Abstract
Physical inactivity and low levels of muscle strength can lead to the early development of sarcopenia and dynapenia, which may increase the number and risk of falls in the elderly population. Meanwhile, exercise programs can stop or even revert the loss of muscle mass, strength, power, and functional capacity and consequently decrease the risk of falls in older adults. However, there is a lack of studies investigating the effect of strengthening programs in octogenarians. The present study investigates the effects of 40 weeks of a training-detraining-retraining cycle of muscle strength exercise program on postural stability and estimated fall risk in octogenarians. Twenty-seven institutionalized participants were allocated into two groups: the muscular strength exercise group (MSEG, n = 14) and control group (CG, n = 13). After the first training period, the MSEG improved postural stability and decreased the estimated fall risk by 7.9% compared to baseline. In comparison, CG worsened their stability and increased their risk of falling by more than 17%. No significant changes were found between groups in the detraining and the retraining period. This study demonstrated that strength exercise effectively improved postural control and reduced fall risk scores. In addition, the interventions were able to reduce the forward speed of postural control deterioration in octogenarians, with great increments in the first months of exercise.
1. Introduction
Life expectancy is growing worldwide, and consequently, many health problems related to the aging process have been drawing greater attention, with the physical, psychological, and physiological degenerative processes standing out. The increase in sedentarism linked to aging is a global health problem [1]. The lack of physical exercise can trigger the early development of some physical and neurodegenerative diseases in older adults leading to the development of sarcopenia and dynapenia [2,3]. The latter phenomena are part of what has been called the frailty syndrome [4], which has been linked to an increase in the number and severity of falls in older adults [5].
The World Health Organization, aware of the importance of the fight against a sedentary lifestyle in the aged population, has published some minimum dose recommendations for health maintenance [6]. These recommendations are to perform at least 150–300 min of moderate physical activity, or 75–150 min of vigorous physical activity per week, including multicomponent exercise focusing on balance and strength, to improve the functional capacity and prevent falls [6].
The fall is conceptualized as an unintentional body displacement to a lower level than the initial position, determined by multifactorial circumstances that compromise stability [7]. Aging plays a central role as it affects the afferent sensory system (i.e., proprioception, vestibular and visual system), the central neurologic control system (i.e., cognition, attention, fear of falling), and finally, the efferent neuromotor system (i.e., physical function, muscle strength, balance, and stability) [8,9]. Changes in central neural system connectivity have been observed in areas related to the integration of information and in areas associated with motor and sensory information processing, providing evidence of the complex multidimensionality of the neural underpinnings of falls [10]. In addition, diseases, drugs, and environment modulate the age-associated changes in the fall risk pathway.
In this scenario, there are intrinsic causes (deterioration of physiological and neuromuscular changes of aging, muscle dysfunctions, pathologies, medications) and extrinsic causes (environmental hazards, architectural and furniture inadequacies, stairs, high heel shoes) of fall occurrence [11]. The interaction between intrinsic and extrinsic factors compromises the perceptive and neuromuscular systems related to postural stability and balance control, affecting the functionality and quality of life of the older adults [12], being an essential aspect of morbidity and mortality and the leading cause of fatal and non-fatal injuries among older adults [13].
According to a Behavioral Surveillance and Risk System survey, about 26% of older adults reported falling at least once in the last 12 months, resulting in 24.96 million falls in 2020 [14]; likewise, in 2019 [15], more than 3 million fall injuries and more than 34,000 deaths related to falls were recorded, generating an approximate cost of 50 billion dollars in medical care in the USA [15,16]. Sadly, these numbers kept growing and are projected to increase 30% by 2030, resulting in around seven deaths/hour in the USA [16]. Similarly, in Europe, the Western region saw 8.4 million older adults in medical centers due to fall-related injuries in 2017 [17]. In this scenario, institutionalized older people are at a higher risk of falling since the prevalence of frailty in nursing homes is 50%, and approximately 40% are pre-frail individuals [18]. Furthermore, the percentage of fallers increases from 26.7% in older people between 65 and 74 y/o to 29.8% in older people between 75 and 84 y/o [19]. For people over 84 years old, their incidence of falls increases up to 36.5% [19]. Furthermore, this effect in octogenarians is still not well understood, given this population’s difficulty, specificity, and high vulnerability [20].
Meanwhile, some studies have shown that physical exercise can attenuate the speed of evolution of some neurodegenerative processes, such as sarcopenia, dynapenia, and frailty, contributing to balance control and postural stability [21,22,23]. Regular exercise has been proven to reverse the frailty status and decrease the fall risk among older adults, even in those who live in nursing homes or social care institutions [9,18]. Recent studies have shown that institutionalized older adults had lower scores in physical fitness and higher scores for depressive symptoms and comorbidities, with a significant correlation between frailty, fear, and risk of falling and physical fitness [21,24,25,26,27]. It has been demonstrated that professional-oriented multicomponent training for eight weeks has positive outcomes; specifically, it has been indicated that shorter and high-intensity dynamic exercise can be an effective way of improving performance, gait, and balance capacities in older adults at risk of fall [28].
A meta-analysis has shown that exercise-only interventions had a practical effect on fall risk in institutionalized and non-institutionalized older adults, significantly reducing the number of falls [29]. However, this same meta-analysis showed a high percentage of drop-out ratio in the population studied, making it difficult to draw conclusions from this study. This, taken together with the lack of works focused on people over 80 years old, indicates the need to study the chronic adaptations in very old populations after exercise interventions. The benefits of exercise are transient and last as long as it is being performed; thus, the necessity of adherence and progression is crucial [3,6,18,21]. However, adherence is an unresolved matter when training older persons, especially those institutionalized, who interrupt their training programs, for example, when spending holidays with families and other diverse circumstances. Therefore, the study of evolution/involution of neuromuscular adaptations after training-detraining may help prescribe exercise with more prolonged residual effects to overcome detraining periods [30,31]. In this way, some authors have analyzed the effects of the detraining process [32]. Detraining can be defined as training reduction or cessation, which implies temporary discontinuation or complete abandonment of systematic programmed physical exercise, which may cause a partial or complete loss of training-induced adaptations (anatomical, physiological, psychological, and functional performance) produced during a previous training period [33]. Some authors studying this process have indicated that after 12 weeks of detraining, the benefits of exercise started to decline, and even after another 12 weeks of detraining, some of the muscular endurance and strength parameters reduced by ~15% [30]. In another detraining study, strength and gait speed were reduced after 16 weeks of no training but did not return to their baseline values [34]. These data point to a lasting protective effect from exercise, even when these capabilities decline due to the aging process [35]. In fact, as people age, muscle power deteriorates faster than muscle strength [36].
Muscle strength is the amount of tension that a muscle or muscle group can generate in a specific movement; meanwhile, muscle power is the tension generated at a specific velocity [37]. In this context, neuromuscular adaptations and deteriorations, mainly at the level of muscle-tendon units (i.e., reduced tendon stiffness), muscular structure (i.e., reduced number of muscular units, and atrophy of fast-twitch fibers), and neural changes affect strength capabilities [cite] and power output [38]. However, since muscle power is more strongly associated with daily life activities, more attention must be paid to exercise strategies that contribute to power development in older adults.
In this scenario, the specific use of elastic bands in exercise training programs has been shown to improve muscular capacities such as strength, balance, and functional capabilities in older adults, even in the institutionalized ones [22], including those characterized as frail or pre-frail [23]. Considering the reasons given, we aimed to evaluate the effects of forty weeks of a training-detraining-retraining cycle of muscle strength exercise (MSE) program with elastic bands on institutionalized octogenarians and its influence on postural control and estimated fall risk status.
2. Materials and Methods
We have employed a non-probability convenience sampling of octogenarian dwelling older adults living in nursing homes. The institution’s directors and the older adults’ legal representatives revised and signed the consent form before the first testing session. The estimated sample size was calculated using the G*Power software (version 3.1.9.7) [39]. Based on our calculations, for an effect size of 0.30, a sample size of 26 achieves 95% statistical power to detect differences among the means using an ANOVA test with an α-level of 0.05. The sample consisted of 27 participants (7 males, 20 females) aged over 80 (86.37 ± 3.59) years old, institutionalized in nursing homes or social care centers of Coimbra (Portugal). This study is designed as a prospective, naturalistic, controlled clinical trial (treatment vs. care) composed of 3 phases, i.e., training, detraining, and retraining. The participants were stratified randomized into two groups: the muscular strength exercise group (MSEG, n = 14, 4 male and 10 female), who performed an elastic band strength exercise program, and the control group (CG, n = 13, 3 male and 10 female) who continued their usual routine, which does not include any kind of programmed and supervised physical exercise.
The eligible criteria for the participants in this study were that, at the time of first screening, participants had to be: (i) 80 years old or more; (ii) clinically stable with their drug therapy updated; (iii) not participating in another structured program of physical exercise in the last six months; (iv) not presenting any type of health condition or use medication that might prevent the functional self-sufficiency test performance or attention impairment (such as severe cardiopathy, uncontrolled hypertension, uncontrolled asthmatic bronchitis or severe musculoskeletal conditions); (v) not presenting mental disorders or hearing/visual impairment that could prevent the evaluations and activities proposed, according to the institutional medical staff.
Additionally, we should address that in this study, care was taken to exclude as much as possible the factors that differentiated the sample, seeking a homogeneous sample without statistical differences in age, sex, the ability to perform daily life activities independently, and without a history of diseases that could directly affect the balance so that the results are as accurate as possible.
The intervention consisted of a first period of sixteen weeks of resistance training with elastic bands, followed by eight weeks of detraining and a second training period of sixteen weeks. Participants performed a total of 64 sessions, 2 sessions/week of 45 min each on non-consecutive days distributed between the two training periods.
To avoid any bias, all the participants completed the evaluation protocol at the same time period, between 10 am to 11.45 a.m. That protocol was repeated on four occasions: pre-intervention (PRE), postintervention after 16 weeks of training (POST16), after eight weeks of detraining (POST24), and postintervention after 16 weeks of training in the second period (POST40) (Figure 1). The evaluation protocol assessed anthropometric values and estimated fall risk through an index based on the posturography platform (Physiosensing® v.19002, Sensing Future, Coimbra, Portugal) test with four specific conditions (please see details below).

Figure 1.
Graphical representation of the study design.
This study was approved by the Faculty of Sport Science and Physical Education, University of Coimbra Ethics Committee (reference number: CEFCDEF/0028/2018) respecting the Portuguese Resolution (Art. 4th; Law no. 12/2005, 1st series) on ethics in human research [40] and the Helsinki Declaration. Clinical trial register number NCT04376463.
2.1. Postural Stability and Fall Risk Assessment
The fall risk assessment allows the identification of potential fall candidates. The protocol employed in the present study, the Physiosensing® Fall Risk test, has been validated and described elsewhere [41,42]. Postural stability assessment was performed employing a specialized force platform (Physiosensing® v.19002, Sensing Future, Coimbra, Portugal) that measured the participants’ sway center of pressure. Each participant stood barefoot on the force platform and tried to be as stable as possible in a static upright position, directing their gaze to a point located at 2 m, for 45 s under four pre-established conditions [41,42]: (1) comfortable stance with eyes open (CSEO); (2) comfortable stance with eyes closed (CSEC); (3) narrow stance with eyes open (NSEO); and (4) narrow stance with eyes closed (NSEC).
Data were stored and analyzed with commercial software (Physiosensing® v.19.0.1.0) that calculated the speed index for each condition to estimate fall risk. The speed index is calculated as the displacement velocity of the center of pressure (i.e., distance traveled in the sagittal plane divided by the test time (mm/s), normalized by the participant’s height, and transformed with the natural logarithm function [1]. The fall risk estimation is based on the assumption that an increment in the participant’s sway velocity denotes a postural control deficit. The software also calculates the composite speed index score as the mean of the scores obtained in the four conditions. The higher scores in the composite index and within each of the four conditions indicate higher fall risk.
2.2. Muscular Strength Exercise Protocol
An exercise expert supervised the MSE program. Exercise prescription was based on the recommendations of the American College of Sports Medicine guidelines and previously published exercise prescription guidelines for older adults [43,44]. Furthermore, participants from the MSE group could choose their preferred music to increase the adherence rate [45].
The sessions comprised five minutes of a general warm-up with mobility exercises, and the main part involved resistance exercises with an elastic band for 35 min (see the detailed program in Table 1). At the end of the session, participants completed a cooldown with stretching exercises for 5 min. The program consisted of organized and planned exercises performed with a chair to ensure the safety of the participants. The intensity of the resistance training program was controlled by a rate perceived exertion (RPE) scale (Borg 0–10 scale [46]). During the exercise sessions, participants wore a heart rate monitor (Polar, model M200, Polar Electro Oy, Kempele, Finland), and heart rate (HR) was estimated using Karvonen’s formula where HRmax was calculated using a specific formula for older populations [47]. Heart rate was controlled jointly with the observation of facial flushing or hyperventilation to identify possible adverse events during exercise training. The MSE program consisted of 9 elastic bands exercises per session of progressively increased intensity (TheraBand, Akron, OH, USA). The exercises’ execution targeted truncal musculature, so the proposed exercises, when possible, were executed safely and correctly in stand positions, adding some balance and stability needs, leading to a higher stimulation of the proprioceptive system.

Table 1.
Protocol for the muscular strength exercise program (MSE).
The progression intensity was based on the OMNI table [48], which indicates the intensity progression throughout a colored band progression (soft-to-hard). The exercise protocol consisted initially of 2 sets of 10 to 20 repetitions with a light intensity band during the adaptation period and progressed gradually every 2 to 3 weeks. Finally, for the last four weeks, some free weights (dumbbells and ankle weights) were added to exercises, which allowed for a more intense and diversified spectrum of exercises. The participants performed all exercises in sequential order within each set and employed a cadence of 2 s in the concentric phase and 3 s in the eccentric phase of the movement. A minimum of two days between sessions was provided to ensure sufficient recovery.
The minimum adherence to be considered for analysis was set up to 80% of training sessions. When participants missed two consecutive sessions, a researcher contacted them and offered help to return to the group class; in case of a negative response, they were excluded from the analysis.
2.3. Anthropometric Assessment
Participants’ body mass and stature were measured in a portable scale (Seca, model 770, Hamburg, Germany) with 0.1 kg of precision and a portable stadiometer (Seca Body meter®, model 208, Hamburg, Germany) with 0.1 cm of precision, respectively. Body mass index (BMI) was calculated as the body mass in kilograms divided by the square of height in meters. Standardized procedures were followed as previously recommended [49].
2.4. Statistical Analyses
All descriptive data are presented as estimated marginal means and the 95% confidence interval (CI). Normality was assessed through standard distribution measures, visual inspection of Q–Q plots and box plots, and the Shapiro–Wilk test. Changes within and between groups were analyzed by employing mixed models for repeated measures designs with the module GAMLj [50], which uses the R formulation of random effects as implemented by the function lme4, an R package, in Jamovi software (The jamovi project, v1.6, 2021). GAMLj estimates variance components with restricted (residual) maximum likelihood (REML), producing unbiased estimates of variance and covariance parameters, unlike earlier maximum likelihood estimation. The inter-subject factor group (MSE and CG), the intra-subject factor time (i.e., PRE, POST16, POST24, and POST40) and condition (i.e., CSEO, CSEC, NSEO, NSEC; when appliable), and the interaction (group × time) were set as fixed effects. Sex and age were not introduced as a fixed factor and covariate, respectively, because these variables did not improve the model (i.e., parsimonious method), as evaluated by the Akaike information criterion (AIC). The participants’ intercepts were set as a random effect. Time slope was not included as a random coefficient since this factor’s variance was small in composite scores and speed index by condition.
Within-subject and between-subject changes were evaluated by ANOVA F omnibus test employing the Satterthwaite approximation of degrees of freedom and estimating the coefficients with their 95% confidence intervals for the fixed effects in the mixed model. When a significant interaction was detected, paired and independent comparisons were made with a t-test with the Bonferroni–Holm correction for within-subject and between-group changes, respectively. Furthermore, the variance of the random coefficients was obtained. Simple effects analysis was applied with ANOVA (type III sums of squares) and the Kenward-Roger method for degrees of freedom calculation. The level of significance was established at p < 0.05.
3. Results
Simple effects analysis revealed that MSEG and CG groups participants did not present significant differences in the anthropometric variables, age, sex distribution, and any postural control conditions and composite index (Table 2) at baseline.

Table 2.
Sample characteristics, postural control, and composite index outcomes at baseline (i.e., PRE).
Body mass index did not change from the baseline to POST16, POST24, and POST40 weeks of intervention (p = 0.477). The composite index evolution and its percentual delta changes are presented in Table 3. The percentual delta changes (∆%) represent the comparison with the precedent moment of measurement.

Table 3.
Composite index (i.e., fall risk estimation) for both groups in each moment of measurement.
In relation to the composite index, we found a main effect of group (F1,25 = 7.80, p = 0.010), moment (F3,399 = 15.15, p < 0.001) and interaction of group by moment (F3,399= 31.75, p < 0.001). Estimated difference between MSEG vs. CG was −2.35 a.u. (95%CI [−4.0 to −0.7]), p = 0.010), and the difference between groups (i.e., MSEG vs. CG) in changes of POST16 vs. PRE (β = −2.6, 95%CI = −3.3 to −1.9, p < 0.001), POST24 vs. PRE (β = −2.4, 95%CI = −3.0 to −1.7, p < 0.001) and POST 40 vs. PRE (β = −2.9, 95%CI = −3.6 to −2.3, p < 0.001) indicate lower composite index in MSEG than CG during the entire intervention. Mainly, CG progressively increased their composite index throughout the intervention period (Table 3 and Figure 2).
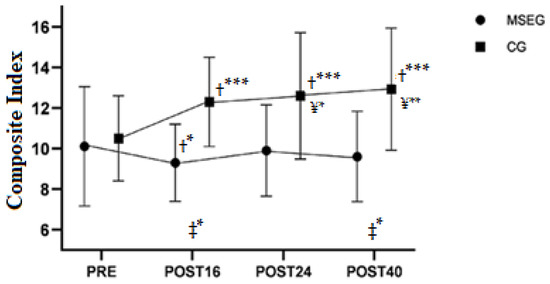
Figure 2.
The composite index in the four moments of measurement. PRE: pre-intervention test; POST16: after sixteen weeks of intervention; POST24: after eight weeks of detraining; POST40: after the second training period (retraining). ‡ = difference between groups at that specific time; † = difference versus PRE; ¥ = Difference versus MSEG-POST16. * = p < 0.05, ** = p < 0.01; *** = p < 0.001.
Mean and individual responses to every moment of postural control conditions (CSEO, CSEC, NSEO, NSEC) are illustrated in Figure 3. We observed a significant difference in postural control between groups (F1,25 = 6.44, p = 0.018), moments (F3,375 = 6.52, p < 0.001), conditions (F3,375 = 9.16, p < 0.001) and moment x group (F3,375 = 11.34, p < 0.001). There was no triple interaction group x moment x condition (F9,375 = 0.53, p = 0.853). However, simple effects analysis of group moderated by moment and condition revealed that eyes closed conditions (CSEC and NSEC) showed differences between MSEG and GC (−2.4 a.u., 95%CI = −4.6 to −0.1; and −2.8 a.u., 95%CI = −5.0 to −0.5; respectively), conversely to eyes open condition (CSEO and NSEO). Moreover, differences between groups were evident (p < 0.05) in POST24 and POST40 in all conditions. The random intercept (i.e., participants intercept) presented higher variance (σ2 = 5.30) than residual variance (σ2 = 3.08), which justifies the employment of setting participants as clustered random component. Individual and mean responses can be seen in Figure 3.
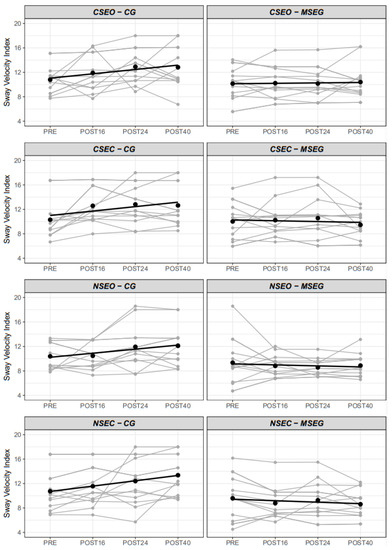
Figure 3.
Individual and mean sway velocity index in the four conditions by the group. CSEO = Comfortable Stance Eyes Open; CSEC = Comfortable Stance Eyes Closed; NSEO = Narrow Stance Eyes Open; NSEC = Narrow Stance Eyes Closed.
4. Discussion
The main findings of this study can highlight that (1) 16 weeks of training were sufficient to improve body control in octogenarians, (2) 8 weeks of detraining were sufficient to observe reductions in the improvements see after the training period, but not strong enough do not return to baseline values, (3) retraining process was able to start reverting the reduction seen after detraining, promoting improvements, and (4) the lack of exercise in the CG led to a trend in a decrease/worsen in all body control parameters.
In this way, the main findings of this study can contribute to the growing body of evidence signaling the protective benefits of strength exercise in the very old populations [30,51]. Our results showed that very old adults performing 45 min of muscular strength exercises, two times a week, for 16 weeks could improve stability and body control, represented by a reduction of 7.9% in the composite index. Moreover, even after the performance decline observed after the detraining process, an increment of 6.4% in the composite index was still present, the exercise program showing a protective effect and avoiding returning to baseline values. Additionally, the MSEG increased their stability, reducing 3.1% their composite index, during the retraining process; meanwhile, CG showed a very marked increase of instability after the first 16 weeks (17.1%) followed by a more gradual increase during the subsequent measuring moments (2.4% at POST24, and 2.3% at POST40). These results agree with those reporting an association between lack of physical activity or regular exercise training with a decrement of postural control and a concomitant worsening in the performance of gait and instrumental activities [52,53].
Therefore, exercise programs have shown positive results, especially those focused on increasing strength combined with functional exercise [21,51,54,55]. Accordingly, in a similar design study [28], it has been reported a 14% improvement in balance after a training period, a reduction of stability of 7% after the detraining period, and a new improvement of 18% after retraining. Taken together, these results confirm a positive effect of strength training over the postural control of older adults [56,57]. Moreover, older adults who did not exercise (CG) increased their sway velocity and the composite index, which is interpreted as an increment of their risk of falls.
We also observed that the CG presented a substantial increment of their sway velocity composite index (17.1%) at POST16, when on the other hand, MSEG presented a 7.9% decrease of sway velocity composite index, indicating an improvement in the estimated risk of falls after that period. A similar pattern was observed in the postural control (in the four conditions (Figure 3), with the CG always showing a worsening trend when compared to the MSEG. It has to be emphasized that MSEG trained resistance exercise with elastic bands for two sessions per week, with similar training configurations producing similar results, showing that groups who performed physical exercise demonstrate a trend to decrease de fall risk by improving their balance [24,57].
In this scenario, other studies comparing older adults who practiced physical activities with those who did not practice [54,55] observed that many older adults were prone to falls in both groups. However, those who practiced physical activity regularly showed a higher level of mobility and less propensity to fall when compared to the inactive group.
After the first training period (POST16), we observed the biggest difference between groups in CSEC (p < 0.001) and NSEC (p < 0.001), these evaluates postural control when visual capacity was taken off, indicating the positive effects of exercise on proprioception, sensorial information, and the vestibular system. Therefore, our results are in line with the study [58] where they found that physical exercise was able to improve significantly the proprioception related to sensorial status in older persons, which is also closely related to the risk of fall [59] and to another study [60] where the practice of exercise apparently was helpful to attenuate the deleterious effect of eye closure on postural control.
After the detraining phase, we expected that the physical capacities of the participants would worsen because of the withdrawal of the exercise program, and consequently, they would diminish their postural control and stability, increasing the risk of fall [19]. However, our study was also able to identify some possible protective effects of exercise because the postural control of the stability test barely varied from POST16 to POST24 in MSEG (0.53%, 0.43%, 0.01%, and 0.09% for CSEO, CSEC, NSEO, and NSEC, respectively). In comparison, percentual increments in instability were observed in CG (10.3%, −1.02%, 9.89, and 0.05% for CSEO, CSEC, NSEO, and NSEC, respectively). These results show a possible protective effect of the exercise program delaying the expected age deterioration of neuromuscular system controlling posture when aging.
In this way, a study [31] observed significant reductions in strength and power after six weeks of detraining, but balance and neuromuscular function did not return to the baseline levels. Therefore, health professionals and researchers have pointed out the relevance of putting their efforts into establishing strategies to delay the aging process or at least maintain the quality of life of people cushioning the consequences of chronic degenerative diseases [22,57,61]. These authors suggested that strength training programs are essential for maintaining muscle strength, balance, functional performance, and independence in older adults.
The results of the present study, when indicating a significant difference in postural stability and fall risk, agree with several studies [24,31,54,62], where physical activity contributes to a lower incidence of falls in older persons. Among the strategies to reduce the action of risk factors for falls, the practice of exercise, like in our study, has been proven as an effective intervention proposal [34,63]. Moreover, most of these studies defend the importance of physical activity and exercise and being active as a method of prevention [6,64,65].
Even if arguing that only the practice of physical activities can reduce the risk of falls by improving body stability and postural control is inconsistent [66,67] because even in the second exercise intervention (retraining), the difference between both groups was not significant, and also, there were cases of older adults in MSEG in whom the score was worse even after the exercise program, this only helps to highlight the multifactorial nature of the process involved. Medications, psychological condition, or even nutritional status, all can affect the risk of fall.
5. Conclusions
Our study revealed that sixteen weeks of two 45 min sessions/week of a resistance training program with elastic bands effectively improved balance control and exerted a protective effect reducing fall risk in very old adults (i.e., > 80 y/o).
During the retraining period, both groups did not change significantly in any variable. However, the MSEG obtained better stability outcomes during this period compared to PRE values. Meanwhile, the CG kept a worsening trend during the eight weeks that this period lasted. The delayed beneficial effects produced on the stability of the MSEG group during the first training period meant that at the end of the detraining period, the participants of this group were at a lower risk of falls than the CG. In addition, we should highlight that we observed greater improvements in CSEC and NSEC conditions, where the visual capacity was taken off during the evaluation, showing a possible positive effect of exercise concerning proprioception and the vestibular system, which should be further studied.
Our results indicate that a band-based resistance training program can positively affect postural stability and the risk of falls, providing an increase in balance and postural stability, which can positively ameliorate the functional ability and mobility of older adults. Additionally, the stability trends observed during the detraining period highlight the need to develop better and more specific physical activity programs for very older people (i.e., >80 years) to ensure adherence to training programs and avoid the detrimental effect of being inactive.
Our study has some limitations that should be reported. We did not measure the participants’ daily physical activity levels, which could influence the results. However, since they were older adults living in nursing homes or social care centers, certain stability can be expected in carrying out their usual physical activities since they were prescribed by the different institutions of origin. In this study, we did not control the incidence of falls prospectively in a follow-up period that would allow us to study the effects of the intervention on the incidence of falls. In future studies, it should be considered to include a follow-up period after intervention with different doses of strength training and functional activities to determine those training configurations that most favor the reduction of falls. In addition, other variables that may affect falls (changes in the medication regimen, aspects related to the context of the participants, psychometric evaluations…) must be recorded to analyze holistically the determining factors that reduce the number of falls in octogenarian adults.
Author Contributions
Conceptualization: R.N.R., G.E.F., C.A. and A.M.T. Methodology: R.N.R., A.C.-S. and G.E.F. Software: R.N.R. and E.C. Validation: R.N.R., E.C. and F.S. analysis: R.N.R. and E.C. Investigation: R.N.R., F.S. and A.C.-S. Data curation: R.N.R., F.S. and E.C. Writing—original draft preparation: R.N.R., E.C., G.E.F. Writing—review and editing: F.S., A.C.-S., Cidalina Abreu, A.M.T. Supervision: C.A., G.E.F. and A.M.T. Project administration: A.M.T. Funding acquisition: E.C., G.E.F. and A.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
Financed by the Portuguese Foundation for Science and Technology (CIDAF- UID/DTP/04213/2020).
Institutional Review Board Statement
This study was approved by the University regional committee (reference number: CEFCDEF/0028/2018) respecting the Portuguese Resolution (Art. 4th; Law no. 12/2005, 1st series) on ethics in human research (Braga, 2013) and the Helsinki Declaration. Clinical trial register number NCT04376463.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to [restrictions e.g. their containing information that could compromise the privacy of research participants].
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization Regional Office for Europe. World Health Statistics. 2018. Available online: https://www.euro.who.int/en/data-and-evidence/european-health-report/european-health-report-2018 (accessed on 22 July 2021).
- Macklai, N.S.; Spagnoli, J.; Junod, J.; Santos-Eggimann, B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: Evidence from 60+ community-dwelling Europeans living in 11 countries. BMC Geriatr. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An overview on current definitions, diagnosis and treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Kearney, F.C.; Harwood, R.H.; Gladman, J.R.; Lincoln, N.; Masud, T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013, 36, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rapp, K.; Becker, C.; Cameron, I.D.; König, H.-H.; Büchele, G. Epidemiology of falls in residential aged care: Analysis of more than 70,000 falls from residents of Bavarian nursing homes. J. Am. Med Dir. Assoc. 2012, 13, 187.e1–187.e6. [Google Scholar] [CrossRef]
- Nnodim, J.O. Balance and its Clinical Assessment in Older Adults-A Review. J. Geriatr. Med. Gerontol. 2015, 1, 3. [Google Scholar] [CrossRef]
- MacKinnon, C.D. Sensorimotor anatomy of gait, balance, and falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar] [CrossRef]
- Maidan, I.; Droby, A.; Jacob, Y.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. The neural correlates of falls: Alterations in large-scale resting-state networks in elderly fallers. Gait Posture 2020, 80, 56–61. [Google Scholar] [CrossRef]
- Almeida, R.; Abreu, C.; Mendes, A. Quedas em doentes hospitalizados: Contributos para uma prática baseada na prevenção. Rev. Enferm. Ref. 2010, 3, 163–172. [Google Scholar] [CrossRef]
- De Menezes, R.L.; Bachion, M.M. Estudo da presença de fatores de riscos intrínsecos para quedas, em idosos institucionalizados. Cien. Saude Colet. 2008, 13, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and fall injuries among adults aged ≥65 years—United States, 2014. Morb. Mortal. Wkly Rep. 2016, 65, 938–983. [Google Scholar] [CrossRef] [PubMed]
- Center of Disease Control and Prevention. Behavioral Risk Factor Surveillance System, LLCP 2020 Codebook Report. 2021. Available online: https://www.cdc.gov/brfss/annual_data/2020/pdf/codebook20_llcp-v2-508.pdf (accessed on 8 March 2022).
- Center of Disease Control and Prevention. Fact About Falls. 2021. Available online: https://www.cdc.gov/falls/facts.html (accessed on 11 March 2022).
- Center of Disease Control and Prevention. Older Adult Fall Prevention. 2019. Available online: https://www.cdc.gov/falls/ (accessed on 13 March 2022).
- Haagsma, J.A.; Olij, B.F.; Majdan, M.; Van Beeck, E.F.; Vos, T.; Castle, C.D.; Dingels, Z.V.; Fox, J.T.; Hamilton, E.B.; Liu, Z.; et al. Falls in older aged adults in 22 European countries: Incidence, mortality and burden of disease from 1990 to 2017. Inj. Prev. 2020, 26 (Suppl. S1), i67–i74. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabil. 2015, 39, 1897–1908. [Google Scholar] [CrossRef]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly Balance Falls Older adults Risk factors. Clin. Geriatr. Med. 2019, 35, 173–183. Available online: https://www.tandfonline.com/doi/full/10.1080/09638288.2016.1212282 (accessed on 13 November 2020). [CrossRef] [PubMed]
- Gotzmeister, D.; Zecevic, A.A.; Klinger, L.; Salmoni, A. People are Getting Lost a Little Bit: Systemic Factors that Contribute to Falls in Community-Dwelling Octogenarians. Can. J. Aging 2015, 34, 397–410. [Google Scholar] [CrossRef]
- Åhlund, K.; Ekerstad, N.; Bäck, M.; Karlson, B.W.; Öberg, B. Preserved physical fitness is associated with lower 1-year mortality in frail elderly patients with a severe comorbidity burden. Clin. Interv. Aging 2019, 14, 577–586. Available online: https://www.dovepress.com/preserved-physical-fitness-is-associated-with-lower-1-year-mortality-i-peer-reviewed-article-CIA (accessed on 26 November 2021). [CrossRef]
- García-Molina, R.; Ruíz-Grao, M.C.; Noguerón-García, A.; Martínez-Reig, M.; Esbrí-Víctor, M.; Izquierdo, M.; Abizanda, P. Benefits of a multicomponent Falls Unit-based exercise program in older adults with falls in real life. Exp. Gerontol. 2018, 110, 79–85. [Google Scholar] [CrossRef]
- Chupel, M.U.; Direito, F.; Furtado, G.E.; Minuzzi, L.G.; Pedrosa, F.M.; Colado, J.C.; Ferreira, J.P.; Filaire, E.; Teixeira, A.M. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front. Physiol. 2017, 8, 377. [Google Scholar] [CrossRef]
- Rieping, T.; Furtado, G.E.; Letieri, R.V.; Chupel, M.U.; Colado, J.C.; Hogervorst, E.; Filaire, E.; Teixeira, A.; Ferreira, J.P. Effects of Different Chair-Based Exercises on Salivary Biomarkers and Functional Autonomy in Institutionalized Older Women. Res. Q. Exerc. Sport 2019, 90, 36–45. [Google Scholar] [CrossRef]
- Figliolino, J.A.M.; Morais, T.B.; Berbel, A.M.; Dal Corso, S. Análise da influência do exercício físico em idosos com relação a equilíbrio, marcha e atividade de vida diária. Rev. Bras Geriatr. Gerontol. 2009, 12, 227–238. [Google Scholar] [CrossRef]
- Freiberger, E.; Haeberle, L.; Spirduso, W.W.; Rixt Zijlstra, G.A. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bruton, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; Vila-Maldonado, S.; Gesteiro, E.; Gusi, N.; Villa-Vicente, J.G.; Espino, L.; Gonzalez-Gross, M.; Casajus, J.A.; et al. The effects of age, organized physical activity and sedentarism on fitness in older adults: An 8-year longitudinal study. Int. J. Environ. Res. Public Health 2020, 17, 4312. [Google Scholar] [CrossRef]
- Casas, A.; Izquierdo, M. Physical exercise as an efficient intervention in frail elderly persons physical exercise as an efficient intervention in frail elderly persons [English;Spanish] Ejercicio fisico como intervencion eficaz en el anciano fragil. An. Sist. Sanit. Navar. 2012, 35, 69–85. [Google Scholar]
- Bruininks, B.D.; Hansen, D.; Wikstrom, L.; Korak, J.A. Short-term Multicomponent Exercise: Effective For Addressing Major Variables That Influence Fall Risk In Older Adults. Med. Sci. Sport Exerc. 2020, 52, 736. [Google Scholar] [CrossRef]
- Guo, J.-L.; Tsai, Y.-Y.; Liao, J.-Y.; Tu, H.-M.; Huang, C.-M. Interventions to reduce the number of falls among older adults with/without cognitive impairment: An exploratory meta-analysis. Int. J. Geriatr. Psychiatry 2014, 29, 661–669. [Google Scholar] [CrossRef] [PubMed]
- de Souza Bezerra, E.; Diefenthaeler, F.; Sakugawa, R.L.; Cadore, E.L.; Izquierdo, M.; Moro, A.R.P. Effects of different strength training volumes and subsequent detraining on strength performance in aging adults. J. Bodyw. Mov. Ther. 2019, 23, 466–472. [Google Scholar] [CrossRef]
- Sakugawa, R.L.; Moura, B.M.; Orssatto, L.B.D.R.; Bezerra, E.D.S.; Cadore, E.L.; Diefenthaeler, F. Effects of resistance training, detraining, and retraining on strength and functional capacity in elderly. Aging Clin. Exp. Res. 2019, 31, 31–39. [Google Scholar] [CrossRef]
- Dipietro, L.; Campbell, W.W.; Buchner, D.M.; Erickson, K.I.; Powell, K.E.; Bloodgood, B.; Hughes, T.; Day, K.R.; Piercy, K.L.; Vaux-Bjerke, A.; et al. Physical Activity, Injurious Falls, and Physical Function in Aging: An Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1303–1313. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I. Sport Med. 2000, 30, 79–87. [Google Scholar] [CrossRef]
- Albornos-Muñoz, L.; Moreno-Casbas, M.T.; Sánchez-Pablo, C.; Bays-Moneo, A.; Fernández-Domínguez, J.C.; Rich-Ruiz, M.; Gea-Sánchez, M.; the Otago Project Working Group. Efficacy of the Otago Exercise Programme to reduce falls in community-dwelling adults aged 65–80 years old when delivered as group or individual training. J. Adv. Nurs. 2018, 74, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ditroilo, M.; Delahunt, E.; De Vito, G. Age Related Changes in Motor Function (II)Decline in Motor Performance Outcomes. Int. J. Sports Med. 2021, 42, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Taani, M.H.; Kovach, C.R.; Buehring, B. Muscle Mechanography: A Novel Method to Measure Muscle Function in Older Adults. Res. Gerontol. Nurs. 2017, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Dynapenia and Aging: An Update. J. Gerontol. Ser. A 2011, 67, 28–40. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Braga, R. Ética na publicação de trabalhos científicos. Rev. Port. Med. Geral. Fam. 2013, 29, 354–356. [Google Scholar] [CrossRef]
- Bigelow, K.E.; Berme, N. Development of a Protocol for Improving the Clinical Utility of Posturography as a Fall-Risk Screening Tool. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 228–233. Available online: https://academic.oup.com/biomedgerontology/article-lookup/doi/10.1093/gerona/glq202 (accessed on 18 January 2020). [CrossRef]
- Pajala, S.; Era, P.; Koskenvuo, M.; Kaprio, J.; Törmäkangas, T.; Rantanen, T. Force Platform Balance Measures as Predictors of Indoor and Outdoor Falls in Community-Dwelling Women Aged 63–76 Years. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 171–178. Available online: https://academic.oup.com/biomedgerontology/article-lookup/doi/10.1093/gerona/63.2.171 (accessed on 20 January 2020). [CrossRef]
- King, A.C.; Whitt-Glover, M.C.; Marquez, D.X.; Buman, M.P.; Napolitano, M.A.; Jakicic, J.; Fulton, J.E.; Tennant, B.L. ACSM Physical Activity Promotion: Highlights from the 2018 Physical Activity Guidelines Advisory Committee Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1340–1353. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Morley, J.E.; Chodzko-Zajko, W.; Pitkala, K.H.; Weening-Djiksterhuis, E.; Rodriguez-Mañas, L.; Barbagallo, M.; Rosendahl, E.; Sinclair, A.; Landi, F.; et al. Recommendations on Physical Activity and Exercise for Older Adults Living in Long-Term Care Facilities: A Taskforce Report. J. Am. Med. Dir. Assoc. 2016, 17, 381–392. [Google Scholar] [CrossRef]
- Ziv, G.; Lidor, R. Music, exercise performance, and adherence in clinical populations and in the elderly: A review. J. Clin. Sport Psychol. 2011, 5, 1–23. [Google Scholar] [CrossRef]
- Borg, G.A.V. Psychophysical Bases of Perception Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Colado, J.C.; Pedrosa, F.M.; Juesas, A.; Gargallo, P.; Carrasco, J.J.; Flandez, J.; Chupel, M.U.; Teixeira, A.M.; Naclerio, F. Concurrent validation of the OMNI-Resistance Exercise Scale of perceived exertion with elastic bands in the elderly. Exp. Gerontol. 2018, 103, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, F. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Gallucci, M. GAMLj: General Analyses for Linear Models. [jamovi module]. 2019. Available online: https://gamlj.github.io/ (accessed on 5 January 2022).
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Bohm, S.; Mersmann, F.; Arampatzis, A. Follow-up efficacy of physical exercise interventions on fall incidence and fall risk in healthy older adults: A systematic review and meta-analysis. Sport Med-Open 2018, 4, 56. [Google Scholar] [CrossRef]
- Wiesmeier, I.K.; Dalin, D.; Maurer, C. Elderly use proprioception rather than visual and vestibular cues for postural motor control. Front. Aging Neurosci. 2015, 7, 97. [Google Scholar] [CrossRef]
- Spink, M.J.; Menz, H.B.; Fotoohabadi, M.R.; Wee, E.; Landorf, K.B.; Hill, K.D.; Lord, S.R. Effectiveness of a multifaceted podiatry intervention to prevent falls in community dwelling older people with disabling foot pain: Randomised controlled trial. BMJ 2011, 342. [Google Scholar] [CrossRef]
- Chan, W.C.; Yeung, J.W.F.; Wong, C.S.M.; Lam, L.C.W.; Chung, K.F.; Luk, J.K.H.; Lee, J.S.W.; Law, A.C.K. Efficacy of physical exercise in preventing falls in older adults with cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2015, 16, 149–154. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Inokuti, T.T.; da Costa Bispo, N.N.; de Almeida Pires Oliveira, D.A.; de Oliveira, R.F.; da Silva, R.A., Jr. Elderly individuals with increased risk of falls show postural balance impairment. Fisioter. Mov. 2015, 28, 269–276. [Google Scholar] [CrossRef]
- De Carvalho, M.P.; Luckow, E.L.T.; Siqueira, F.V. Quedas e fatores associados em idosos institucionalizados no município de Pelotas (RS, Brasil). Cien. Saude Colet. 2011, 16, 2945–2952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Padoin, P.G.; Gonçalves, M.P.; Comaru, T.; Silva, A.M.V. Análise comparativa entre idosos praticantes de exercício físico e sedentários quanto ao risco de quedas. O Mundo. Saúde. 2010, 35, 158–164. [Google Scholar] [CrossRef]
- Ribeiro, F.; Gomes, S.; Teixeira, F.; Brochado, G.; Oliveira, J. Impacto da prática regular de exercício físico no equilíbrio, mobilidade funcional e risco de queda em idosos institucionalizados. Rev. Port. Ciências Desporto. 2016, 9, 36–42. [Google Scholar] [CrossRef]
- Prioli, A.C.; Freitas Júnior, P.B.; Barela, J.A. Physical activity and postural control in the elderly: Coupling between visual information and body sway. Gerontology 2005, 51, 145–148. [Google Scholar] [CrossRef]
- Lelard, T.; Doutrellot, P.L.; David, P.; Ahmaidi, S. Effects of a 12-Week Tai Chi Chuan Program Versus a Balance Training Program on Postural Control and Walking Ability in Older People. Arch. Phys. Med. Rehabil. 2010, 91, 9–14. [Google Scholar] [CrossRef]
- Tokmakidis, S.P.; Kalapotharakos, V.I.; Smilios, I.; Parlavantzas, A. Effects of detraining on muscle strength and mass after high or moderate intensity of resistance training in older adults. Clin. Physiol. Funct. Imaging 2009, 29, 316–319. [Google Scholar] [CrossRef]
- Salzman, B. Gait and balance disorders in older adults. Am. Fam. Physician 2011, 82, 61–68. [Google Scholar]
- Ramalho, F.; Santos-Rocha, R.; Branco, M.; Moniz-Pereira, V.; André, H.I.; Veloso, A.P.; Carnide, F. Effect of 6-month community-based exercise interventions on gait and functional fitness of an older population: A quasi-experimental study. Clin. Interv. Aging 2018, 13, 595–606. [Google Scholar] [CrossRef]
- Johnson, M.; George, A.; Tran, D.T. Analysis of falls incidents: Nurse and patient preventive behaviours. Int. J. Nurs. Pract. 2011, 17, 60–66. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef]
- Bamidis, P.D.; Vivas, A.B.; Styliadis, C.; Frantzidis, C.; Klados, M.; Schlee, W.; Siountas, A.; Papageorgiou, S.G. A review of physical and cognitive interventions in aging. Neurosci. Biobehav. Rev. 2014, 44, 206–220. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).