New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix—Case Report and Review of Literature
Abstract
:1. Introduction
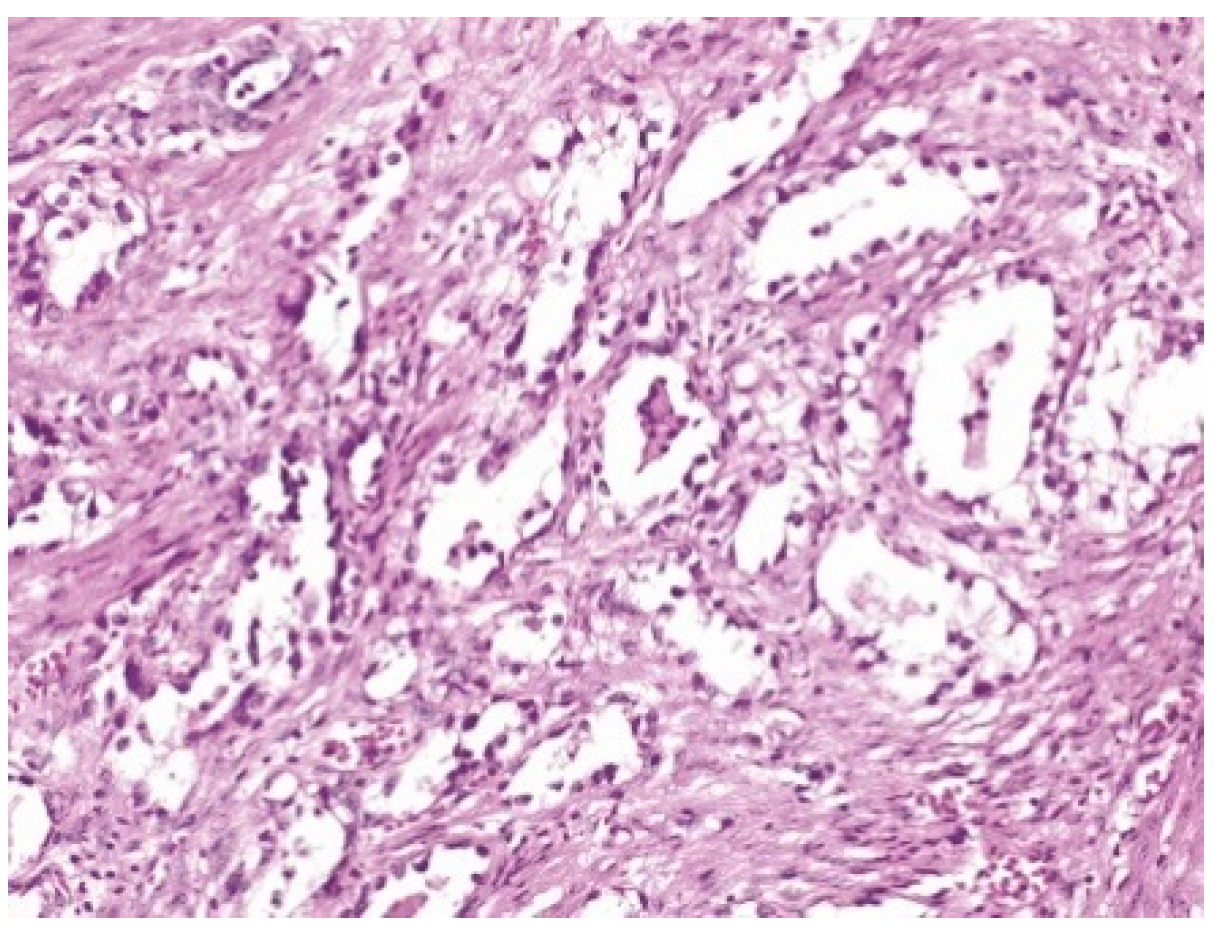
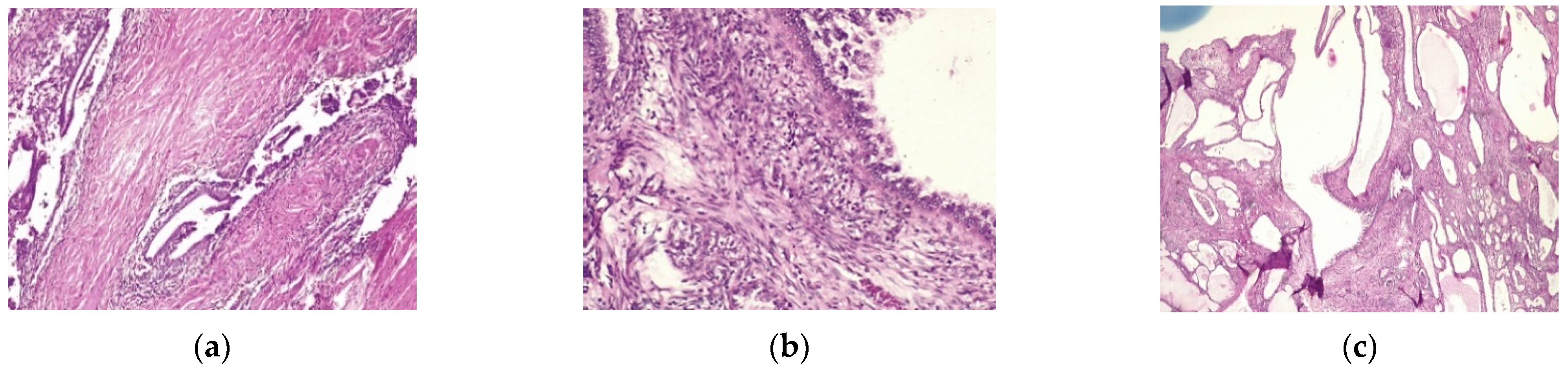
2. Case Report
3. Discussions and Literature Review
3.1. Definitions
3.2. Presentation
3.3. Incidence
3.4. Age
3.5. Cervical Cytology
3.6. HPV Genotyping
3.7. Pathogenesis
3.8. Pathology
3.9. Immunohistochemical Study
3.10. Prognosis
3.11. Minimally Invasive or Open Surgery Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; Collas De Souza, S.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Castro Sánchez, M.; et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar]
- Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. ObGyn. 2018, 10, 107–113. [Google Scholar]
- Limaiem, F.; Mahdy, H. Cervical Clear Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546591/ (accessed on 12 March 2022).
- Tantitamit, T.; Hamontri, S.; Rangsiratanakul, L. Clear cell adenocarcinoma of the cervix in second generation young women who are without maternal exposure to diethylstilbestrol: A case report. Gynecol. Oncol. Rep. 2017, 20, 34–36. [Google Scholar]
- Sawaya, G.F.; Kuppermann, M. Identifying a “range of reasonable options” for cervical cancer screening. Obstet. Gynecol. 2015, 125, 308–310. [Google Scholar]
- Houghton, O.; Jamison, J.; Wilson, R.; Carson, J.; McCluggage, W.G. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology 2010, 57, 342–350. [Google Scholar]
- Glasbey, J.C.; Nepogodiev, D.; Omar, O.; Simoes, J.F.F.; Ademuyiwa, A.; Fiore, M. COVIDSurg Collaborative. Delaying surgery for patients with a previous SARS-CoV-2 infection. Br. J. Surg. 2020, 107, e601–e602. [Google Scholar]
- Goyal, A.; Yang, B. Differential patterns of PAX8, p16, and ER immunostains in mesonephric lesions and adenocarcinomas of the cervix. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2014, 33, 613–619. [Google Scholar]
- Stolnicu, S.; Park, K.J.; Kiyokawa, T.; Oliva, E.; McCluggage, W.G.; Soslow, R.A. Tumor Typing of Endocervical Adenocarcinoma: Contemporary Review and Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2021, 40 (Suppl. S1), 75–91. [Google Scholar]
- Moch, H. IAFROC-I. In Female Genital Tumours: WHO Classification of Tumours, 5th ed.; WHO: Geneva, Switzerland, 2020; Volume 4, pp. 407–414. [Google Scholar]
- PathologyOutlines.com. Available online: https://www.pathologyoutlines.com/topic/cervixWHO.html.website (accessed on 24 February 2022).
- Montalvo, N.; Redrobán, L.; Galarza, D. Mesonephric adenocarcinoma of the cervix: A case report with a three-year follow-up, lung metastases, and next-generation sequencing analysis. Diagn Pathol. 2019, 14, 71. [Google Scholar]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Chiriboga, L.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; et al. Diagnostic Algorithmic Proposal Based on Comprehensive Immunohistochemical Evaluation of 297 Invasive Endocervical Adenocarcinomas. Am. J. Surg. Pathol. 2018, 42, 989–1000. [Google Scholar]
- Dierickx, A.; Göker, M.; Braems, G.; Tummers, P.; Van den Broecke, R. Mesonephric adenocarcinoma of the cervix: Case report and literature review. Gynecol. Oncol. Rep. 2016, 17, 7–11. [Google Scholar]
- Park, K.J.; Kiyokawa, T.; Soslow, R.A.; Lamb, C.A.; Oliva, E.; Zivanovic, O.; Juretzka, M.M.; Pirog, E.C. Unusual endocervical adenocarcinomas: An immunohistochemical analysis with molecular detection of human papillomavirus. Am. J. Surg. Pathol. 2011, 35, 633–646. [Google Scholar]
- Silver, S.A.; Devouassoux-Shisheboran, M.; Mezzetti, T.P.; Tavassoli, F.A. Mesonephric adenocarcinomas of the uterine cervix: A study of 11 cases with immunohistochemical findings. Am. J. Surg. Pathol. 2001, 25, 379–387. [Google Scholar]
- Bergström, R.; Sparén, P.; Adami, H.O. Trends in cancer of the cervix uteri in Sweden following cytological screening. Br. J. Cancer 1999, 81, 159–166. [Google Scholar]
- Smith, H.O.; Tiffany, M.F.; Qualls, C.R.; Key, C.R. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—A 24-year population-based study. Gynecol. Oncol. 2000, 78, 97–105. [Google Scholar]
- Zappa, M.; Visioli, C.B.; Ciatto, S.; Iossa, A.; Paci, E.; Sasieni, P. Lower protection of cytological screening for adenocarcinomas and shorter protection for younger women: The results of a case-control study in Florence. Br. J. Cancer 2004, 90, 1784–1786. [Google Scholar]
- Miller, B.E.; Flax, S.D.; Arheart, K.; Photopulos, G. The presentation of adenocarcinoma of the uterine cervix. Cancer 1993, 72, 1281–1285. [Google Scholar]
- Vizcaino, A.P.; Moreno, V.; Bosch, F.X.; Muñoz, N.; Barros-Dios, X.M.; Parkin, D.M. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int. J. Cancer 1998, 75, 536–545. [Google Scholar]
- Arraiz, G.A.; Wigle, D.T.; Mao, Y. Is cervical cancer increasing among young women in Canada? Can. J. Public Health 1990, 81, 396–397. [Google Scholar]
- Chilvers, C.; Mant, D.; Pike, M.C. Cervical adenocarcinoma and oral contraceptives. Br. Med. J. (Clin. Res. Ed.) 1987, 295, 1446–1447. [Google Scholar]
- Eide, T.J. Cancer of the uterine cervix in Norway by histologic type, 1970–1984. J. Natl. Cancer Inst. 1987, 79, 199–205. [Google Scholar]
- Hart, W.R. Symposium part II: Special types of adenocarcinoma of the uterine cervix. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2002, 21, 327–346. [Google Scholar]
- Ferry, J.A.; Scully, R.E. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am. J. Surg. Pathol. 1990, 14, 1100–1111. [Google Scholar]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar]
- Hanselaar, A.; van Loosbroek, M.; Schuurbiers, O.; Helmerhorst, T.; Bulten, J.; Bernhelm, J. Clear cell adenocarcinoma of the vagina and cervix. An update of the central Netherlands registry showing twin age incidence peaks. Cancer 1997, 79, 2229–2236. [Google Scholar]
- Pirog, E.C.; Kleter, B.; Olgac, S.; Bobkiewicz, P.; Lindeman, J.; Quint, W.G.; Richart, R.M.; Isacson, C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am. J. Pathol. 2000, 157, 1055–1062. [Google Scholar]
- Stolnicu, S.; Talia, K.L.; McCluggage, W.G. The Evolving Spectrum of Precursor Lesions of Cervical Adenocarcinomas. Adv. Anat. Pathol. 2020, 27, 278–293. [Google Scholar]
- Nishio, S.; Mikami, Y.; Tokunaga, H.; Yaegashi, N.; Satoh, T.; Saito, M.; Okamoto, A.; Kasamatsu, T.; Miyamoto, T.; Shiozawa, T.; et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix—An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol. Oncol. 2019, 153, 13–19. [Google Scholar]
- McCluggage, W.G. Recent Developments in Non-HPV-related Adenocarcinomas of the Lower Female Genital Tract and Their Precursors. Adv. Anat. Pathol. 2016, 23, 58–69. [Google Scholar]
- Talia, K.L.; McCluggage, W.G. The developing spectrum of gastric-type cervical glandular lesions. Pathology 2018, 50, 122–133. [Google Scholar]
- Mikami, Y.; McCluggage, W.G. Endocervical glandular lesions exhibiting gastric differentiation: An emerging spectrum of benign, premalignant, and malignant lesions. Adv. Anat. Pathol. 2013, 20, 227–237. [Google Scholar]
- McCluggage, W.G. Progress in the pathological arena of gynecological cancers. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2021, 155, 107–114. [Google Scholar]
- Nomoto, K.; Hayashi, S.; Tsuneyama, K.; Hori, T.; Ishizawa, S. Cytopathology of cervical mesonephric adenocarcinoma: A report of two cases. Cytopathology 2013, 24, 129–131. [Google Scholar]
- Anagnostopoulos, A.; Ruthven, S.; Kingston, R. Mesonephric adenocarcinoma of the uterine cervix and literature review. BMJ Case Rep. 2012, 2012, bcr0120125632. [Google Scholar]
- Stolnicu, S.; Hoang, L.; Soslow, R.A. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019, 475, 537–549. [Google Scholar]
- Wang, D.; Zhao, C.; Fu, L.; Liu, Y.; Zhang, W.; Xu, T. Primary Clear Cell Adenocarcinoma of the Cervix: A Clinical Analysis of 18 Cases without Exposure to Diethylstilbestrol. Obstet. Gynecol. Int. 2019, 2019, 9465375. [Google Scholar]
- Zhu, Y.; Ren, C.; Yang, L.; Zhang, X.; Liu, L.; Wang, Z. Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC Cancer 2019, 19, 271. [Google Scholar]
- Hiromura, T.; Tanaka, Y.O.; Nishioka, T.; Satoh, M.; Tomita, K. Clear cell adenocarcinoma of the uterine cervix arising from a background of cervical endometriosis. Br. J. Radiol. 2009, 82, e20–e22. [Google Scholar]
- Nili, F.; Salarvand, S.; Saffar, H.; Kalaghchi, B.; Ghalehtaki, R. Mesonephric Adenocarcinoma of Uterine Cervix: A Case Report and Review of the Literature. Iran. J. Pathol. 2021, 16, 227–231. [Google Scholar]
- Menon, S.; Kathuria, K.; Deodhar, K.; Kerkar, R. Mesonephric adenocarcinoma (endometrioid type) of endocervix with diffuse mesonephric hyperplasia involving cervical wall and myometrium: An unusual case report. Indian J. Pathol. Microbiol. 2013, 56, 51–53. [Google Scholar]
- Tan, Y.T.; Zhang, X.; Lin, Z.Q.; Chen, Q.; Wang, L.J.; Zhang, B.Z. Primary clear cell carcinoma of the cervix: Report of five cases and review of the literature. Zhonghua Fu Chan Ke Za Zhi 2008, 43, 120–123. [Google Scholar]
- Clement, P.B.; Young, R.H.; Keh, P.; Ostör, A.G.; Scully, R.E. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am. J. Surg. Pathol. 1995, 19, 1158–1171. [Google Scholar]
- Lang, G.; Dallenbach-Hellweg, G. The histogenetic origin of cervical mesonephric hyperplasia and mesonephric adenocarcinoma of the uterine cervix studied with immunohistochemical methods. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1990, 9, 145–157. [Google Scholar]
- Fregnani, J.H.T.G.; Soares, F.A.; Novik, P.R.; Lopes, A.; Latorre, M.R.D.O. Comparison of biological behavior between early-stage adenocarcinoma and squamous cell carcinoma of the uterine cervix. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 215–223. [Google Scholar]
- Mukonoweshuro, P.; McCluggage, W.G. Clear Cell Carcinoma of the Cervix with Choriocarcinomatous Differentiation: Report of an Extremely Rare Phenomenon associated with Mismatch Repair Protein Abnormality. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2017, 36, 323–327. [Google Scholar]
- Liang, J.; Mittal, K.R.; Wei, J.J.; Yee, H.; Chiriboga, L.; Shukla, P. Utility of p16INK4a, CEA, Ki67, P53 and ER/PR in the differential diagnosis of benign, premalignant, and malignant glandular lesions of the uterine cervix and their relationship with Silverberg scoring system for endocervical glandular lesions. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2007, 26, 71–75. [Google Scholar]
- Chen, C.C.; Huang, L.W.; Bai, C.H.; Lee, C.C. Predictive value of p16/Ki-67 immunocytochemistry for triage of women with abnormal Papanicolaou test in cervical cancer screening: A systematic review and meta-analysis. Ann. Saudi Med. 2016, 36, 245–251. [Google Scholar]
- Volkova, L.V.; Pashov, A.I.; Omelchuk, N.N. Cervical Carcinoma: Oncobiology and Biomarkers. Int. J. Mol. Sci. 2021, 22, 12571. [Google Scholar]
- Thomas, M.B.; Wright, J.D.; Leiser, A.L.; Chi, D.S.; Mutch, D.G.; Podratz, K.C.; Dowdy, S.C. Clear cell carcinoma of the cervix: A multi-institutional review in the post-DES era. Gynecol. Oncol. 2008, 109, 335–339. [Google Scholar]
- Singh, P.; Nicklin, J.; Hassall, T. Neoadjuvant chemotherapy followed by radical vaginal trachelectomy and adjuvant chemotherapy for clear cell cancer of the cervix: A feasible approach and review. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 137–140. [Google Scholar]
- Jiang, X.; Jin, Y.; Li, Y.; Huang, H.F.; Wu, M.; Shen, K.; Pan, L.Y. Clear cell carcinoma of the uterine cervix: Clinical characteristics and feasibility of fertility-preserving treatment. Onco Targets Ther. 2014, 7, 111–116. [Google Scholar]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar]
- Kim, J.H.; Kim, K.; Park, S.J.; Lee, J.Y.; Kim, K.; Lim, M.C.; Kim, J.W. Comparative Effectiveness of Abdominal versus Laparoscopic Radical Hysterectomy for Cervical Cancer in the Postdissemination Era. Cancer Res. Treat. 2019, 51, 788–796. [Google Scholar]
- Mendivil, A.A.; Rettenmaier, M.A.; Abaid, L.N.; Brown, J.V., III; Micha, J.P.; Lopez, K.L.; Goldstein, B.H. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: A five year experience. Surg. Oncol. 2016, 25, 66–71. [Google Scholar]
- Gil-Moreno, A.; Carbonell-Socias, M.; Salicrú, S.; Centeno-Mediavilla, C.; Franco-Camps, S.; Colas, E.; Oaknin, A.; Pérez-Benavente, A.; Díaz-Feijoo, B. Radical Hysterectomy: Efficacy and Safety in the Dawn of Minimally Invasive Techniques. J. Minim. Invasive Gynecol. 2019, 26, 492–500. [Google Scholar]
- Corrado, G.; Vizza, E.; Legge, F.; Pedone Anchora, L.; Sperduti, I.; Fagotti, A.; Mancini, E.; Gallotta, V.; Zampa, A.; Chiofalo, B.; et al. Comparison of Different Surgical Approaches for Stage IB1 Cervical Cancer Patients: A Multi-institution Study and a Review of the Literature. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 1020–1028. [Google Scholar]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 1269–1277. [Google Scholar]
- Chacon, E.; Manzour, N.; Zanagnolo, V.; Querleu, D.; Núñez-Córdoba, J.M.; Martin-Calvo, N.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; et al. SUCCOR cone study: Conization before radical hysterectomy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2022, 32, 117–124. [Google Scholar]
- Yu, L.; Fei, L.; Liu, X.; Pi, X.; Wang, L.; Chen, S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J. Cancer 2019, 10, 2654–2660. [Google Scholar]
- Ravarino, A.; Nemolato, S.; Macciocu, E.; Fraschini, M.; Senes, G.; Faa, G.; Negri, G. CINtec PLUS immunocytochemistry as a tool for the cytologic diagnosis of glandular lesions of the cervix uteri. Am. J. Clin. Pathol. 2012, 138, 652–656. [Google Scholar]
- Nucci, M.R. Symposium part III: Tumor-like glandular lesions of the uterine cervix. Int. J. Gynecol. Pathol. 2002, 21, 347–359. [Google Scholar]


| ER | PR | CD10 | CK7 | CK20 | mCEA | Inhibin | |
|---|---|---|---|---|---|---|---|
| First laboratory | neg | neg | neg | - | - | neg | focal pos |
| Second laboratory | neg | - | focal pos | pos | neg | - | - |
| Third laboratory | neg | neg | neg | - | - | - | - |
| Mesonephric adenocarcinoma | neg | neg | pos | pos | - | neg | variably pos |
| Mesonephric hyperplasia | neg | neg | pos | - | - | - | - |
| Clear-cell carcinoma | neg | neg | pos | neg | |||
| TTF1 | p53 | GATA3 | Ki-67 | p16 | PAX8 | PAX2 | |
| First laboratory | neg | focal pos | intense diffuse pos | 12% | - | - | - |
| Second laboratory | - | - | - | - | - | - | - |
| Third laboratory | neg | - | neg | - | - | focal positive | - |
| Mesonephric adenocarcinoma | variably pos | neg | pos | 15–20% | - | pos | pos |
| Mesonephric hyperplasia | - | - | pos | less than 1% | neg | pos | - |
| Clear-cell carcinoma | - | pos | neg | - | neg/pos | - | - |
| Calretinin | Napsin A | HNF 1B | ARID1A | Racemase | KRAS/ NRAS mutation | AE1/AE3 | |
| First laboratory | - | - | - | - | - | - | - |
| Second laboratory | - | - | - | - | - | - | focal positive |
| Third laboratory | - | diffuse pos | diffusepos | retention of nuclear staining | focal positive | not found | - |
| Mesonephric adenocarcinoma | variably pos | - | - | - | - | Canonical activating KRAS and NRAS mutations not found | - |
| Mesonephric hyperplasia | 10% | - | - | - | - | not found | - |
| Clear-cell carcinoma | - | pos | pos | - | - | - | - |
| Five years prior to first consultation in our office | Cervical cytology: H-SIL LEEP proposed, patient refused; opted for electrocoagulation; another cervical cytology in the following 5 years: NILM |
| First consultation in our office | Cervical cytology: L-SIL HPV genotyping: negative CINtec test: positive Colposcopy: severe dysplastic lesion LEEP proposed |
| LEEP | atypical mesonephric hyperplasia with a malignant transformation zone—mesonephric adeno-carcinoma with moderate cell pleomorphism, moderate mitotic activity, without invasion of the lymphovascular space, resection limits tangential to the lesion |
| Abdomino-pelvic MRI after LEEP | 2.2/1.6/1.6 cm formation with suspicion of malignancy which does not exceed the contour of the cervical wall, and no radiologically detectable pelvic lymphadenopathy. |
| Radical vaginal trachelectomy with laparoscopic pelvic lymphadenectomy | Uterine isthmus—endocervix—upper limit of resection with benign mesonephric hyperplasia with areas of atypical mesonephric hyperplasia, showing moderate atypia; |
| Cervix with previous conization –appearance of atypical mesonephric hyperplasia; zone of stromal invasion and malignant transformation—endocervical mesonephric adenocarcinoma with moderate cell pleomorphism and mitotic activity, intraluminal detritus, added inflammation | |
| Right ilioobturator lymphadenctomy specimen—eleven lymphonodules with sinus histiocytosis, lipomatosis, no tumor metastasis; left ilioouturator lymphadenctomy specimen—seven lymphonodules with sinus histiocytosis, lipomatosis, no tumor metastasis. The stage according to FIGO classification was pT1b1 N0 Mx. | |
| Abdomino-pelvic MRI performed after trachelectomy | Modification of the anatomy of the cervix in the postoperative context; area (10–12 mm) at the junction with the uterine body with appearance similar to the lesion described previously (MRI prior to trachelectomy)—tumor remains? No pelvic lymphadenopathy. Moderate fluid accumulations noted in the pouch of Douglas. |
| Final MDT decision | laparoscopic hysterectomy with vaginal cuff, left adnexectomy and transposition of right ovary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secosan, C.; Balint, O.; Ilian, A.; Balan, L.; Balulescu, L.; Motoc, A.; Zahoi, D.; Grigoras, D.; Pirtea, L. New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix—Case Report and Review of Literature. Healthcare 2022, 10, 1410. https://doi.org/10.3390/healthcare10081410
Secosan C, Balint O, Ilian A, Balan L, Balulescu L, Motoc A, Zahoi D, Grigoras D, Pirtea L. New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix—Case Report and Review of Literature. Healthcare. 2022; 10(8):1410. https://doi.org/10.3390/healthcare10081410
Chicago/Turabian StyleSecosan, Cristina, Oana Balint, Aurora Ilian, Lavinia Balan, Ligia Balulescu, Andrei Motoc, Delia Zahoi, Dorin Grigoras, and Laurentiu Pirtea. 2022. "New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix—Case Report and Review of Literature" Healthcare 10, no. 8: 1410. https://doi.org/10.3390/healthcare10081410
APA StyleSecosan, C., Balint, O., Ilian, A., Balan, L., Balulescu, L., Motoc, A., Zahoi, D., Grigoras, D., & Pirtea, L. (2022). New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix—Case Report and Review of Literature. Healthcare, 10(8), 1410. https://doi.org/10.3390/healthcare10081410








