Therapeutic Outcome of MR-Guided High-Intensity Focused Ultrasound (MR-HIFU) in Solitary versus Multiple Uterine Fibroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MR-Guided HIFU Ablation
2.3. MRI Data Acquisition and Postprocessing
2.3.1. Data Acquisition
2.3.2. MRI Postprocessing
2.4. Disease-Specific Symptom and Health-Related Quality of Life Questionnaire for Leiomyomata
2.5. Statistical Analysis
3. Results
3.1. Patients
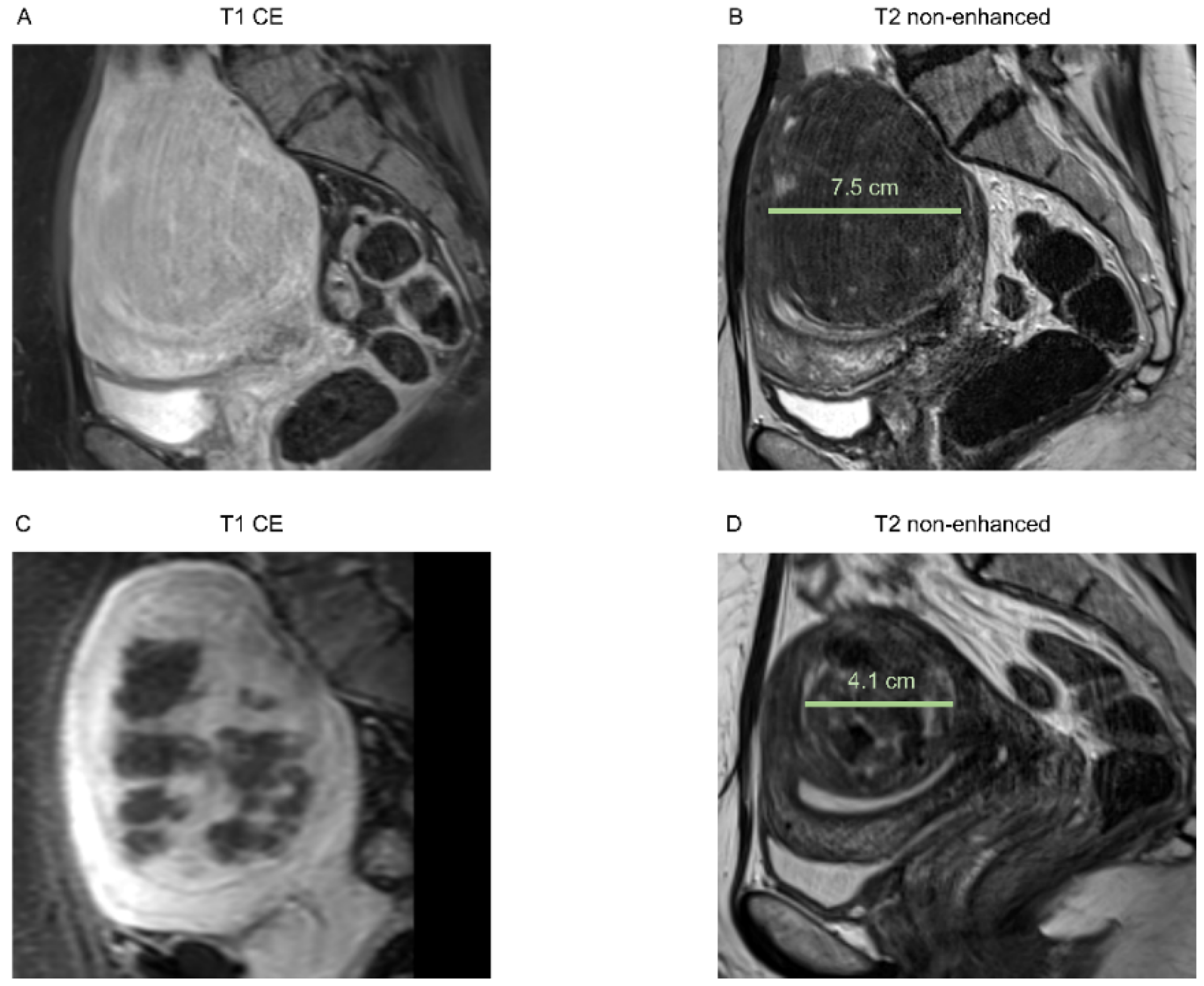
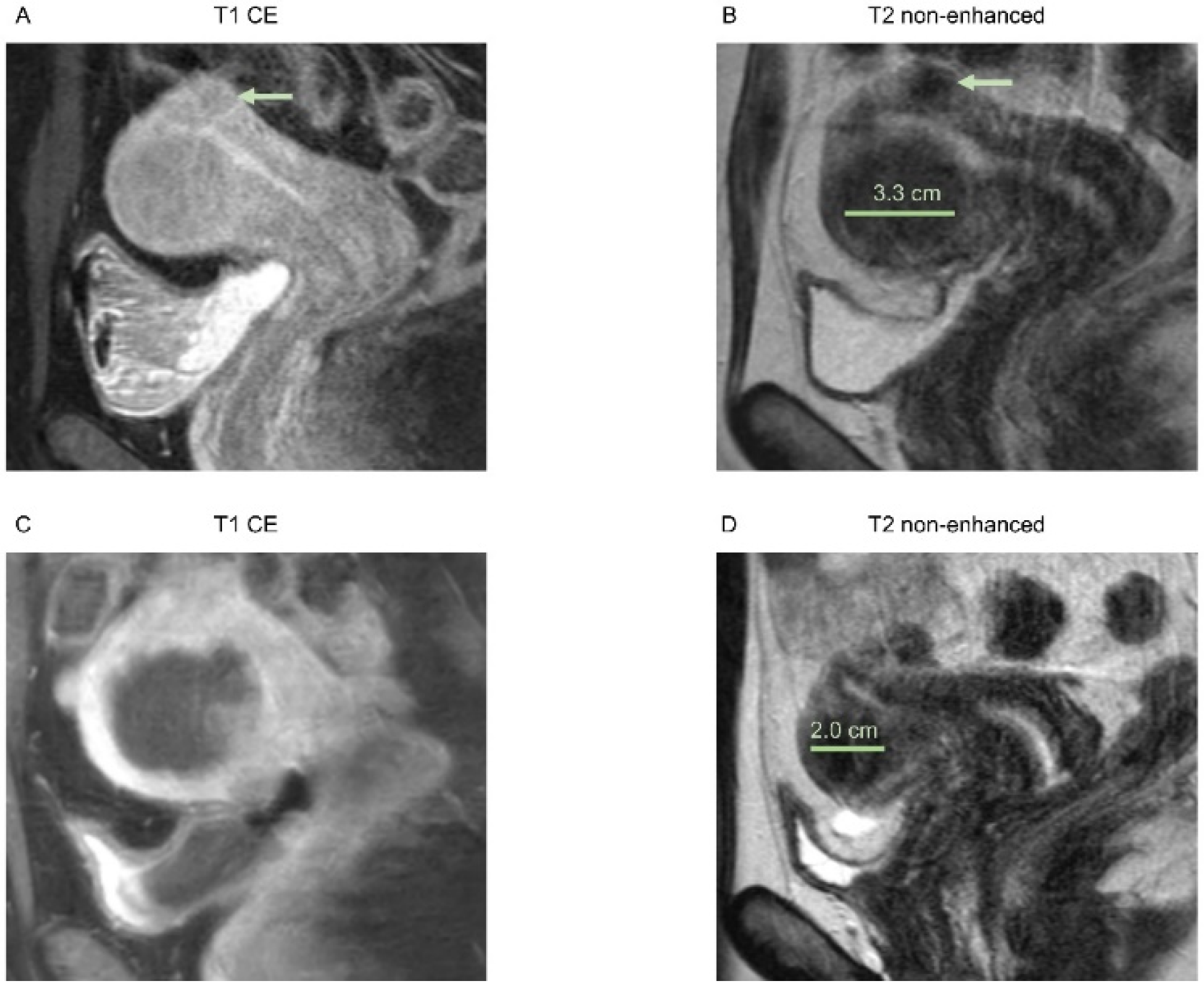
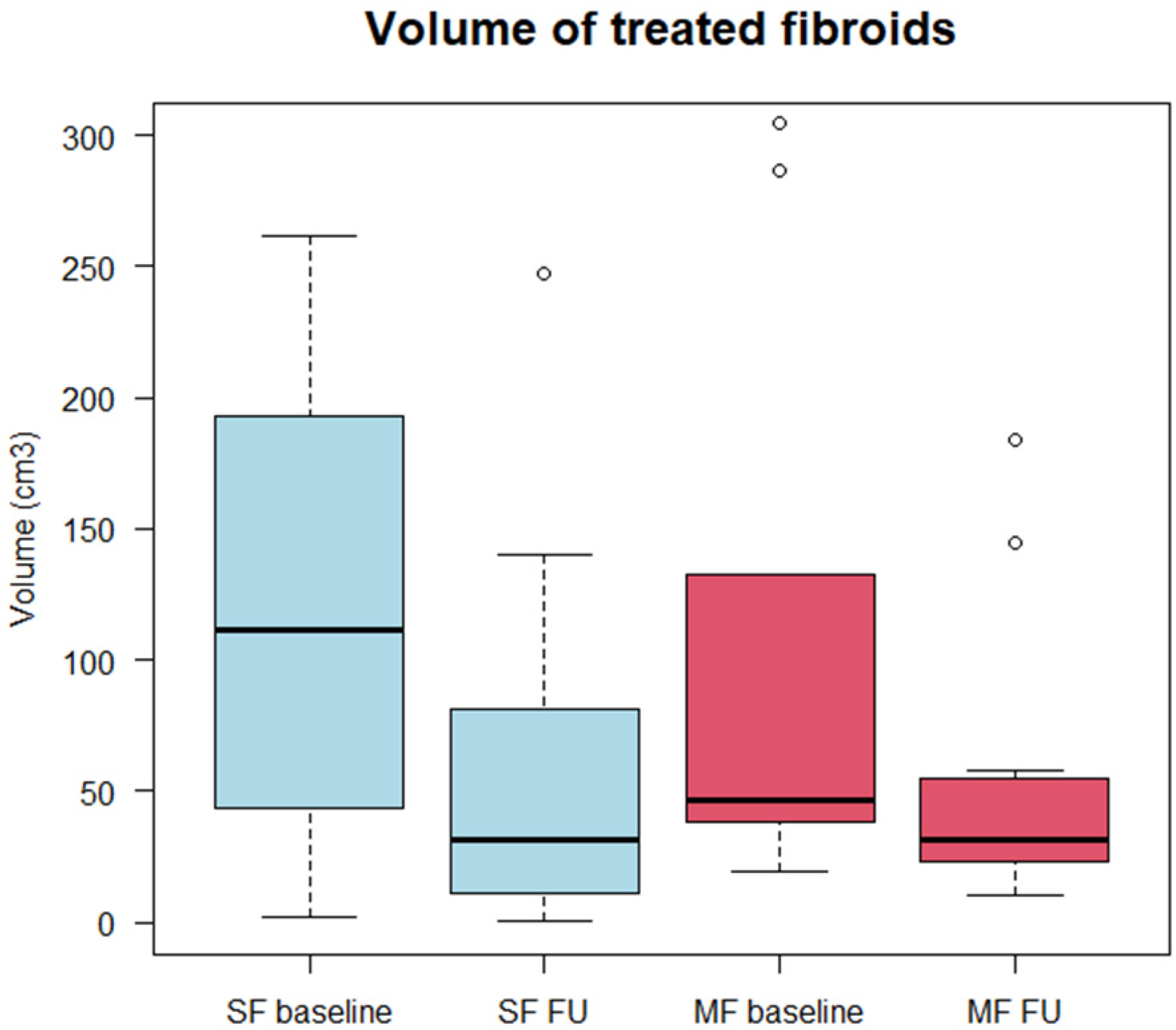
3.2. Morphological Assessment
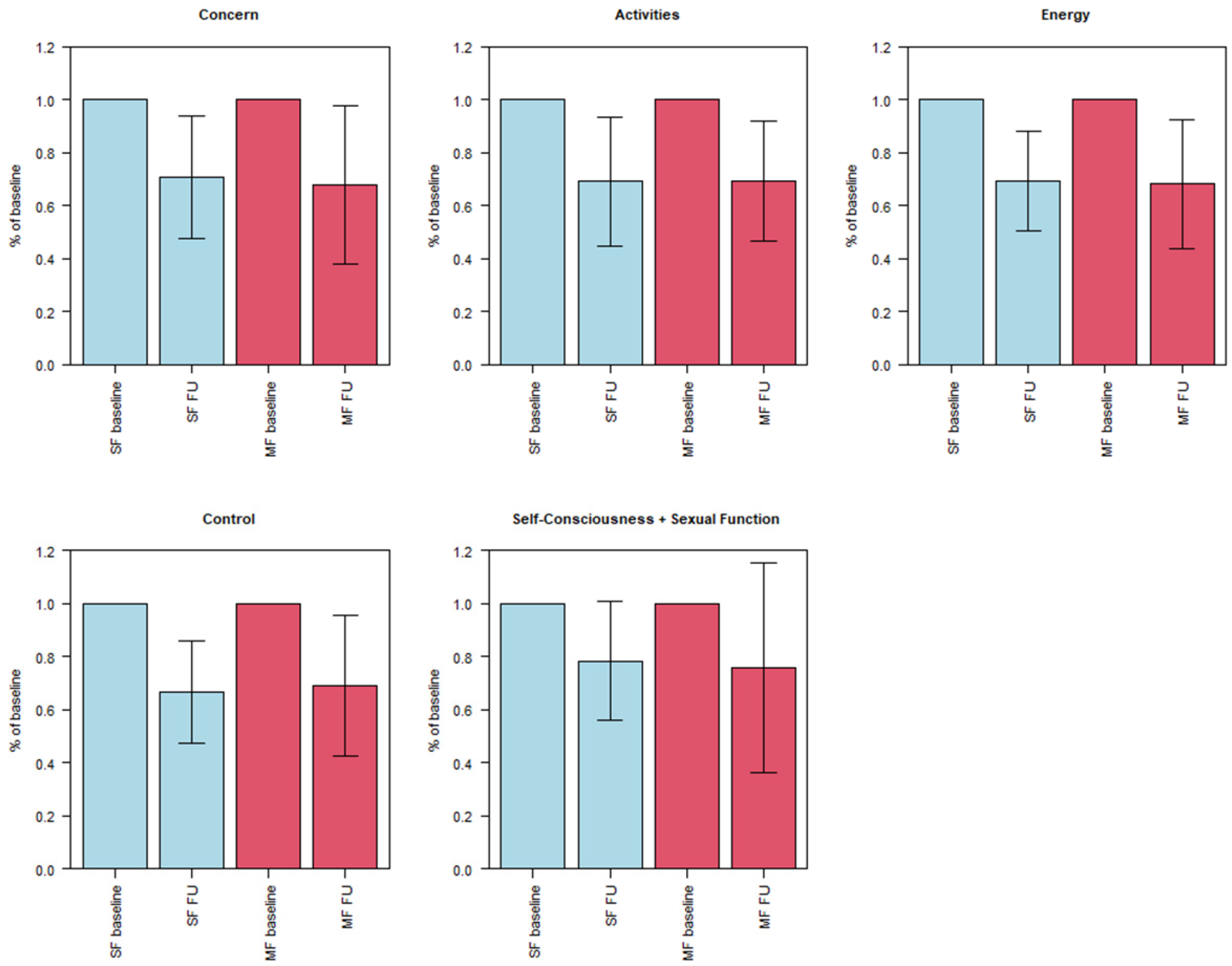
3.3. Symptom Severity (UFS-QOL)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallach, E.E.; Vlahos, N.F. Uterine myomas: An overview of development, clinical features, and management. Obstet. Gynecol. 2004, 104, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Buttram, V.C., Jr.; Reiter, R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981, 36, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.F.; Patel, A. The frequency of uterine leiomyomas. Am. J. Clin. Pathol. 1990, 94, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Farrer-Brown, G.; Beilby, J.O.; Tarbit, M.H. The vascular patterns in myomatous uteri. J. Obstet. Gynaecol. Br. Commonw. 1970, 77, 967–975. [Google Scholar] [CrossRef]
- Stewart, E.A.; Nowak, R.A. Leiomyoma-related bleeding: A classic hypothesis updated for the molecular era. Hum. Reprod. Update 1996, 2, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Mourgues, J.; Villot, A.; Thubert, T.; Fauvet, R.; Pizzoferrato, A.C. Uterine myomas and lower urinary tract dysfunctions: A literature review. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 771–774. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Lei, B. Uterine Artery Embolization Compared with High-intensity Focused Ultrasound Ablation for the Treatment of Symptomatic Uterine Myomas: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 218–227. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Luo, Y.; Wang, Y.; Song, L.; Wang, W. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: A retrospective study. BJOG—Int. J. Obstet. Gynaecol. 2020, 127, 1422–1428. [Google Scholar] [CrossRef]
- Yan, L.; Huang, H.; Lin, J.; Yu, R. High-intensity focused ultrasound treatment for symptomatic uterine fibroids: A systematic review and meta-analysis. Int. J. Hyperth. 2022, 39, 230–238. [Google Scholar] [CrossRef]
- Gao, H.; Li, T.; Fu, D.; Wei, J. Uterine artery embolization, surgery and high intensity focused ultrasound in the treatment of uterine fibroids: A network meta-analysis. Quant. Imaging Med. Surg. 2021, 11, 4125–4136. [Google Scholar] [CrossRef]
- Chen, R.; Keserci, B.; Bi, H.; Han, X.; Wang, X.; Bai, W.; Wang, Y.; Yang, X.; Yang, J.; Wei, J.; et al. The safety and effectiveness of volumetric magnetic resonance-guided high-intensity focused ultrasound treatment of symptomatic uterine fibroids: Early clinical experience in China. J. Ther. Ultrasound 2016, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.T.; Jeng, C.J.; Long, C.Y.; Chuang, L.T.; Shen, J. High-intensity focused ultrasound treatment for large and small solitary uterine fibroids. Int. J. Hyperth. 2022, 39, 485–489. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, P.H.; Zhang, R.; An, P.L. Predictive Value of Quantitative Uterine Fibroid Perfusion Parameters From Contrast-Enhanced Ultrasound for the Therapeutic Effect of High-Intensity Focused Ultrasound Ablation. J. Ultrasound Med. 2019, 38, 1511–1517. [Google Scholar] [CrossRef]
- Dorenberg, E.J.; Courivaud, F.; Ring, E.; Hald, K.; Jakobsen, J.A.; Fosse, E.; Hol, P.K. Volumetric ablation of uterine fibroids using Sonalleve high-intensity focused ultrasound in a 3 Tesla scanner—First clinical assessment. Minim. Invasive Ther. Allied Technol. 2013, 22, 73–79. [Google Scholar] [CrossRef]
- Hindley, J.; Gedroyc, W.M.; Regan, L.; Stewart, E.; Tempany, C.; Hynyen, K.; McDannold, N.; Inbar, Y.; Itzchak, Y.; Rabinovici, J.; et al. MRI guidance of focused ultrasound therapy of uterine fibroids: Early results. AJR Am. J. Roentgenol. 2004, 183, 1713–1719. [Google Scholar] [CrossRef]
- Spies, J.B.; Coyne, K.; Guaou Guaou, N.; Boyle, D.; Skyrnarz-Murphy, K.; Gonzalves, S.M. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet. Gynecol. 2002, 99, 290–300. [Google Scholar] [CrossRef]
- Taran, F.A.; Tempany, C.M.; Regan, L.; Inbar, Y.; Revel, A.; Stewart, E.A.; Group, M.R. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet. Gynecol. 2009, 34, 572–578. [Google Scholar] [CrossRef]
- Lyon, P.C.; Rai, V.; Price, N.; Shah, A.; Wu, F.; Cranston, D. Ultrasound-Guided High Intensity Focused Ultrasound Ablation for Symptomatic Uterine Fibroids: Preliminary Clinical Experience. Ultraschall Med.-Eur. J. Ultrasound 2020, 41, 550–556. [Google Scholar] [CrossRef]
- Mohr-Sasson, A.; Machtinger, R.; Mashiach, R.; Nir, O.; Inbar, Y.; Maliyanker, N.; Goldenberg, M.; Rabinovici, J. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am. J. Obstet. Gynecol. 2018, 219, 375.e371–375.e377. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 March 2021).
- Funaki, K.; Fukunishi, H.; Funaki, T.; Sawada, K.; Kaji, Y.; Maruo, T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am. J. Obstet. Gynecol. 2007, 196, 184.e181–184.e186. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Wu, A.; Gong, C.; Wang, Z.; Zhang, L. Ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids: Long-term outcomes and factors affecting local recurrence. Int. J. Hyperth. 2021, 38, 1341–1348. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, C.; Tian, J. Correlation between uterine fibroids with various magnetic resonance imaging features and therapeutic effects of high-intensity focused ultrasound ablation. Pak. J. Med. Sci. 2015, 31, 869–873. [Google Scholar] [CrossRef]
- Parker-Autry, C.; Harvie, H.; Arya, L.A.; Northington, G.M. Lower urinary tract symptoms in patients with uterine fibroids: Association with fibroid location and uterine volume. Female Pelvic Med. Reconstr. Surg. 2011, 17, 91–96. [Google Scholar] [CrossRef]
- Ekin, M.; Cengiz, H.; Ozturk, E.; Kaya, C.; Yasar, L.; Savan, K. Genitourinary symptoms and their effects on quality of life in women with uterine myomas. Int. Urogynecol. J. 2014, 25, 807–810. [Google Scholar] [CrossRef]
- Kim, H.S.; Baik, J.H.; Pham, L.D.; Jacobs, M.A. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: Long-term outcomes. Acad. Radiol. 2011, 18, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Keung, J.J.; Spies, J.B.; Caridi, T.M. Uterine artery embolization: A review of current concepts. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 66–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erber, B.; Schwarze, V.; Strobl, F.; Burges, A.; Mahner, S.; Goller, S.S.; Rudolph, J.; Ricke, J.; Sabel, B.O. Therapeutic Outcome of MR-Guided High-Intensity Focused Ultrasound (MR-HIFU) in Solitary versus Multiple Uterine Fibroids. Healthcare 2022, 10, 1471. https://doi.org/10.3390/healthcare10081471
Erber B, Schwarze V, Strobl F, Burges A, Mahner S, Goller SS, Rudolph J, Ricke J, Sabel BO. Therapeutic Outcome of MR-Guided High-Intensity Focused Ultrasound (MR-HIFU) in Solitary versus Multiple Uterine Fibroids. Healthcare. 2022; 10(8):1471. https://doi.org/10.3390/healthcare10081471
Chicago/Turabian StyleErber, Bernd, Vincent Schwarze, Frederik Strobl, Alexander Burges, Sven Mahner, Sophia Samira Goller, Jan Rudolph, Jens Ricke, and Bastian Oliver Sabel. 2022. "Therapeutic Outcome of MR-Guided High-Intensity Focused Ultrasound (MR-HIFU) in Solitary versus Multiple Uterine Fibroids" Healthcare 10, no. 8: 1471. https://doi.org/10.3390/healthcare10081471
APA StyleErber, B., Schwarze, V., Strobl, F., Burges, A., Mahner, S., Goller, S. S., Rudolph, J., Ricke, J., & Sabel, B. O. (2022). Therapeutic Outcome of MR-Guided High-Intensity Focused Ultrasound (MR-HIFU) in Solitary versus Multiple Uterine Fibroids. Healthcare, 10(8), 1471. https://doi.org/10.3390/healthcare10081471





