Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components
Abstract
1. Introduction
2. Materials and Methods
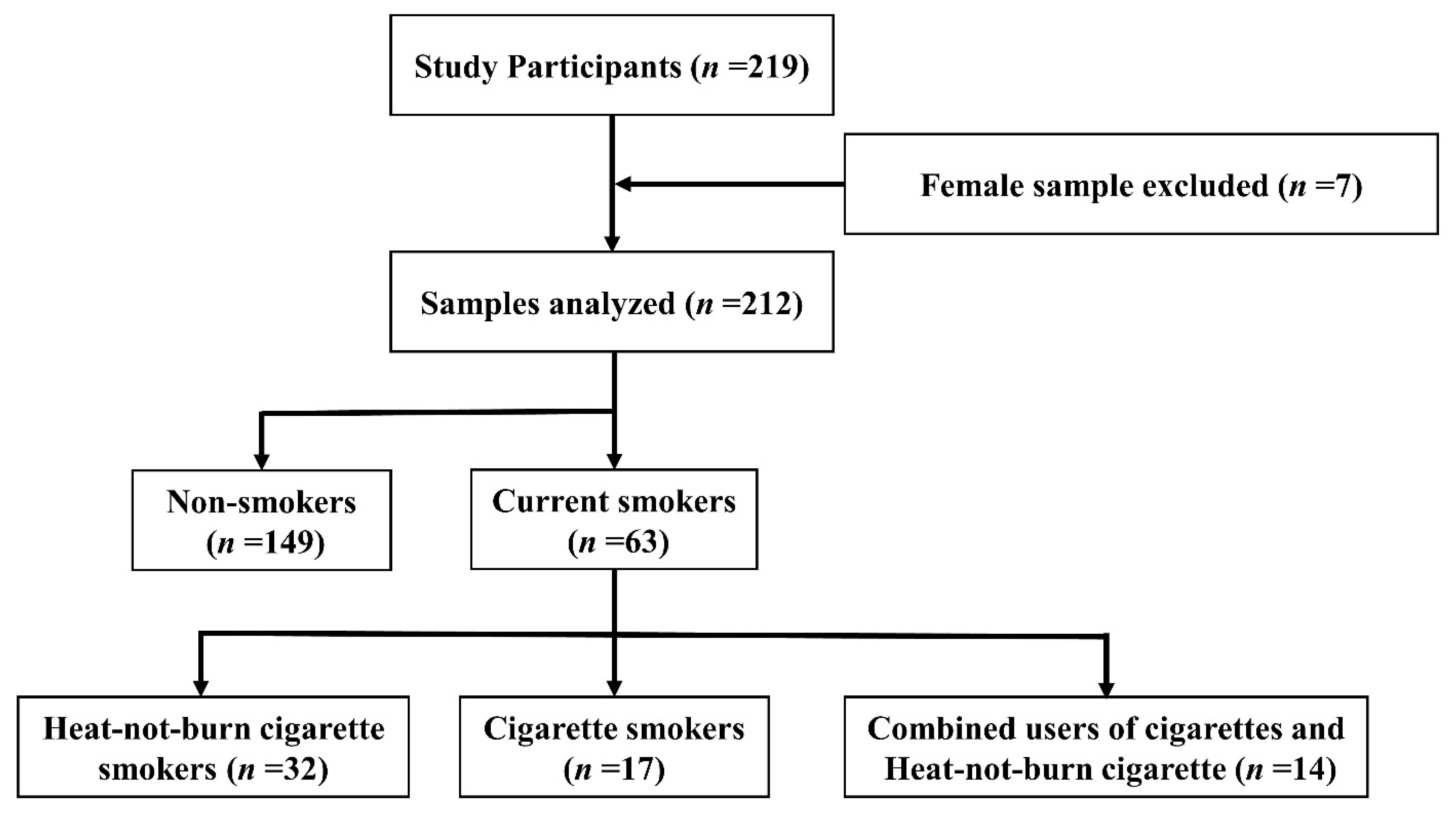
2.1. Study Participants
2.2. Saliva Collection
2.3. Lac and Lys Rating
2.4. Survey
2.5. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Saliva Secretion Rate
3.3. Lac Secretion Rate
3.4. Lys Secretion Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K. Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison With a Tobacco Cigarette and E-Cigarettes. Nicotine Tob. Res. 2018, 20, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, T.; Gallus, S.; Shinozaki, T.; Nakaya, T.; Kunugita, N.; Colwell, B. Heat-not-burn tobacco product use in Japan: Its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol. Tob. Control 2018, 27, e25–e33. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, A.; Kuwabara, Y.; Fujii, M.; Imamoto, A.; Osaki, Y.; Minobe, R.; Maezato, H.; Nakayama, H.; Takimura, T.; Higuchi, S. Heated Tobacco Product Smokers in Japan Identified by a Population-Based Survey. J. Epidemiol. 2020, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.C.; Cappella, J.N. Harm Perceptions and Beliefs about Potential Modified Risk Tobacco Products. Int. J. Environ. Res. Public Health 2021, 18, 576. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern. Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Yamashita, Y.; Tomioka, H. Acute eosinophilic pneumonia following heat-not-burn cigarette smoking. Respirol. Case. Rep 2016, 4, e00190. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, P.; Liu, J.; Havel, C.M.; Ibrahim, S.; Derakhshandeh, R.; Jacob Iii, P.; Springer, M.L. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob. Control 2018, 27, s13–s19. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, B.K.; Oh, J.H.; Shim, J.S.; Chang, Y.S.; Cho, S.H.; Yang, M.S. Novel tobacco products including electronic cigarette and heated tobacco products increase risk of allergic rhinitis and asthma in adolescents: Analysis of Korean youth survey. Allergy 2020, 75, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Chestnutt, I.G. Smoking and periodontal disease. Crit. Rev. Oral Biol. Med. 2000, 11, 356–365. [Google Scholar] [CrossRef]
- Palmer, R.M.; Wilson, R.F.; Hasan, A.S.; Scott, D.A. Mechanisms of action of environmental factors--tobacco smoking. J. Clin. Periodontol. 2005, 32 (Suppl. 6), 180–195. [Google Scholar] [CrossRef]
- Kibayashi, M.; Tanaka, M.; Nishida, N.; Kuboniwa, M.; Kataoka, K.; Nagata, H.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Longitudinal Study of the Association Between Smoking as a Periodontitis Risk and Salivary Biomarkers Related to Periodontitis. J. Periodontol. 2007, 78, 859–867. [Google Scholar] [CrossRef]
- Wagner, V.; Skokanová, K.; Wagnerová, M.; Heribanová, A.; Ríha, M. Indicators of humoral immunity in smokers and nonsmokers working underground. J. Hyg. Epidemiol. Microbiol. Immunol. 1983, 27, 129–142. [Google Scholar] [PubMed]
- Edgar, W.M. Saliva: Its secretion, composition and functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Rudney, J.D. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit. Rev. Oral Biol. Med. 1995, 6, 343–367. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, J. Antimicrobial agents in saliva--protection for the whole body. J. Dent. Res. 2002, 81, 807–809. [Google Scholar] [CrossRef]
- Van Nieuw Amerongen, A.; Bolscher, J.G.; Veerman, E.C. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004, 38, 247–253. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Renshaw, G.; Cripps, A.W. Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol. Med. Microbiol. 2006, 48, 293–304. [Google Scholar] [CrossRef]
- Malamud, D.; Abrams, W.R.; Barber, C.A.; Weissman, D.; Rehtanz, M.; Golub, E. Antiviral activities in human saliva. Adv. Dent. Res. 2011, 23, 34–37. [Google Scholar] [CrossRef]
- Gillum, T.; Kuennen, M.; Miller, T.; Riley, L. The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc. Immunol. Rev. 2014, 20, 23–38. [Google Scholar]
- Tonguc Altin, K.; Topcuoglu, N.; Duman, G.; Unsal, M.; Celik, A.; Selvi Kuvvetli, S.; Kasikci, E.; Sahin, F.; Kulekci, G. Antibacterial effects of saliva substitutes containing lysozyme or lactoferrin against Streptococcus mutans. Arch. Oral Biol. 2021, 129, 105183. [Google Scholar] [CrossRef]
- McKenna, Z.; Berkemeier, Q.; Gorini, F.; Kuennen, M.; Naylor, A.; Kleint, A.; Gillum, T. Effects of exercise in hot and humid conditions and bovine colostrum on salivary immune markers. J. Therm. Biol. 2020, 93, 102717. [Google Scholar] [CrossRef] [PubMed]
- Glimvall, P.; Wickström, C.; Jansson, H. Elevated levels of salivary lactoferrin, a marker for chronic periodontitis? J. Periodontal. Res. 2012, 47, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, D.; Kusiak, A.; Kochańska, B.; Ochocińska, J.; Świetlik, D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. Int. J. Environ. Res. Public Health 2019, 16, 4433. [Google Scholar] [CrossRef]
- Shimizu, K.; Kimura, F.; Akimoto, T.; Akama, T.; Otsuki, T.; Nishijima, T.; Kuno, S.; Kono, I. Effects of exercise, age and gender on salivary secretory immunoglobulin A in elderly individuals. Exerc. Immunol. Rev. 2007, 13, 55–66. [Google Scholar] [PubMed]
- Al-Tarawneh, S.K.; Border, M.B.; Dibble, C.F.; Bencharit, S. Defining salivary biomarkers using mass spectrometry-based proteomics: A systematic review. OMICS 2011, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gillum, T.L.; Kuennen, M.R.; Castillo, M.N.; Williams, N.L.; Jordan-Patterson, A.T. Exercise, but not acute sleep loss, increases salivary antimicrobial protein secretion. J. Strength Cond. Res. 2015, 29, 1359–1366. [Google Scholar] [CrossRef]
- Rad, M.; Kakoie, S.; Niliye Brojeni, F.; Pourdamghan, N. Effect of Long-term Smoking on Whole-mouth Salivary Flow Rate and Oral Health. J. Dent. Res. Dent. Clin. Dent. Prospect. 2010, 4, 110–114. [Google Scholar]
- Dyasanoor, S.; Saddu, S.C. Association of Xerostomia and Assessment of Salivary Flow Using Modified Schirmer Test among Smokers and Healthy Individuals: A Preliminutesary Study. J. Clin. Diagn. Res 2014, 8, 211–213. [Google Scholar]
- Petrušić, N.; Posavac, M.; Sabol, I.; Mravak-Stipetić, M. The Effect of Tobacco Smoking on Salivation. Acta Stomatol. Croat. 2015, 49, 309–315. [Google Scholar] [CrossRef]
- Oliver, S.J.; Laing, S.J.; Wilson, S.; Bilzon, J.L.; Walters, R.; Walsh, N.P. Salivary immunoglobulin A response at rest and after exercise following a 48 h period of fluid and/or energy restriction. Br. J. Nutr. 2007, 97, 1109–1116. [Google Scholar] [CrossRef]
- Walsh, N.P.; Laing, S.J.; Oliver, S.J.; Montague, J.C.; Walters, R.; Bilzon, J.L. Saliva parameters as potential indices of hydration status during acute dehydration. Med. Sci. Sport. Exerc. 2004, 36, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Ferragut, J.M.; da Cunha, M.R.; Carvalho, C.A.; Isayama, R.N.; Caldeira, E.J. Epithelial-stromal interactions in salivary glands of rats exposed to chronic passive smoking. Arch. Oral Biol. 2011, 56, 580–587. [Google Scholar] [CrossRef]
- Smith, C.J.; Hansch, C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem. Toxicol. 2000, 38, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Born, I.A.; Mall, G. Effect of chronic ethanol and nicotine consumption on the function and morphology of the salivary glands. Klin. Wochenschr. 1988, 66 (Suppl. 11), 140–150. [Google Scholar] [PubMed]
- Gregory, R.L.; Gfell, L.E. Effect of nicotine on secretory component synthesis by secretory epithelial cells. Clin. Diagn. Lab. Immunol. 1996, 3, 578–583. [Google Scholar] [CrossRef]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef]
- Uchiyama, S.; Noguchi, M.; Takagi, N.; Hayashida, H.; Inaba, Y.; Ogura, H.; Kunugita, N. Simple Determination of Gaseous and Particulate Compounds Generated from Heated Tobacco Products. Chem. Res. Toxicol. 2018, 31, 585–593. [Google Scholar] [CrossRef]
- Dawes, C. Circadian rhythms in human salivary flow rate and composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef]
- Walsh, N.P.; Montague, J.C.; Callow, N.; Rowlands, A.V. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch. Oral Biol. 2004, 49, 149–154. [Google Scholar] [CrossRef]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Kindblom, C.; Davies, J.R.; Herzberg, M.C.; Svensäter, G.; Wickström, C. Salivary proteins promote proteolytic activity in Streptococcus mitis biovar 2 and Streptococcus mutans. Mol. Oral Microbiol. 2012, 27, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, H.; Tenovuo, J.; Waris, M.; Hukkanen, V. Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol. J. 2009, 6, 53. [Google Scholar] [CrossRef]
- Małaczewska, J.; Kaczorek-Łukowska, E.; Wójcik, R.; Siwicki, A.K. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet. Res. 2019, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]


| n | % | |
|---|---|---|
| Age groups | ||
| 20–29 | 67 | 31.6 |
| 30–39 | 75 | 35.4 |
| 40–49 | 52 | 24.5 |
| >50 | 18 | 8.5 |
| Sex | ||
| Male | 212 | 100.0 |
| Smoking habit | ||
| Non-smokers | 149 | 70.3 |
| Current smokers | 63 | 29.7 |
| HNB cigarette smokers | 32 | 51.0 |
| Cigarette smokers | 17 | 27.0 |
| Combined users of cigarettes and HNB cigarette | 14 | 22.0 |
| Saliva Secretion Rate (mL/min) | p-Value † | Lac Concentration (μg/mL) | p-Value † | Lys Concentration (μg/mL) | p-Value † | |
|---|---|---|---|---|---|---|
| Non–smokers | 1.4 (0.9–2.2) | 4.5 (2.5–10.2) | 2.8 (1.5–4.8) | |||
| Current smokers | 1.0 (0.7–1.4) | <0.001 | 4.3 (2.1–7.1) | 0.094 | 2.4 (1.1–3.9) | 0.243 |
| HNB cigarette smokers | 1.0 (0.7–1.5) | 0.010 | 3.6 (2.7–6.1) | 0.054 | 2.3 (1.2–3.4) | 0.152 |
| Cigarette smokers | 1.1 (0.6–1.3) | 0.045 | 6.0 (3.9–8.8) | 0.618 | 3.2 (1.0–5.3) | 0.850 |
| Combined users | 1.1 (0.7–1.5) | 0.100 | 3.5 (1.0–7.5) | 0.125 | 2.6 (1.0–4.5) | 0.738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, Y.; Tanaka, M.; Kozai, H.; Aoyama, Y.; Shigeno, Y.; Hotta, K.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components. Healthcare 2023, 11, 132. https://doi.org/10.3390/healthcare11010132
Mori Y, Tanaka M, Kozai H, Aoyama Y, Shigeno Y, Hotta K, Aoike M, Kawamura H, Tsurudome M, Ito M. Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components. Healthcare. 2023; 11(1):132. https://doi.org/10.3390/healthcare11010132
Chicago/Turabian StyleMori, Yukihiro, Mamoru Tanaka, Hana Kozai, Yuka Aoyama, Yukihiro Shigeno, Kiyoshi Hotta, Makoto Aoike, Hatsumi Kawamura, Masato Tsurudome, and Morihiro Ito. 2023. "Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components" Healthcare 11, no. 1: 132. https://doi.org/10.3390/healthcare11010132
APA StyleMori, Y., Tanaka, M., Kozai, H., Aoyama, Y., Shigeno, Y., Hotta, K., Aoike, M., Kawamura, H., Tsurudome, M., & Ito, M. (2023). Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components. Healthcare, 11(1), 132. https://doi.org/10.3390/healthcare11010132








