, J.; Gruber, M.; Leivseth, G.; Hoff, J. Enhanced Neural Drive after Maximal Strength Training in Multiple Sclerosis Patients. Eur. J. Appl. Physiol. 2010, 110, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kerling, A.; Keweloh, K.; Tegtbur, U.; Kück, M.; Grams, L. Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). Int. J. Mol. Sci. 2015, 0067, 15761–15775. [Google Scholar] [CrossRef] [PubMed]
- Strube, W.; Bunse, T.; Nitsche, M.A.; Nikolaeva, A.; Palm, U.; Padberg, F.; Falkai, P.; Hasan, A. Bidirectional Variability in Motor Cortex Excitability Modulation Following 1 MA Transcranial Direct Current Stimulation in Healthy Participants. Physiol. Rep. 2016, 4, e12884. [Google Scholar] [CrossRef]
- Nguemeni, C.; Homola, G.A.; Nakchbandi, L.; Pham, M.; Volkmann, J.; Zeller, D. A Single Session of Anodal Cerebellar Transcranial Direct Current Stimulation Does Not Induce Facilitation of Locomotor Consolidation in Patients with Multiple Sclerosis. Front. Hum. Neurosci. 2020, 14, 588671. [Google Scholar] [CrossRef]
- Marotta, N.; De Sire, A.; Marinaro, C.; Moggio, L.; Inzitari, M.T.; Russo, I.; Tasselli, A.; Paolucci, T.; Valentino, P.; Ammendolia, A. Efficacy of Transcranial Direct Current Stimulation (TDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach. J. Clin. Med. 2022, 11, 3505. [Google Scholar] [CrossRef]
- Bowman, T.; Gervasoni, E.; Amico, A.P.; Antenucci, R.; Benanti, P.; Boldrini, P.; Bonaiuti, D.; Burini, A.; Castelli, E.; Draicchio, F.; et al. What Is the Impact of Robotic Rehabilitation on Balance and Gait Outcomes in People with Multiple Sclerosis? A Systematic Review of Randomized Control Trials. Eur. J. Phys. Rehabil. Med. 2021, 57, 246–253. [Google Scholar] [CrossRef]
- Edwards, T.; Pilutti, L.A. The Effect of Exercise Training in Adults with Multiple Sclerosis with Severe Mobility Disability: A Systematic Review and Future Research Directions. Mult. Scler. Relat. Disord. 2017, 16, 31–39. [Google Scholar] [CrossRef]
- Binshalan, T.; Nair, K.P.S.; McNeill, A. The Effectiveness of Physiotherapy Interventions for Mobility in Severe Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Int. 2022, 2022, 2357785. [Google Scholar] [CrossRef]
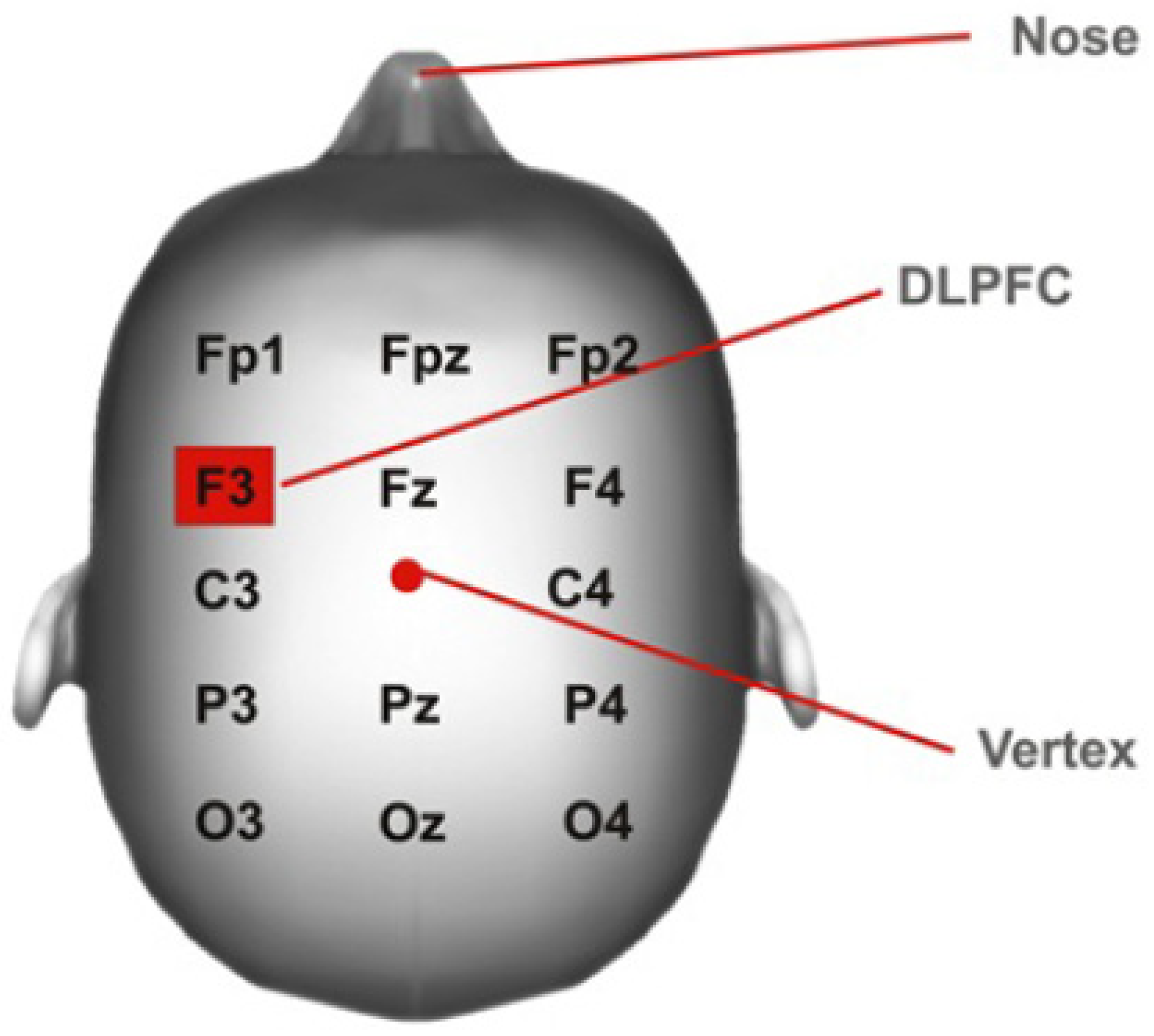

| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| Endurance | 1 Ss | 2 Ss | 2 Ss | 2–3 Ss |
| 10 min | 15 min | 20 min (5 min of rest in the middle of the session) | 30 min (5 min of rest in the middle of the session) | |
| RPE 3–5 | RPE 3–5 | RPE 3–5 | RPE 3–5 | |
| Static bike/MOTOmed | Static bike/MOTOmed | Static bike/MOTOmed | Static bike/MOTOmed | |
| Strength | 2 Ss | 2 Ss | 3 Ss | 3 Ss |
| 15 Rep (rest 2 min) | 15 Rep (rest 2 min) | 10 Rep (rest 3 min) | 10 Rep (rest 3 min) | |
| 2 Circuits (rest 3 min) | 2 Circuits (rest 3 min) | 3 Circuits (rest 5 min) | 3 Circuits (rest 5 min) |
| Variables | N [Min-Max]; Mean ± (SD) | Frequency (%) |
|---|---|---|
| Age | 12 [35–66]; 48.0 ± (8.5) | |
| Diagnosis (years) | 12 [0.8–28]; 16.65 ± (7.44) | |
| Outbreaks (years) | 11 [0–2]; 0.36 ± (0.67) | |
| Kurtzke Disability Scale EDSS | 12 [0.5–5]; 3.66 ± (1.30) | |
| Walking time (minutes) | 9 [0.0–120]; 51.11 ± (41.06) | |
| Sitting time (minutes) | 9 [0–960]; 466.667 ± (304.13) | |
| 6MWT | 12 [66.9–669]; 411 ± (207.67) | |
| 2MWT | 12 [25.8–292.3]; 139.27 ± (80.98) | |
| MFIS | 12 [38–69]; 44.5 ± (9.69) | |
| IPAQ Level | ||
| High level | 4 (33.3%) | |
| Moderate level | 5 (41.7%) | |
| Low or inactive level | 3 (25%) | |
| Type of sclerosis | ||
| Relapsing–Remitting | 7 (58.3%) | |
| Progressive Secondary | 5 (41.7%) | |
| Outbreak intensity | ||
| Mild | 2 (18.2%) | |
| Moderate | 1 (9.1%) | |
| Intense | 1 (9.1%) | |
| No outbreaks | 7 (63.6%) | |
| Medical recommendation | ||
| Physical activity | 6 (11.3%) | |
| Other | 1 (8.3%) | |
| No recommendation | 5 (41.7%) | |
| Fatigue medication | ||
| Yes | 5 (41.7%) | |
| No | 7 (58.3%) | |
| Rehabilitation | ||
| Yes | 11 (91.7%) | |
| No | 1 (8.3%) | |
| Intensity rehabilitation | ||
| Occasional | 5 (41.7%) | |
| Periodic | 7 (58.3%) | |
| Exercise habits | ||
| Occasional | 2 (16.7%) | |
| Periodically | 10 (83.3%) | |
| T0 Median [Range] | T1 Median [Range] | p | Size Effect Hedges’ g | T2 Median [Range] | T3 Median [Range] | p | Size Effect Hedges’ g | |
|---|---|---|---|---|---|---|---|---|
| 6MWT | 504 [602.1] | 467.1 [610] | 0.388 | 0.632 | 362.8 [552] | 409.65 [566] | 0.004 ** | 0.418 |
| 2MWT | 144.45 [266.5] | 149.95 [201.35] | 0.347 | 0.027 | 107.45 [175.7] | 119.7 [213.8] | 0.002 ** | 0.182 |
| MFIS | 39.5 [31] | 38.5 [45] | 0.028 * | 0.525 | 43 [33] | 36 [52] | 0.003 ** | 0.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Paredes, I.; Herrero, A.J.; Seco-Calvo, J. Influence of Transcranial Direct Current Stimulation and Exercise on Physical Capacity and Gait in Multiple Sclerosis: A Cross-Over Pilot Study. Healthcare 2023, 11, 1384. https://doi.org/10.3390/healthcare11101384
Muñoz-Paredes I, Herrero AJ, Seco-Calvo J. Influence of Transcranial Direct Current Stimulation and Exercise on Physical Capacity and Gait in Multiple Sclerosis: A Cross-Over Pilot Study. Healthcare. 2023; 11(10):1384. https://doi.org/10.3390/healthcare11101384
Chicago/Turabian StyleMuñoz-Paredes, Inés, Azael J. Herrero, and Jesús Seco-Calvo. 2023. "Influence of Transcranial Direct Current Stimulation and Exercise on Physical Capacity and Gait in Multiple Sclerosis: A Cross-Over Pilot Study" Healthcare 11, no. 10: 1384. https://doi.org/10.3390/healthcare11101384
APA StyleMuñoz-Paredes, I., Herrero, A. J., & Seco-Calvo, J. (2023). Influence of Transcranial Direct Current Stimulation and Exercise on Physical Capacity and Gait in Multiple Sclerosis: A Cross-Over Pilot Study. Healthcare, 11(10), 1384. https://doi.org/10.3390/healthcare11101384







