Validation and Reliability of Polish Version of the Reflux Symptoms Index and Reflux Finding Score
Abstract
:1. Introduction
2. Materials and Methods
2.1. RSI and RFS Translation
2.2. Participants
2.3. RSI and RFS Procedure
2.4. pH-Metry and LPR Diagnosis
2.5. Other Measurement and Diagnostic Tools
2.6. Reliability Analysis
2.7. Validity Assessment
- Comparison with the control group. The rationale behind conducting PL-RSI and RFS in the healthy subjects was two-fold: (i) it allowed us to establish a country-specific baseline and reference group; (ii) the difference between the group with voice disorders and a group without symptoms shows the relevance of the scale to the problem it was meant to solve.
- Concordance with other LPR diagnostic tools. One of the aims was to determine the extent that measures from other LPR scales relate to PL-RSI and PL-RFS.
- Relationship to pH-metry measurements. The gold standard of acid LPR detection is assumed to be pharyngeal pH-metry measurements. Hence, the basal validity and usability of PL-RSI and PL-RFS was evaluated by their predictive power for diagnosis of acid LPR and by the correlation between the value of pH-metry and the strength of symptoms as indicated by the scales.
2.8. Statistical Method
2.9. Ethics Statement
3. Results
3.1. Translation and Validation Procedure
3.2. Study Group: PL-RSI and PL-RFS Scores
3.3. Consistency and Reliability of PL-RFS
3.4. Consistency and Reliability of PL-RFS
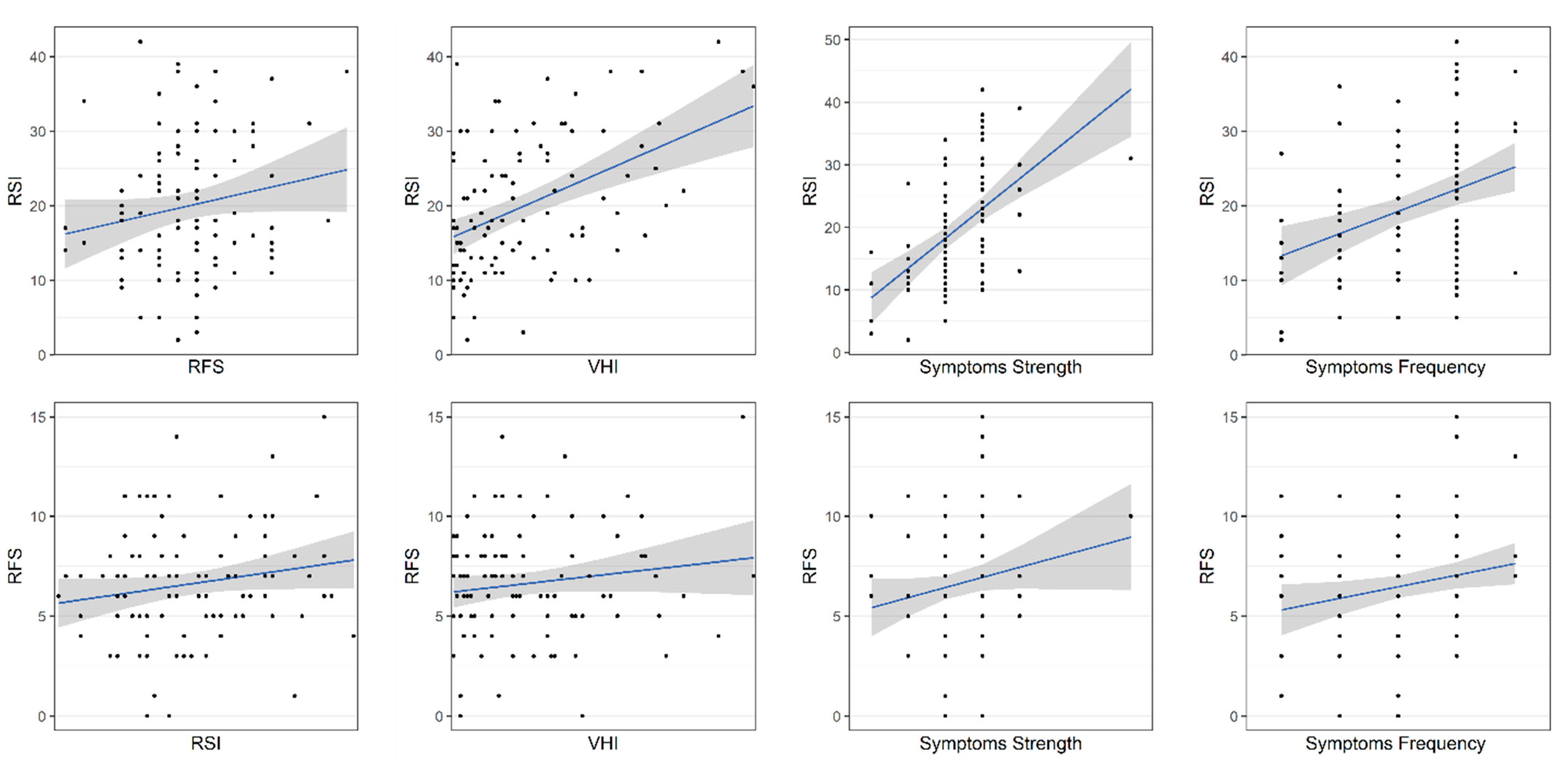
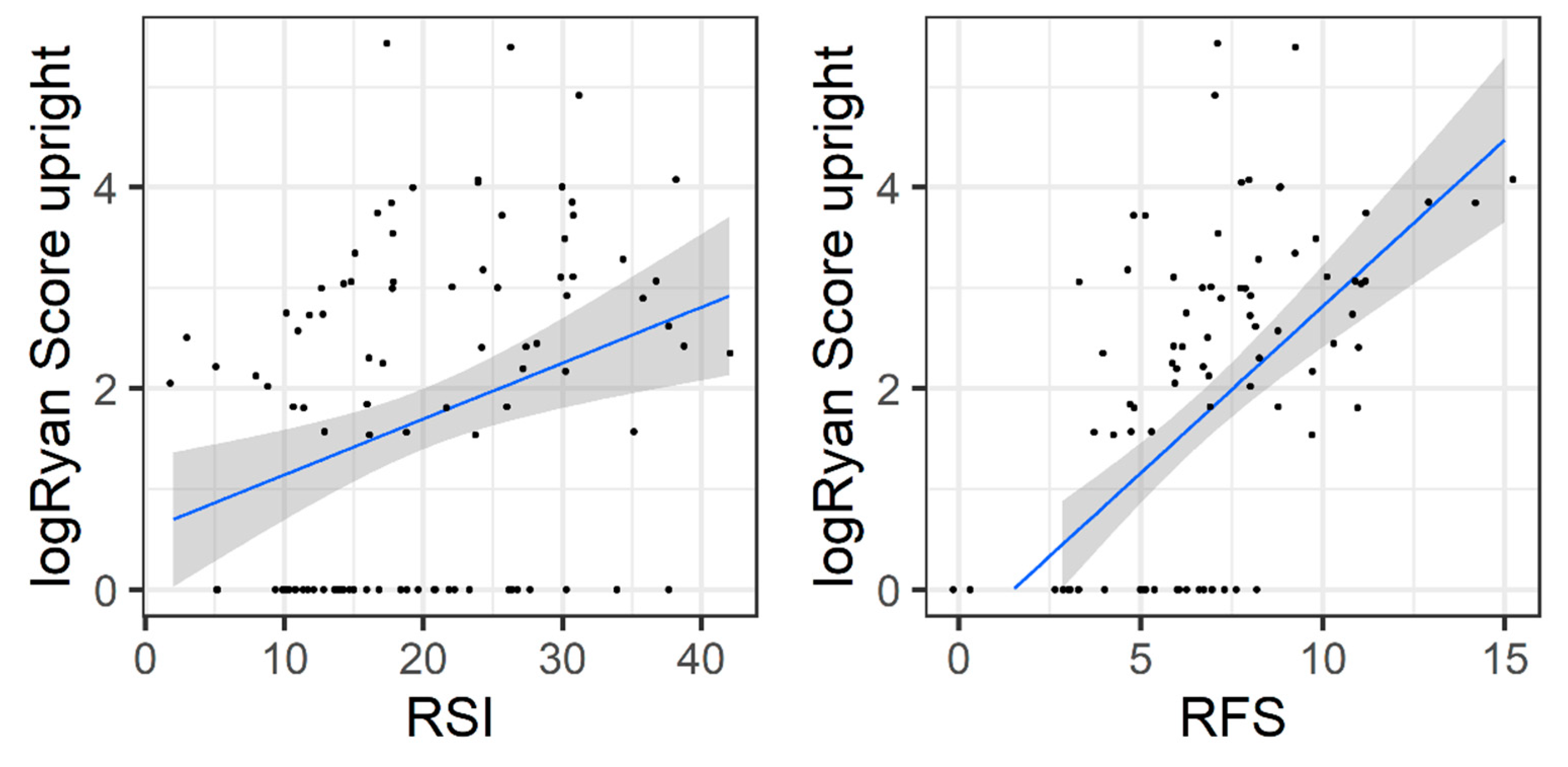
3.5. Validity of PL-RFS and PL-RSI
4. Discussion
5. Conclusions
- High reliability and consistency;
- Correlation with the objective clinical metrics of acid LPR;
- Adequate distinction between patients with and without severe acid LPR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The Validity and Reliability of the Reflux Finding Score (RFS). The Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice Off. J. Voice Found. 2002, 16, 274–277. [Google Scholar] [CrossRef]
- Włodarczyk, E.; Miaśkiewicz, B.; Szkiełkowska, A.; Skarżyński, P.H.; Skarżyński, H. Applicability of RSI and RFS Questionnaires in the Polish Language Version. Pol. J. Otolaryngol. 2019, 73, 17–21. [Google Scholar]
- Włodarczyk, E.; Miaśkiewicz, B.; Raj-Koziak, D.; Szkiełkowska, A.; Skarżyński, P.H.; Skarżyński, H. The Application of 24-Hour Pharyngeal PH-Monitoring and Reflux Finding Score and Reflux Symptom Index Questionnaires in the Diagnostics of Laryngopharyngeal Reflux. Przeglad Gastroenterol. 2019, 14, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, E.; Jetka, T.; Raj-Koziak, D.; Panasiewicz, A.; Szkiełkowska, A.; Skarżyński, P.H.; Skarżyński, H. Diagnosis of Laryngopharyngeal Reflux in Children with Voice Disorders Using 24-Hour Pharyngeal PH Monitoring. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Rees, C.J. Laryngopharyngeal Reflux: The Value of Otolaryngology Examination. Curr. Gastroenterol. Rep. 2008, 10, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Mozzanica, F.; Ginocchio, D.; Peri, A.; Bottero, A.; Ottaviani, F. Reliability and Clinical Validity of the Italian Reflux Symptom Index. J. Voice Off. J. Voice Found. 2010, 24, 354–358. [Google Scholar] [CrossRef]
- Printza, A.; Kyrgidis, A.; Oikonomidou, E.; Triaridis, S. Assessing Laryngopharyngeal Reflux Symptoms with the Reflux Symptom Index: Validation and Prevalence in the Greek Population. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2011, 145, 974–980. [Google Scholar] [CrossRef]
- Farahat, M.; Malki, K.H.; Mesallam, T.A. Development of the Arabic Version of Reflux Symptom Index. J. Voice Off. J. Voice Found. 2012, 26, 814.e15–814.e19. [Google Scholar] [CrossRef]
- Lapeña, J.F.F.; Ambrocio, G.M.C.; Carrillo, R.J.D. Validity and Reliability of the Filipino Reflux Symptom Index. J. Voice Off. J. Voice Found. 2017, 31, 387.e11–387.e16. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Li, J.; Li, J.; Cheng, C.-F.J. Chinese version of the reflux symptom index was evaluated for reliability and validity. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012, 47, 894–898. [Google Scholar]
- Lechien, J.R.; Huet, K.; Finck, C.; Khalife, M.; Fourneau, A.-F.; Delvaux, V.; Piccaluga, M.; Harmegnies, B.; Saussez, S. Validity and Reliability of a French Version of Reflux Symptom Index. J. Voice Off. J. Voice Found. 2017, 31, 512.e1–512.e7. [Google Scholar] [CrossRef]
- Akbulut, S.; Aydinli, F.E.; Kuşçu, O.; Özcebe, E.; Yilmaz, T.; Rosen, C.A.; Gartner-Schmidt, J. Reliability and Validity of the Turkish Reflux Symptom Index. J. Voice Off. J. Voice Found. 2020, 34, 965.e23–965.e28. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Henríquez, C.; Ruano-Ravina, A.; Vaamonde, P.; Lattomus, K.; Sebio, A.; Fernández-Rodríguez, R.; Martin-Martin, C. Translation and Validation of the Reflux Symptom Index to Spanish. J. Voice Off. J. Voice Found. 2019, 33, 807.e1–807.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kwon, O.E.; Park, J.M.; Eun, Y.G. Do Laryngoscopic Findings Reflect the Characteristics of Reflux in Patients with Laryngopharyngeal Reflux? Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2018, 43, 137–143. [Google Scholar] [CrossRef]
- Wu, W.; Li, L.; Qu, C.; Wang, M.; Liang, S.; Gao, X.; Bao, X.; Wang, L.; Liu, H.; Han, H.; et al. Reflux Finding Score Is Associated with Gastroesophageal Flap Valve Status in Patients with Laryngopharyngeal Reflux Disease: A Retrospective Study. Sci. Rep. 2019, 9, 15744. [Google Scholar] [CrossRef]
- Mesallam, T.A.; Stemple, J.C.; Sobeih, T.M.; Elluru, R.G. Reflux Symptom Index versus Reflux Finding Score. Ann. Otol. Rhinol. Laryngol. 2007, 116, 436–440. [Google Scholar] [CrossRef]
- Karakaya, N.E.; Akbulut, S.; Altıntaş, H.; Demir, M.G.; Demir, N.; Berk, D. The Reflux Finding Score: Reliability and Correlation to the Reflux Symptom Index. J. Acad. Res. Med. 2015, 5, 68–74. [Google Scholar] [CrossRef]
- Eckley, C.A.; Tangerina, R. Sensitivity, Specificity, and Reproducibility of the Brazilian Portuguese Version of the Reflux Symptom Index. J. Voice Off. J. Voice Found. 2021, 35, 161.e15–161.e19. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, R.; Pandolfino, J.E.; Lidder, A.K.; Shabeeb, N.; Jaiyeola, D.-M.; Adkins, C.; Agrawal, N.; Cooper, A.; Price, C.P.E.; Ciolino, J.D.; et al. Oropharyngeal PH Testing Does Not Predict Response to Proton Pump Inhibitor Therapy in Patients with Laryngeal Symptoms. Am. J. Gastroenterol. 2016, 111, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Nunes, H.S.; Pinto, J.A.; Zavanela, A.R.; Cavallini, A.F.; Freitas, G.S.; Garcia, F.E. Comparison between the Reflux Finding Score and the Reflux Symptom Index in the Practice of Otorhinolaryngology. Int. Arch. Otorhinolaryngol. 2016, 20, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugras, M.K.; Dogan, M.; Pata, D.Y.S.; Ozkan, F. Can the Reflux Finding Score and Reflux Symptom Index Be Used to Evaluate the Severity of Esophagitis in Children? J. Voice Off. J. Voice Found. 2021, 35, 157.e7–157.e10. [Google Scholar] [CrossRef] [PubMed]
- Brauer, D.L.; Tse, K.Y.; Lin, J.C.; Schatz, M.X.; Simon, R.A. The Utility of the Reflux Symptom Index for Diagnosis of Laryngopharyngeal Reflux in an Allergy Patient Population. J. Allergy Clin. Immunol. Pract. 2018, 6, 132–138.e1. [Google Scholar] [CrossRef]
- Fritz, M.A.; Persky, M.J.; Fang, Y.; Simpson, C.B.; Amin, M.R.; Akst, L.M.; Postma, G.N. The Accuracy of the Laryngopharyngeal Reflux Diagnosis: Utility of the Stroboscopic Exam. Otolaryngol.--Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2016, 155, 629–634. [Google Scholar] [CrossRef]
- Mosli, M.; Alkhathlan, B.; Abumohssin, A.; Merdad, M.; Alherabi, A.; Marglani, O.; Jawa, H.; Alkhatib, T.; Marzouki, H.Z. Prevalence and Clinical Predictors of LPR among Patients Diagnosed with GERD According to the Reflux Symptom Index Questionnaire. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2018, 24, 236–241. [Google Scholar] [CrossRef]
- Pokharel, M.; Shrestha, I.; Dhakal, A.; Purbey, B.; Amatya, R.C. Reflux Symptom Index and Reflux Finding Score in Diagnosis of Laryngopharyngeal Reflux. Kathmandu Univ. Med. J. KUMJ 2016, 14, 332–336. [Google Scholar] [PubMed]
- Agrawal, N.; Yadlapati, R.; Shabeeb, N.; Price, C.P.; Lidder, A.; Shintani-Smith, S.; Bové, M.; Pandolfino, J.; Tan, B. Relationship between Extralaryngeal Endoscopic Findings, Proton Pump Inhibitor (PPI) Response, and PH Measures in Suspected Laryngopharyngeal Reflux. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2019, 32, doy072. [Google Scholar] [CrossRef] [PubMed]
- Mesallam, T.A.; Malki, K.H.; Farahat, M.; Bukhari, M.; Alharethy, S. Voice Problems among Laryngopharyngeal Reflux Patients Diagnosed with Oropharyngeal PH Monitoring. Folia Phoniatr. Logop. Off. Organ Int. Assoc. Logop. Phoniatr. IALP 2013, 65, 280–287. [Google Scholar] [CrossRef]
- Maldhure, S.; Chandrasekharan, R.; Dutta, A.-K.; Chacko, A.; Kurien, M. Role of PH Monitoring in Laryngopharyngeal Reflux Patients with Voice Disorders. Iran. J. Otorhinolaryngol. 2016, 28, 377–383. [Google Scholar]
- Habermann, W.; Schmid, C.; Neumann, K.; Devaney, T.; Hammer, H.F. Reflux Symptom Index and Reflux Finding Score in Otolaryngologic Practice. J. Voice Off. J. Voice Found. 2012, 26, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- Reichel, O.; Issing, W.J. Impact of Different PH Thresholds for 24-Hour Dual Probe PH Monitoring in Patients with Suspected Laryngopharyngeal Reflux. J. Laryngol. Otol. 2008, 122, 485–489. [Google Scholar] [CrossRef]
- Vailati, C.; Mazzoleni, G.; Bondi, S.; Bussi, M.; Testoni, P.A.; Passaretti, S. Oropharyngeal PH Monitoring for Laryngopharyngeal Reflux: Is It a Reliable Test before Therapy? J. Voice Off. J. Voice Found. 2013, 27, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, C.; Ogüt, F.; Kesimli-Dinç, H.; Kirazli, T.; Bor, S. The Comparison of an Empiric Proton Pump Inhibitor Trial vs 24-Hour Double-Probe Ph Monitoring in Laryngopharyngeal Reflux. J. Laryngol. Otol. 2003, 117, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.W.; Vaezi, M.F. Laryngopharyngeal Reflux: More Questions than Answers. Cleve. Clin. J. Med. 2010, 77, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kelchner, L.N.; Horne, J.; Lee, L.; Klaben, B.; Stemple, J.C.; Adam, S.; Kereiakes, T.; Levin, L. Reliability of Speech-Language Pathologist and Otolaryngologist Ratings of Laryngeal Signs of Reflux in an Asymptomatic Population Using the Reflux Finding Score. J. Voice Off. J. Voice Found. 2007, 21, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Choi, S.M.; Kwon, S.U.K.; Yoon, S.W.; Kim, S.U.K. Diagnosis of Laryngopharyngeal Reflux among Globus Patients. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2006, 134, 81–85. [Google Scholar] [CrossRef]
- Musser, J.; Kelchner, L.; Neils-Strunjas, J.; Montrose, M. A Comparison of Rating Scales Used in the Diagnosis of Extraesophageal Reflux. J. Voice Off. J. Voice Found. 2011, 25, 293–300. [Google Scholar] [CrossRef]
- Friedman, M.; Hamilton, C.; Samuelson, C.G.; Kelley, K.; Taylor, R.; Darling, R.; Taylor, D.; Fisher, M.; Maley, A. The Value of Routine PH Monitoring in the Diagnosis and Treatment of Laryngopharyngeal Reflux. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2012, 146, 952–958. [Google Scholar] [CrossRef]
- Sousa, V.D.; Rojjanasrirat, W. Translation, Adaptation and Validation of Instruments or Scales for Use in Cross-Cultural Health Care Research: A Clear and User-Friendly Guideline. J. Eval. Clin. Pract. 2011, 17, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Canino, G.; Bravo, M. The Translation and Adaptation of Diagnostic Instruments for Cross-Cultural Use. In Diagnostic Assessment in Child and Adolescent Psychopathology; The Guilford Press: New York, NY, USA, 1999; pp. 285–298. ISBN 978-1-57230-502-1. [Google Scholar]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Jacobson, B.; Grywalski, C.; Silbergleit, A.; Jacobson, G.; Benninger, M. The Voice Handicap Index (VHI): Development and Validation. Am. J. Speech Lang. Pathol. 1997, 6, 66–70. [Google Scholar]
- Domeracka-Kołodziej, A.; Grabczak, E.; Dabrowska, M.; Lachowska, M.; Osuch-Wójcikiewicz, E. Skala Warszawska A–E Oceny Endoskopowych Zmian w Krtani Związanych z Refluksem Gardłowo-Krtaniowym. Korelacja Ze Skalą Patologii Refluksowej (RFS). Otolaryngol. Pol. 2013, 68, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, R.T.; Hawkshaw, M.J.; Gupta, R. Laryngopharyngeal Reflux and Voice Disorders: An Overview on Disease Mechanisms, Treatments, and Research Advances. Discov. Med. 2010, 10, 213–224. [Google Scholar] [PubMed]

| Item Number | Item in English RSI | Item in Polish PL-RSI | Scale |
|---|---|---|---|
| 1 | Hoarseness or a problem with your voice | Chrypka lub problemy głosowe | 0–5 |
| 2 | Clearing your throat | Chrząkanie | 0–5 |
| 3 | Excess throat mucus or postnasal drip | Nadmierna ilość wydzieliny w gardle lub spływania wydzieliny po tylnej ścianie gardła | 0–5 |
| 4 | Difficulty swallowing food, liquids, or pills | Zaburzenia połykania | 0–5 |
| 5 | Coughing after you eat or after lying down | Kaszel po jedzeniu lub położeniu się | 0–5 |
| 6 | Breathing difficulties or choking episodes | Zaburzenia oddychania | 0–5 |
| 7 | Troublesome or annoying cough | Męczący kaszel | 0–5 |
| 8 | Sensation of something sticking to your throat or a lump in your throat | Uczucie przeszkody lub ciasnoty w gardle | 0–5 |
| 9 | Heartburn, chest pain, indigestion, or stomach acid coming up | Pieczenie/ból w klatce piersiowej, niestrawność, uczucie cofania się treści żołądkowej | 0–5 |
| Total RSI | 0–45 | ||
| Item Number | Item in English RFS | Item in Polish RFS | Scores |
|---|---|---|---|
| 1 | Pseudosulcus (infraglottic edema) | Obrzęk okolicy podgłośniowej | 0, 2 |
| 2 | Ventricular obliteration | Obliteracja kieszonki krtaniowej | 0, 2, 4 |
| 3 | Erythema and hyperemia | Rumień/przekrwienie | 0, 2, 4 |
| 4 | Vocal fold edema | Obrzęk fałdów głosowych | 0–4 |
| 5 | Diffuse laryngeal edema | Rozległy obrzęk krtani | 0–4 |
| 6 | Posterior commissure hypertrophy | Przerost spoidła tylnego | 0–4 |
| 7 | Granuloma/granulation tissue | Ziarniniak/ziarnina | 0, 2 |
| 8 | Thick endolaryngeal mucus | Gęsty wydzielina śluzowa w okolicy zanalewkowej i/lub zachyłków gruszkowatych | 0, 2 |
| Total RSI | 0–26 | ||
| All | Female | Male | ||
|---|---|---|---|---|
| N | 100 (100%) | 65 (65%) | 35 (35%) | |
| Age | Mean: 49.55 (SD: 13.84) | Mean: 49.23 (SD: 13.14) | Mean: 50.14 (SD: 15.22) | |
| RSI | TEST | Mean: 20.2 (SD: 8.5) | Mean: 20.55 (SD: 8.41) | Mean: 19.54 (SD: 8.75) |
| RE-TEST | Mean: 20.02 (SD: 9.13) | Mean: 20.66 (SD: 9.04) | Mean: 18.83 (SD: 9.3) | |
| RFS (L1) | TEST | Mean: 6.62 (SD: 2.8) | Mean: 6.66 (SD: 2.53) | Mean: 6.54 (SD: 3.28) |
| RE-TEST | Mean: 6.7 (SD: 3) | Mean: 6.8 (SD: 2.82) | Mean: 6.51 (SD: 3.33) | |
| RFS (L2) | TEST | Mean: 6.51 (SD: 2.82) | Mean: 6.52 (SD: 2.78) | Mean: 6.49 (SD: 2.93) |
| RE-TEST | Mean: 6.62 (SD: 2.67) | Mean: 6.65 (SD: 2.62) | Mean: 6.57 (SD: 2.8) | |
| VHI | overall | Mean: 20.49 (SD: 20.29) | Mean: 22.48 (SD: 21.71) | Mean: 16.8 (SD: 17.04) |
| functional | Mean: 5.28 (SD: 6.65) | Mean: 5.55 (SD: 7.15) | Mean: 4.77 (SD: 5.67) | |
| emotional | Mean: 9.65 (SD: 9.4) | Mean: 10.92 (SD: 9.83) | Mean: 7.29 (SD: 8.17) | |
| physical | Mean: 5.56 (SD: 6.35) | Mean: 6 (SD: 6.79) | Mean: 4.74 (SD: 5.42) | |
| Symptom perception | strength | Mean: 3.37 (SD: 0.98) | Mean: 3.35 (SD: 1.01) | Mean: 3.4 (SD: 0.95) |
| frequency | Mean: 3.26 (SD: 1.08) | Mean: 3.15 (SD: 1.09) | Mean: 3.46 (SD: 1.04) | |
| Ryan score | upright | Mean: 16.9 (SD: 35.58) | Mean: 21.48 (SD: 42.69) | Mean: 8.39 (SD: 11.93) |
| supine | Mean: 1.21 (SD: 4.84) | Mean: 1.08 (SD: 3.98) | Mean: 1.43 (SD: 6.19) | |
| LPR status | LPR | 43 | 30 | 13 |
| no LPR | 57 | 35 | 22 | |
| RSI Item | Cronbach Alpha (if Item Deleted) | Correlation with Total RSI | Test–Retest Reliability | ||
|---|---|---|---|---|---|
| TEST | RE-TEST | TEST | RE-TEST | ||
| Item 1 | 0.76 (SE: 0.037) | 0.83 (SE: 0.026) | 0.50 | 0.58 | 0.80 |
| Item 2 | 0.75 (SE: 0.037) | 0.82 (SE: 0.026) | 0.52 | 0.58 | 0.74 |
| Item 3 | 0.75 (SE: 0.037) | 0.83 (SE: 0.026) | 0.56 | 0.61 | 0.80 |
| Item 4 | 0.75 (SE: 0.038) | 0.82 (SE: 0.027) | 0.53 | 0.61 | 0.86 |
| Item 5 | 0.73 (SE: 0.04) | 0.8 (SE: 0.031) | 0.65 | 0.79 | 0.70 |
| Item 6 | 0.73 (SE: 0.04) | 0.81 (SE: 0.028) | 0.57 | 0.62 | 0.79 |
| Item 7 | 0.72 (SE: 0.042) | 0.81 (SE: 0.028) | 0.71 | 0.69 | 0.85 |
| Item 8 | 0.75 (SE: 0.038) | 0.82 (SE: 0.027) | 0.60 | 0.63 | 0.73 |
| Item 9 | 0.76 (SE: 0.036) | 0.82 (SE: 0.027) | 0.48 | 0.61 | 0.74 |
| Cronbach’s alpha | Test–retest reliability | ||||
| TEST | RE-TEST | ||||
| Total RSI | 0.77 (95% CI: 0.7–0.84) | 0.83 (95% CI: 0.79–0.88) | 0.83 | ||
| RFS Item | Intra-Rater Reliability | Inter-Rater Reliability | ||||
|---|---|---|---|---|---|---|
| Expert 1 | Expert 2 | |||||
| ICC | p-Value | ICC | p-Value | ICC | p-Value | |
| Item 1 | 1.00 | 0.00 × 100 | 0.84 | 1.50 × 10−18 | 0.57 | 1.93 × 10−5 |
| Item 2 | 0.76 | 6.10 × 10−20 | 0.81 | 1.80 × 10−16 | 0.66 | 1.00 × 10−7 |
| Item 3 | 0.83 | 1.80 × 10−26 | 0.85 | 5.70 × 10−19 | 0.78 | 5.78 × 10−13 |
| Item 4 | 0.89 | 8.00 × 10−35 | 0.80 | 9.20 × 10−16 | 0.84 | 2.40 × 10−18 |
| Item 5 | 0.89 | 1.30 × 10−35 | 0.84 | 2.50 × 10−18 | 0.83 | 1.03 × 10−16 |
| Item 6 | 0.92 | 3.10 × 10−42 | 0.86 | 2.80 × 10−20 | 0.83 | 3.51 × 10−17 |
| Item 7 | NA | NA | NA | NA | NA | NA |
| Item 8 | 0.83 | 3.50 × 10−26 | 0.69 | 8.30 × 10−11 | 0.84 | 1.11 × 10−17 |
| RFS Total | 0.91 | 1.38 × 10−38 | 0.84 | 8.52 × 10−19 | 0.88 | 9.47 × 10−23 |
| PL-RSI | PL-RFS | ||||
|---|---|---|---|---|---|
| r | p-Value | r | p-Value | ||
| PL-RSI | 1.00 | 0.00 | 0.15 | 0.14 | |
| PL-RFS | 0.15 | 0.14 | 1.00 | 0.00 | |
| VHI | overall | 0.40 | 4.00 × 10−5 | 0.11 | 0.26 |
| functional | 0.34 | 5.80 × 10−4 | 0.16 | 0.11 | |
| physical | 0.39 | 6.60 × 10−5 | 0.13 | 0.19 | |
| emotional | 0.36 | 2.30 × 10−4 | 0.09 | 0.39 | |
| Symptom perception | strength | 0.54 | 8.40 × 10−9 | 0.20 | 0.05 |
| frequency | 0.33 | 8.80 × 10−4 | 0.21 | 0.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, E.; Jetka, T.; Miaśkiewicz, B.; Skarzynski, P.H.; Skarzynski, H. Validation and Reliability of Polish Version of the Reflux Symptoms Index and Reflux Finding Score. Healthcare 2022, 10, 1411. https://doi.org/10.3390/healthcare10081411
Włodarczyk E, Jetka T, Miaśkiewicz B, Skarzynski PH, Skarzynski H. Validation and Reliability of Polish Version of the Reflux Symptoms Index and Reflux Finding Score. Healthcare. 2022; 10(8):1411. https://doi.org/10.3390/healthcare10081411
Chicago/Turabian StyleWłodarczyk, Elżbieta, Tomasz Jetka, Beata Miaśkiewicz, Piotr Henryk Skarzynski, and Henryk Skarzynski. 2022. "Validation and Reliability of Polish Version of the Reflux Symptoms Index and Reflux Finding Score" Healthcare 10, no. 8: 1411. https://doi.org/10.3390/healthcare10081411
APA StyleWłodarczyk, E., Jetka, T., Miaśkiewicz, B., Skarzynski, P. H., & Skarzynski, H. (2022). Validation and Reliability of Polish Version of the Reflux Symptoms Index and Reflux Finding Score. Healthcare, 10(8), 1411. https://doi.org/10.3390/healthcare10081411








