A Weighted Stochastic Conjugate Direction Algorithm for Quantitative Magnetic Resonance Images—A Pattern in Ruptured Achilles Tendon T2-Mapping Assessment
Abstract
1. Introduction
2. Materials and Methods
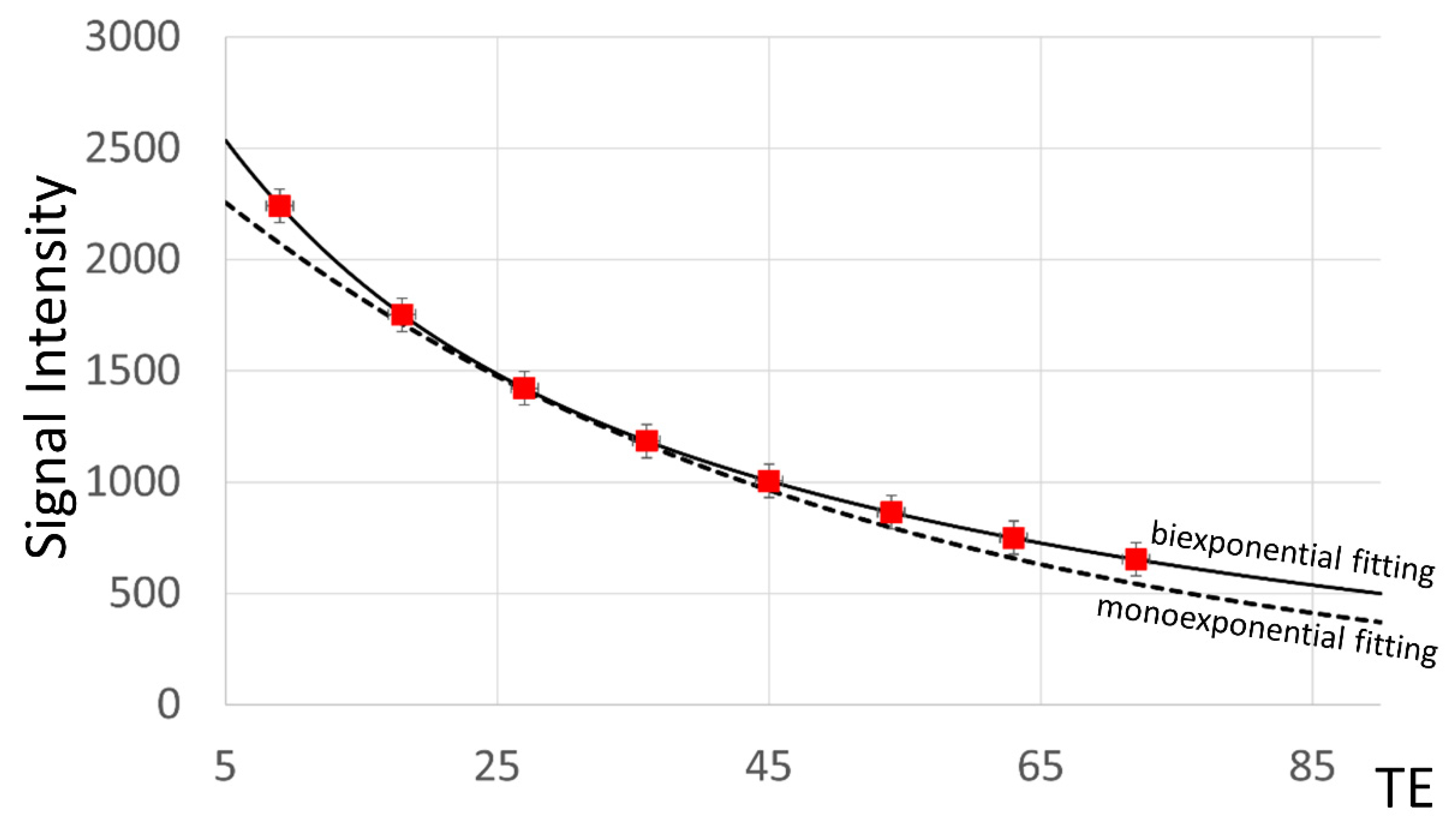
2.1. Calculation of T2 Time
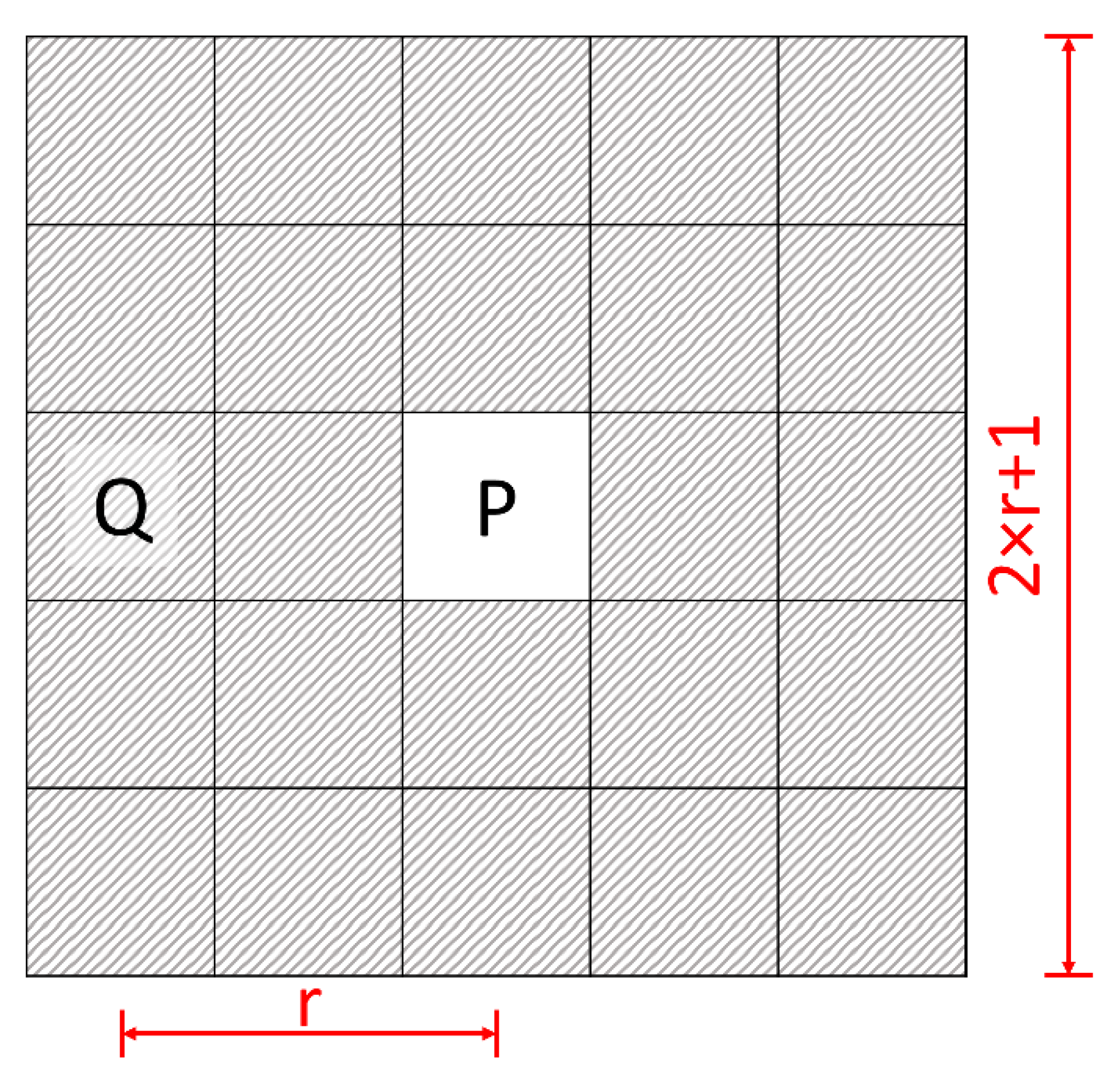
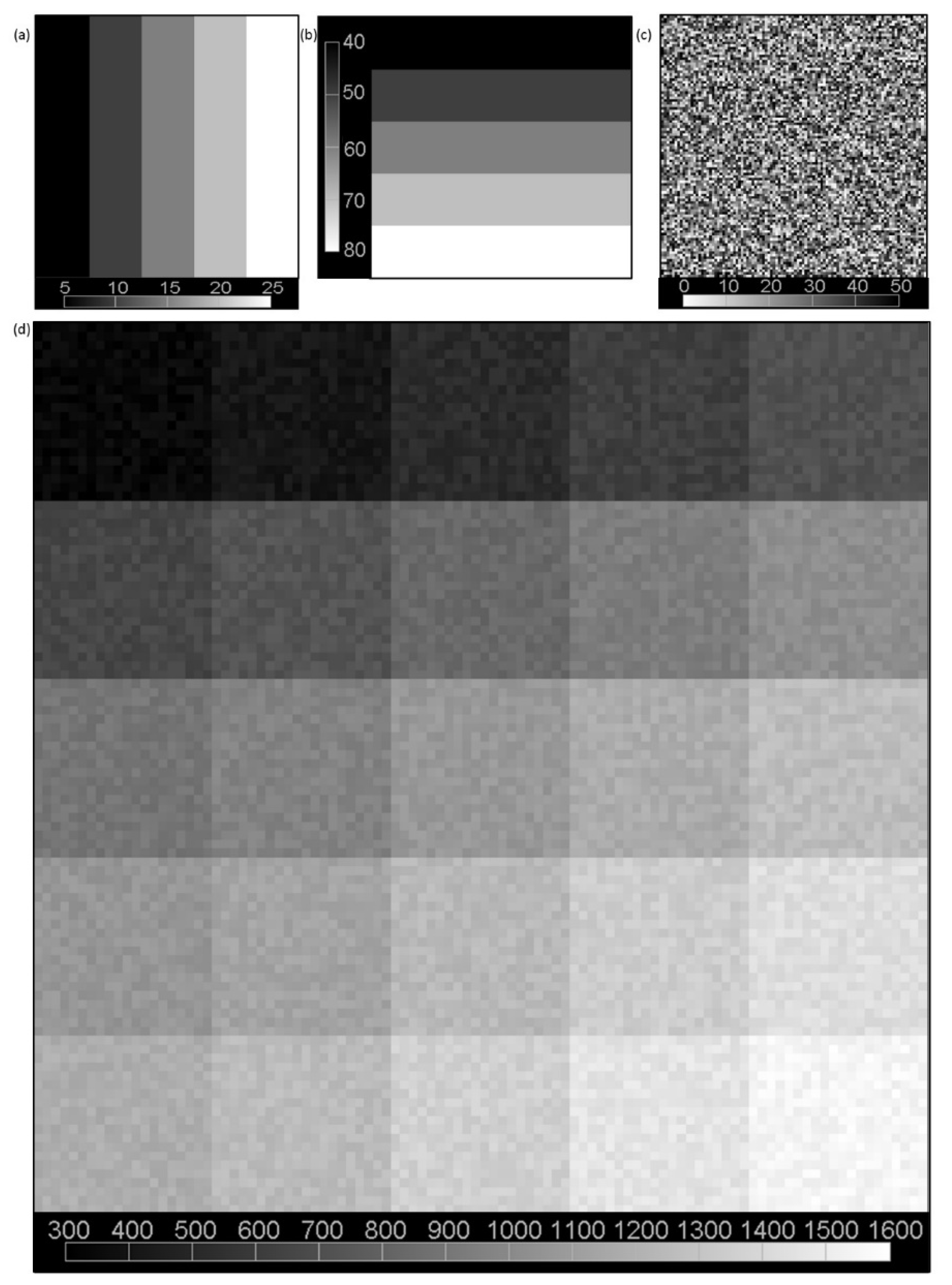
2.2. Simulation Data
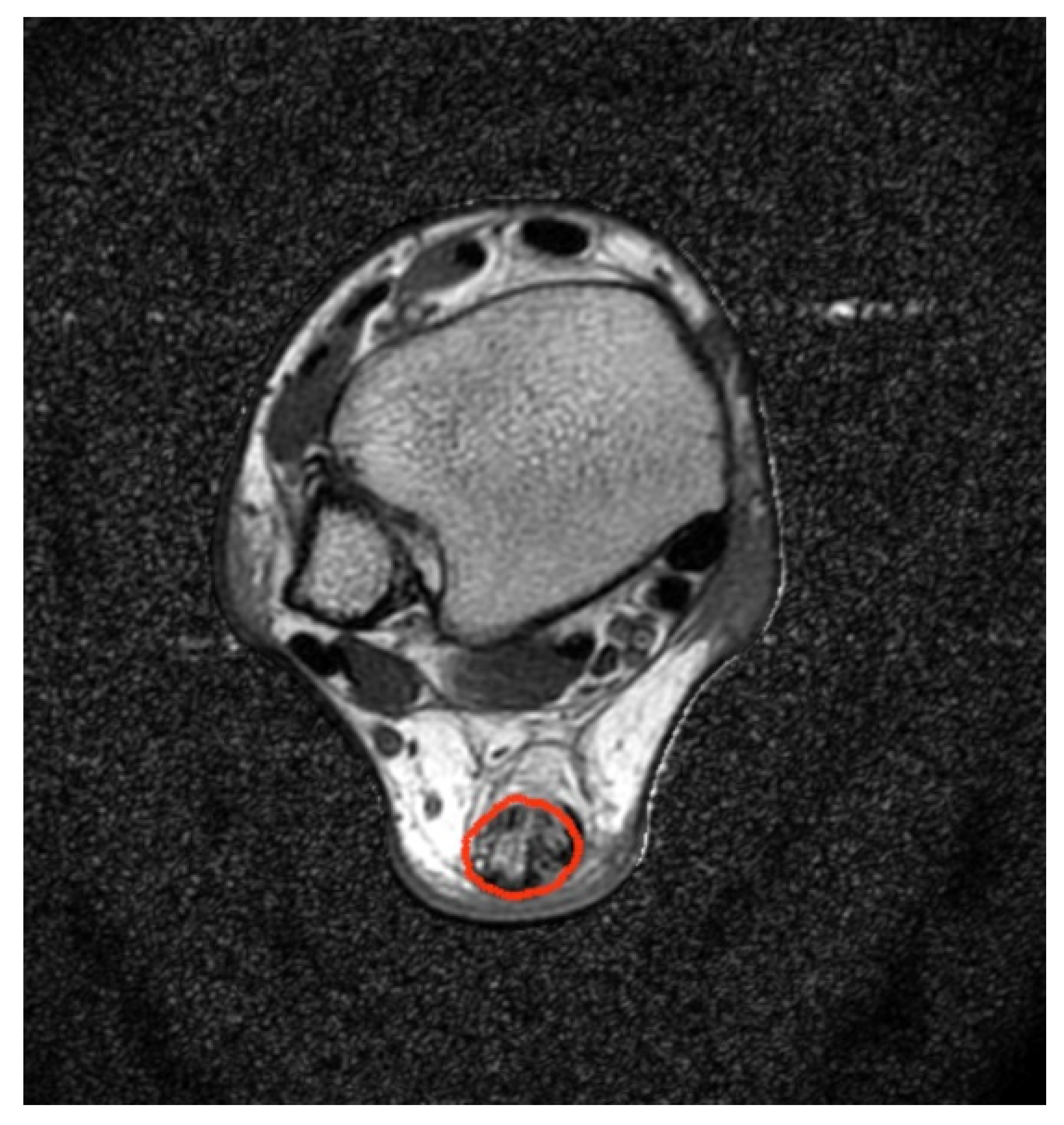
2.3. MRI Acquisition Protocol
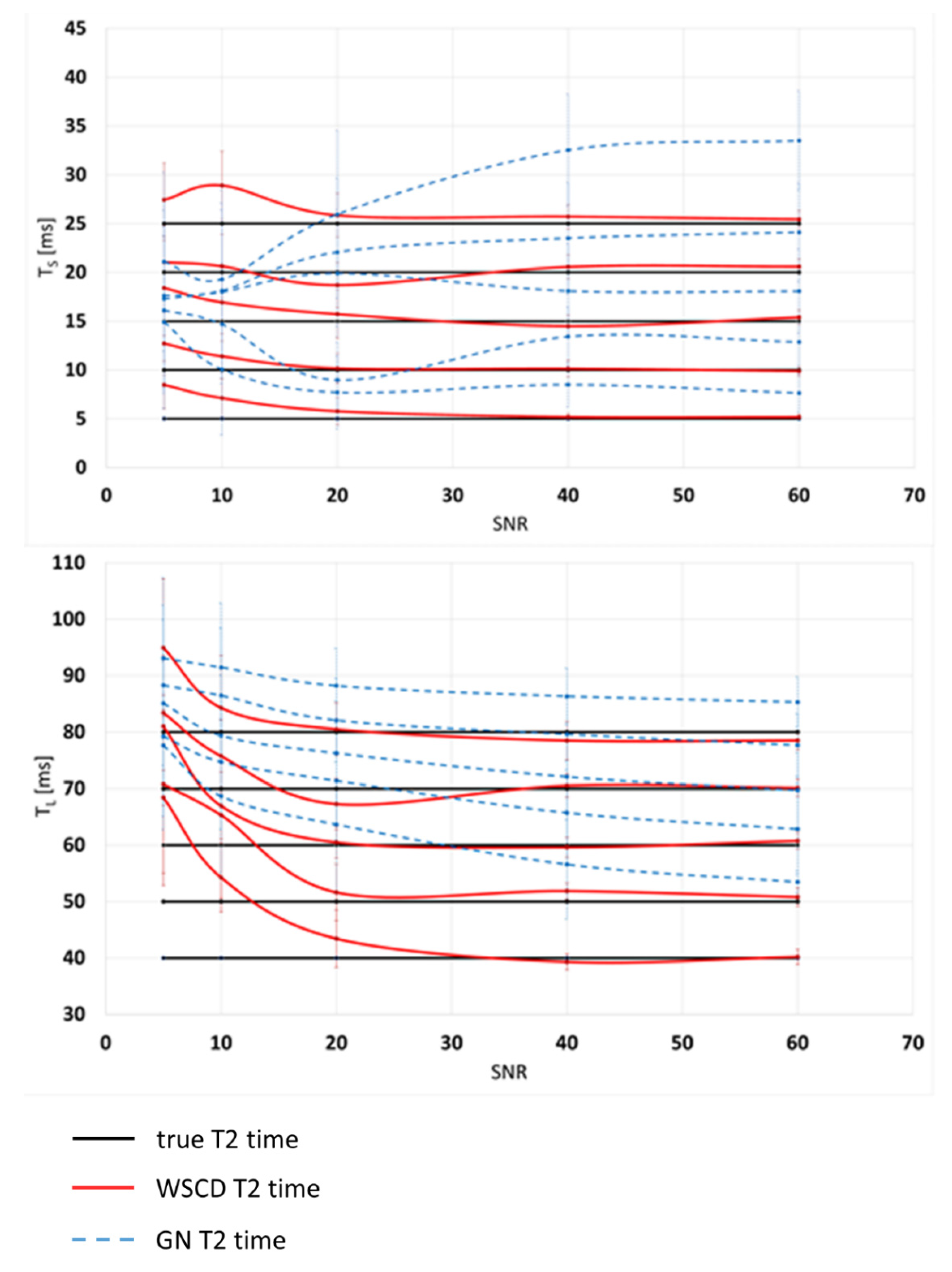
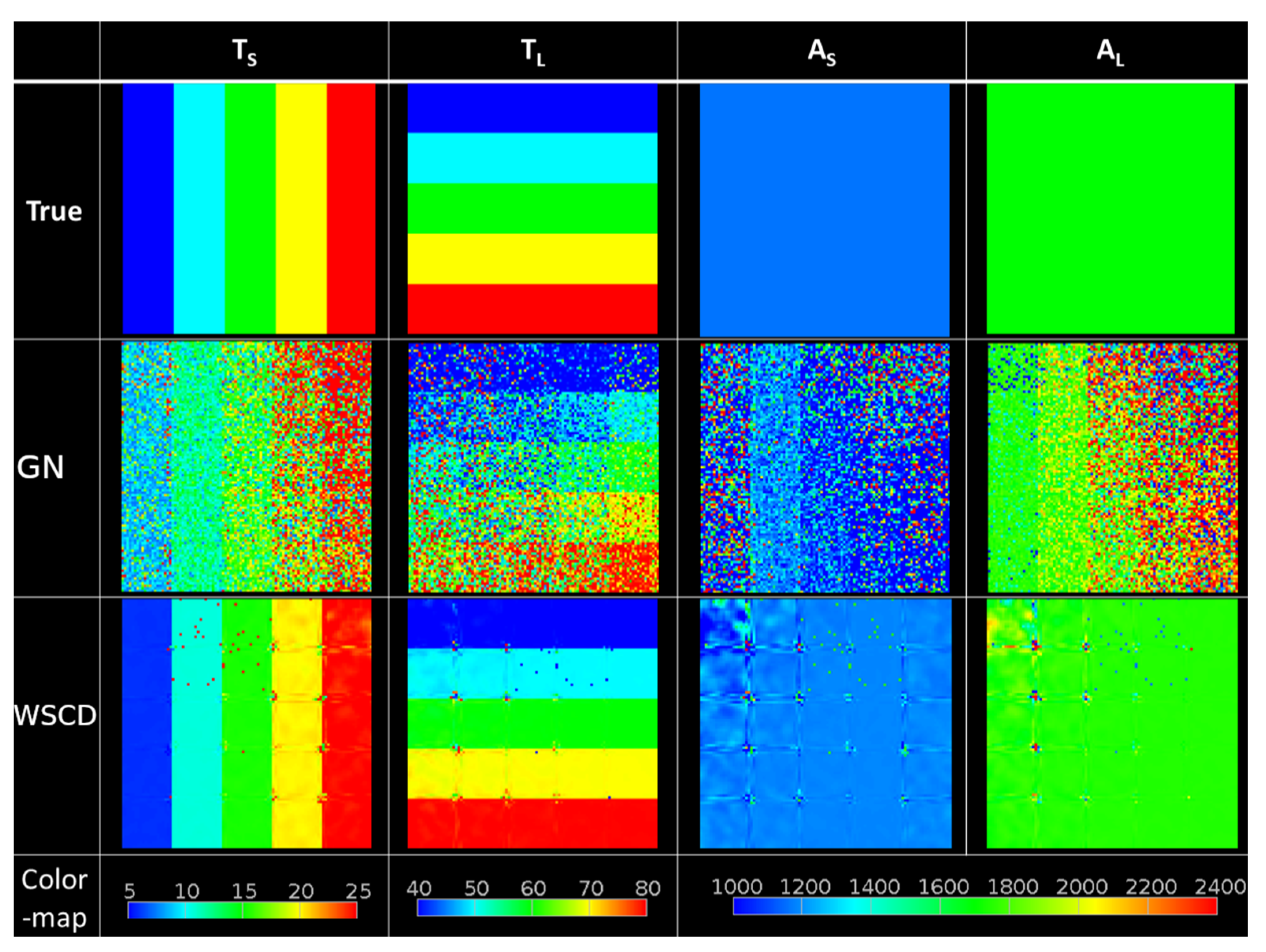
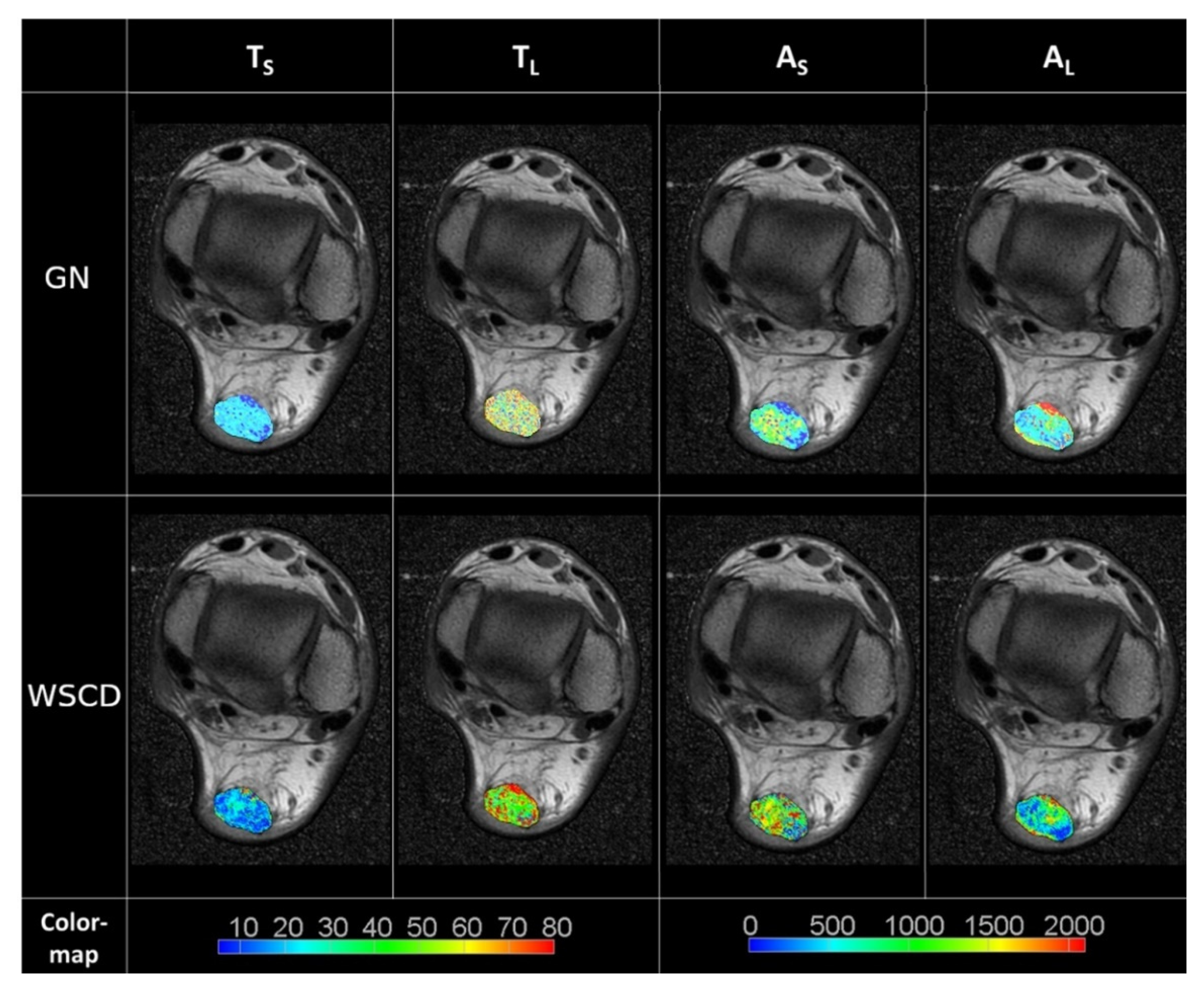
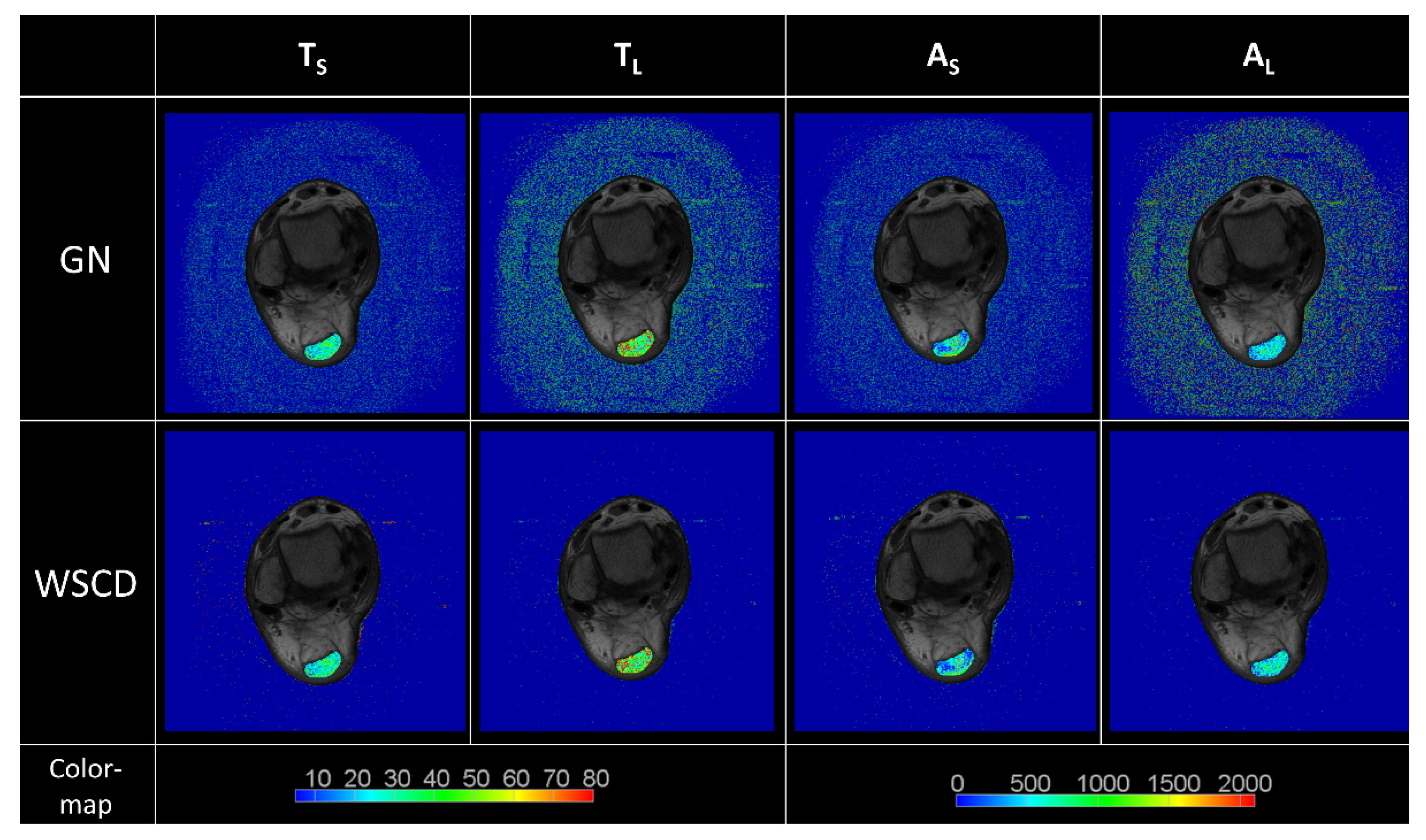
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tawara, N.; Nitta, O.; Kuruma, H.; Niitsu, M.; Itoh, A. T2 mapping of muscle activity using ultrafast imaging. Magn. Reson. Med. Sci. 2011, 10, 85–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anz, A.W.; Lucas, E.P.; Fitzcharles, E.K.; Surowiec, R.K.; Millett, P.J.; Ho, C.P. MRI T2 mapping of the asymptomatic supraspinatus tendon by age and imaging plane using clinically relevant subregions. Eur. J. Radiol. 2014, 83, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Apprich, S.; Pressl, C.; Zbyn, S.; Szomolanyi, P.; Domayer, S.; Hofstaetter, J.G.; Trattnig, S. Histological correlation of 7 T multi-parametric MRI performed in ex-vivo Achilles tendon. Eur. J. Radiol. 2013, 82, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Biercevicz, A.M.; Walsh, E.G.; Murray, M.M.; Akelman, M.R.; Fleming, B.C. Improving the clinical efficiency of T2* mapping of ligament integrity. J. Biomech. 2014, 47, 2522–2525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, W.; Zhang, K.; Zhao, C.Q.; Ding, W.; Yuan, J.J.; Sun, Q.; Sun, X.J.; Xie, Y.Z.; Li, H.; Zhao, J. Quantitative T2 mapping to characterize the process of intervertebral disc degeneration in a rabbit model. BMC Musculoskelet. Disord. 2013, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Choi, E.Y.; Kwon, H.M.; Hong, B.K.; Lee, B.K.; Yoon, Y.W.; Min, P.K.; Greiser, A.; Paek, M.Y.; Yu, W.; et al. Quantitative T2 mapping for detecting myocardial edema after reperfusion of myocardial infarction: Validation and comparison with T2-weighted images. Int. J. Cardiovasc. Imaging 2013, 29, 65–72. [Google Scholar] [CrossRef]
- Sandino, C.M.; Kellman, P.; Arai, A.E.; Hansen, M.S.; Xue, H. Myocardial T2* mapping: Influence of noise on accuracy and precision. J. Cardiovasc. Magn. Reson. 2015, 17, 7. [Google Scholar] [CrossRef]
- Maizlin, Z.V.; Clement, J.J.; Patola, W.B.; Fenton, D.M.; Gillies, J.H.; Vos, P.M.; Jacobson, J.A. T2 mapping of articular cartilage of glenohumeral joint with routine MRI correlation--initial experience. HSS J. 2009, 5, 61–66. [Google Scholar] [CrossRef]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Raya, J.G.; Dietrich, O.; Horng, A.; Weber, J.; Reiser, M.F.; Glaser, C. T2 measurement in articular cartilage: Impact of the fitting method on accuracy and precision at low SNR. Magn. Reson. Med. 2010, 63, 181–193. [Google Scholar] [CrossRef]
- Juras, V.; Apprich, S.; Szomolanyi, P.; Bieri, O.; Deligianni, X.; Trattnig, S. Bi-exponential T2 analysis of healthy and diseased Achilles tendons: An in vivo preliminary magnetic resonance study and correlation with clinical score. Eur. Radiol. 2013, 23, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Adriaensen, H.; Musse, M.; Quellec, S.; Vignaud, A.; Cambert, M.; Mariette, F. MSE-MRI sequence optimisation for measurement of bi- and tri-exponential T2 relaxation in a phantom and fruit. Magn. Reson. Imaging 2013, 31, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Thevendran, G.; Sarraf, K.M.; Patel, N.K.; Sadri, A.; Rosenfeld, P. The ruptured Achilles tendon: A current overview from biology of rupture to treatment. Musculoskelet. Surg. 2013, 97, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Fukawa, T.; Yamaguchi, S.; Watanabe, A.; Sasho, T.; Akagi, R.; Muramatsu, Y.; Akatsu, Y.; Katsuragi, J.; Endo, J.; Osone, F.; et al. Quantitative assessment of tendon healing by using MR T2 mapping in a rabbit Achilles tendon transection model treated with platelet-rich plasma. Radiology 2015, 276, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Thorpe, A.P.; Smith, E.W. Magnetic resonance imaging after operative repair of Achilles tendon rupture. Scand. J. Med. Sci. Sports 2001, 11, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hayter, C.L.; Linklater, J.M.; Man, K.H. State of the art MR imaging techniques for the foot and ankle. Curr. Radiol. Rep. 2014, 2, 42. [Google Scholar] [CrossRef]
- Tbini, Z.; Mars, M.; Riahi, H.; Bouaziz, M.C.; Ladeb, M.F. Quantitative MRI Measurements of Achilles tendon In Vivo Using T2 Mapping at 3T. In Proceedings of the European Society of Musculoskeletal Radiology Annual Meeting, Bari, Italy, 15–17 June 2017; EPOS, P-0227. pp. S1–S21. [Google Scholar]
- Carmont, M.R.; Highland, A.M.; Rochester, J.R.; Paling, E.M.; Davies, M.B. An anatomical and radiological study of the fascia cruris and paratenon of the Achilles tendon. Foot Ankle Surg. 2011, 17, 186–192. [Google Scholar] [CrossRef]
- De Carli, A.; Lanzetti, R.M.; Ciompi, A.; Lupariello, D.; Vadalà, A.; Argento, G.; Ferretti, A.; Vulpiani, M.C.; Vetrano, M. Can platelet-rich plasma have a role in Achilles tendon surgical repair? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2231–2237. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Andía, I.; Padilla, S.; Mujika, I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am. J. Sports Med. 2007, 35, 245–251. [Google Scholar] [CrossRef]
- Regulski, P.A.; Zielinski, J. Multi-step segmentation algorithm for quantitative magnetic resonance imaging T2 mapping of ruptured Achilles tendons. IEEE Access 2020, 8, 199995–200004. [Google Scholar] [CrossRef]
- Regulski, P.A.; Zielinski, J.; Borucki, B.A.; Nowinski, K.S. Comparison of noise reducing T2-map reconstruction methods in MRI imaging of Achilles tendon. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, S15–S16. [Google Scholar]
- Regulski, P.A.; Zieliński, J.; Borucki, B.; Nowiński, K. Assessment of anisotropic denoiser enhanced cone beam CT for patient dose reduction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 295–296. [Google Scholar]
- Zielinski, J.; Nowinski, K. Multi-step anisotropic denoiser scheme applied for cardiac non-contrast CT image. Int. J. Comput. Assist. Radiol. Surg. 2014, 9, S263–S264. [Google Scholar]
- Hansen, B.E. Least squares model averaging. Econometrica 2007, 75, 1175–1189. [Google Scholar] [CrossRef]
- Alefeld, G.E.; Potra, F.A.; Shi, Y. Algorithm 748: Enclosing zeros of continuous functions. ACM Trans. Math. Softw. 1995, 21, 327–344. [Google Scholar] [CrossRef]
- Anastasiou, A.; Hall, L.D. Optimisation of T2 and M0 measurements of bi-exponential systems. Magn. Reson. Imaging 2004, 22, 67–80. [Google Scholar] [CrossRef]
- Powell, M.J.D. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput. J. 1964, 7, 155–162. [Google Scholar] [CrossRef]
- Biercevicz, A.M.; Walsh, E.G.; Murray, M.M.; Akelman, M.; Fleming, B.C. T2* Mapping of Ligaments: Can We Improve Computational Time with Relaxometry Post-Processing? In Proceedings of the Orthopaedic Research Society Annual Meeting, New Orleans, LA, USA, 15–18 March 2014; p. 1364. [Google Scholar]
- Vergeldt, F.J.; Prusova, A.; Fereidouni, F.; Amerongen, H.V.; Van As, H.; Scheenen, T.W.J.; Bader, A.N. Multi-component quantitative magnetic resonance imaging by phasor representation. Sci. Rep. 2017, 7, 861. [Google Scholar] [CrossRef]
- Akcakaya, M.; Basha, T.A.; Weingartner, S.; Roujol, S.; Berg, S.; Nezafat, R. Improved quantitative myocardial T2 mapping: Impact of the fitting model. Magn. Reson. Med. 2015, 74, 93–105. [Google Scholar] [CrossRef]
- Dula, A.N.; Gochberg, D.F.; Does, M.D. Optimal echo spacing for multi-echo imaging measurements of bi-exponential T2 relaxation. J. Magn. Reson. 2009, 196, 149–156. [Google Scholar] [CrossRef]
- Bonny, J.M.; Boespflug-Tanguly, O.; Zanca, M.; Renou, J.P. Multi-exponential analysis of magnitude MR images using a quantitative multispectral edge-preserving filter. J. Magn. Reson. 2003, 161, 25–34. [Google Scholar] [CrossRef]
- Gensanne, D.; Josse, G.; Lagarde, J.M.; Vincensini, D. A post-processing method for multiexponential spin-spin relaxation analysis of MRI signals. Phys. Med. Biol. 2005, 50, 3755–3772. [Google Scholar] [CrossRef] [PubMed]
- Bjork, M.; Zachariah, D.; Kullberg, J.; Stoica, P. A multicomponent T2 relaxometry algorithm for myelin water imaging of the brain. Magn. Reson. Med. 2016, 75, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Formation of T2* Mapping Using Mixed-Effects Model. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop (NSS/MIC/RTSD), Strasbourg, France, 29 October–6 November 2016; pp. 1–2. [Google Scholar]
- Shao, H.; Chang, E.Y.; Pauli, C.; Zanganeh, S.; Bae, W.; Chung, C.B.; Tang, G.; Du, J. UTE bi-component analysis of T2* relaxation in articular cartilage. Osteoarthr. Cartil. 2016, 24, 364–373. [Google Scholar] [CrossRef]
- Kapinski, N.; Zielinski, J.; Borucki, B.A.; Trzcinski, T.; Ciszkowska-Lyson, B.; Nowinski, K.S. Estimating Achilles tendon healing progress with convolutional neural networks. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2018. Lecture Notes in Computer Science; Frangi, A., Schnabel, J., Davatzikos, C., Alberola-López, C., Fichtinger, G., Eds.; Springer: Cham, Switzerland, 2018; pp. 949–957. [Google Scholar]
- Regulski, P.A.; Zielinski, J.; Szopinski, K.T. Temporomandibular disk dislocation impacts the stomatognathic system: Comparative study based on biexponential quantitative T2 maps. J. Clin. Med. 2022, 11, 1621. [Google Scholar] [CrossRef]
| SNR | Method | Mean Difference | Standard Deviation | Wilcoxon Signed-Rank Test with Continuity Correction | ||||
|---|---|---|---|---|---|---|---|---|
| Pseudo median | CI lower | CI upper | p | |||||
| TS | 5 | GN | −1.14 | 17.06 | −1.38 | −1.82 | −0.93 | 0.0000 |
| WSCD | 3.41 | 5.73 | 1.30 | 0.41 | 2.18 | 0.0000 | ||
| 10 | GN | 0.30 | 15.42 | −0.51 | −0.92 | −0.11 | 0.0000 | |
| WSCD | −2.00 | 5.05 | −0.54 | −0.81 | −0.26 | 0.0000 | ||
| 20 | GN | 2.23 | 11.13 | 1.66 | 1.25 | 2.40 | 0.0000 | |
| WSCD | −0.51 | 5.73 | −0.89 | −2.18 | 0.41 | 0.0895 | ||
| 40 | GN | 2.92 | 7.98 | 2.50 | 2.01 | 2.95 | 0.0000 | |
| WSCD | −0.42 | 4.04 | −0.05 | −0.43 | 0.33 | 0.1315 | ||
| 60 | GN | 2.97 | 6.76 | 2.50 | 2.30 | 2.71 | 0.0000 | |
| WSCD | 0.54 | 3.60 | 0.02 | −0.03 | 0.07 | 0.2112 | ||
| TL | 5 | GN | 20.27 | 33.32 | 14.95 | 10.10 | 40.00 | 0.0000 |
| WSCD | 14.05 | 10.46 | 14.05 | 6.65 | 21.44 | 0.0000 | ||
| 10 | GN | 13.26 | 25.28 | 15.00 | 12.49 | 17.50 | 0.0000 | |
| WSCD | 6.36 | 12.89 | 4.90 | −0.56 | 10.44 | 0.0017 | ||
| 20 | GN | 13.44 | 22.06 | 12.51 | 12.50 | 14.99 | 0.0000 | |
| WSCD | −0.50 | 6.43 | −0.40 | −1.73 | 0.94 | 0.3139 | ||
| 40 | GN | 12.07 | 15.94 | 10.00 | 10.00 | 12.50 | 0.0000 | |
| WSCD | −0.81 | 4.51 | −0.20 | −0.52 | 0.12 | 0.1025 | ||
| 60 | GN | 8.80 | 13.96 | 7.50 | 7.50 | 10.00 | 0.0000 | |
| WSCD | 0.27 | 2.88 | 0.10 | −0.16 | 0.40 | 0.2137 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regulski, P.A.; Zielinski, J.; Borucki, B.; Nowinski, K. A Weighted Stochastic Conjugate Direction Algorithm for Quantitative Magnetic Resonance Images—A Pattern in Ruptured Achilles Tendon T2-Mapping Assessment. Healthcare 2022, 10, 784. https://doi.org/10.3390/healthcare10050784
Regulski PA, Zielinski J, Borucki B, Nowinski K. A Weighted Stochastic Conjugate Direction Algorithm for Quantitative Magnetic Resonance Images—A Pattern in Ruptured Achilles Tendon T2-Mapping Assessment. Healthcare. 2022; 10(5):784. https://doi.org/10.3390/healthcare10050784
Chicago/Turabian StyleRegulski, Piotr A., Jakub Zielinski, Bartosz Borucki, and Krzysztof Nowinski. 2022. "A Weighted Stochastic Conjugate Direction Algorithm for Quantitative Magnetic Resonance Images—A Pattern in Ruptured Achilles Tendon T2-Mapping Assessment" Healthcare 10, no. 5: 784. https://doi.org/10.3390/healthcare10050784
APA StyleRegulski, P. A., Zielinski, J., Borucki, B., & Nowinski, K. (2022). A Weighted Stochastic Conjugate Direction Algorithm for Quantitative Magnetic Resonance Images—A Pattern in Ruptured Achilles Tendon T2-Mapping Assessment. Healthcare, 10(5), 784. https://doi.org/10.3390/healthcare10050784






