A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance
Abstract
1. Introduction
2. Materials and Methods
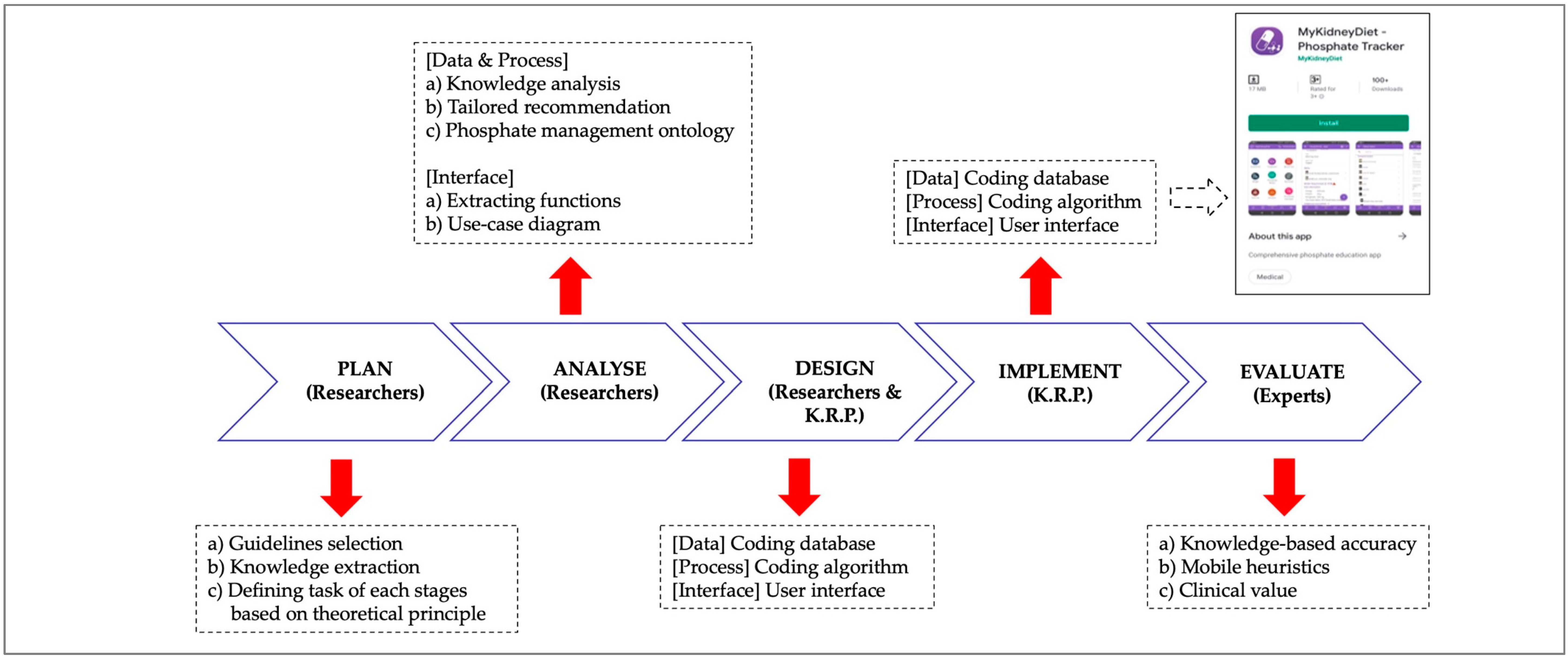
2.1. Development of Phosphate Mobile App (PMA)
2.1.1. Planning of the PMA
- Intestinal absorption for inorganic phosphate sources is 100%, 50% for animal-based dietary phosphate (40–60%), and 10–30% for plant-based dietary phosphate [16]. For a mixed meal (containing more than one dietary phosphate source), the higher intestinal absorption value as per individual food components was referenced.
- Dialysis treatment removes about 2300–2600 mg phosphate per week with the standard 12 h HD treatment a week and blood flow achieving a dialysis blood quotient (Qb) of 300 mL/min on average [45].
- Phosphate binding capacity for calcium carbonate is 19.0 mg per 500 mg tablet and for lanthanum carbonate is 67.5 mg per 500 mg tablet and for sevelamer is 21.4 mg per 800 mg tablet [46].
- Patients have about 40% phosphate removal through bowel output (feces) and do not have constipation issues [47].
2.1.2. Analytical Procedures of the PMA
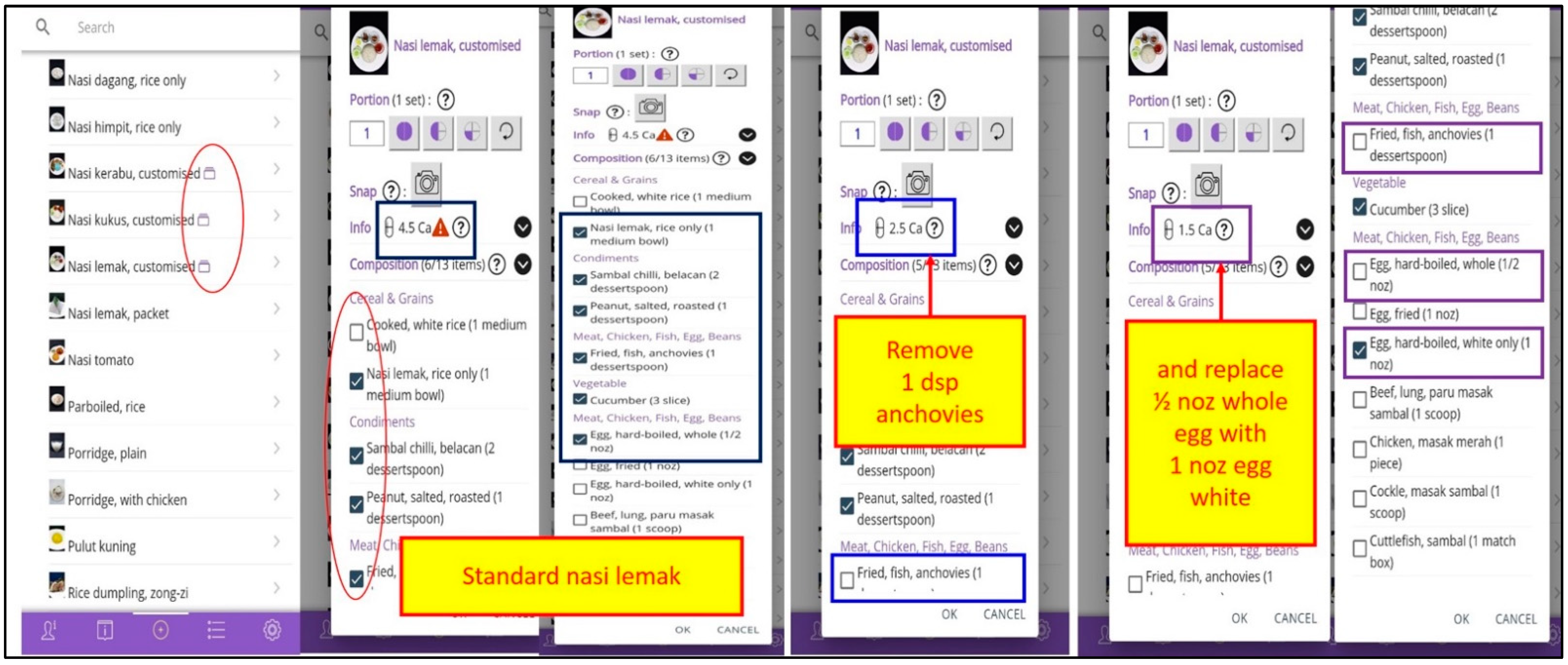
2.1.3. Design of the PMA
- Education videography regarding phosphate and dialysis lifestyle;
- Interactive self-efficacy features included food and beverages nutrient content and choices to reduce dietary phosphate load, goal setting in blood profiles and weight, self-monitoring of dietary phosphate intake, dialysis treatment, phosphate binder use, emotion, exercise and weight, and phosphate calculation for serum phosphorus reduction; and
- Interactive cues to action features included phosphate binder intake reminders and dietary phosphate and phosphate binder adjustments.
2.1.4. Implementation of the PMA
- Profile—providing the user profile information.
- Information—providing seven features of education, which include graphical household Measurement tools, and videography on Phosphate, Dialysis, Phosphate Binder, Dietary Phosphate, Lifestyle, and Responsibility.
- Input—providing nine features that supported self-efficacy and cues to action, for Food/drink, Treatment, Blood test, Weight, Phosphate binder, Phosphate binder reminder, Exercise, Emotion, and Phosphate calculator.
- Log—providing three features for record-keeping that come in different formats of Daily, Periodic, and Feature-based log.
- Setting—providing six features facilitating users for Unit converter, Goal setting, FAQ, Feedback, Adjust font, and Language option.
2.1.5. Validation of the PMA by Expert Reviewers
2.2. Evaluation of PMA Acceptance by Adult HD Patients
2.2.1. Study Design
2.2.2. Patient Recruitment
2.2.3. Demographics, Clinical, and Mobile App Experience
2.2.4. Outcome Measures
2.2.5. Statistical Analysis
3. Results
3.1. Phosphate Mobile App
3.2. Experts Feedback
3.3. Evaluation of PMA Acceptance by HD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Goh, B.L. Twenty Forth Report of the Malaysian Dialysis and Transplant 2016; National Renal Registry-Clinical Research Centre/Ministry of Health: Kuala Lumpur, Malaysia, 2018.
- Palmer, S.C.; Hayen, A.; Macaskill, P.; Pellegrini, F.; Craig, J.C.; Elder, G.J.; Strippoli, G.F. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 2011, 305, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsuruya, K.; Taniguchi, M.; Tokumoto, M.; Fujisaki, K.; Hirakata, H.; Fujimi, S.; Kitazono, T. Association between serum phosphate levels and stroke risk in patients undergoing hemodialysis: The Q-cohort study. Stroke 2016, 47, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Mittalhenkle, A.; Gillen, D.L.; Stehman-Breen, C.O. Increased risk of mortality associated with hip fracture in the dialysis population. Am. J. Kidney Dis. 2004, 44, 672–679. [Google Scholar] [CrossRef]
- Russo, D.; Miranda, I.; Ruocco, C.; Battaglia, Y.; Buonanno, E.; Manzi, S.; Russo, L.; Scafarto, A.; Andreucci, V.E. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007, 72, 1255–1261. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Goh, B.L.; Mushahar, L.; Ching, C.H. 1st Malaysian CKD-MBD & Parathyroidectomy Guidelines and Standard Operating Procedures; Malaysian Society of Nephrology: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding development of malnutrition in hemodialysis patients: A narrative review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Woolf, K.; Pompeii, M.L.; Kalantar-Zadeh, K.; Sevick, M.A. Reexamining the Phosphorus–Protein Dilemma: Does Phosphorus Restriction Compromise Protein Status? J. Ren. Nutr. 2016, 26, 136–140. [Google Scholar] [CrossRef]
- Peter, W.L.S.; Wazny, L.D.; Weinhandl, E.; Cardone, K.E.; Hudson, J.Q. A review of phosphate binders in chronic kidney disease: Incremental progress or just higher costs? Drugs 2017, 77, 1155–1186. [Google Scholar] [CrossRef]
- Hand, R.K.; Steiber, A.; Burrowes, J. Renal dietitians lack time and resources to follow the NKF KDOQI guidelines for frequency and method of diet assessment: Results of a survey. J. Ren. Nutr. 2013, 23, 445–449. [Google Scholar] [CrossRef]
- Ng, E.S.Y.; Wong, P.Y.; Kamaruddin, A.T.H.; Lim, C.T.S.; Chan, Y.M. Poor sleep quality, depression and social support are determinants of serum phosphate level among hemodialysis patients in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5144. [Google Scholar] [CrossRef]
- Pollock, J.B.; Jaffery, J.B. Knowledge of phosphorus compared with other nutrients in maintenance dialysis patients. J. Ren. Nutr. 2007, 17, 323–328. [Google Scholar] [CrossRef]
- Cupisti, A.; Ferretti, V.; D’Alessandro, C.; Petrone, I.; Di Giorgio, A.; Meola, M.; Panichi, V.; Conti, P.; Lippi, A.; Caprioli, R.; et al. Nutritional knowledge in hemodialysis patients and nurses: Focus on phosphorus. J. Ren. Nutr. 2012, 22, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Chan, M.W.; Cheah, H.M.; Padzil, M.B.M. Multidisciplinary education approach to optimize phosphate control among hemodialysis patients. Int. J. Clin. Pharm. 2019, 41, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Okpechi, I.G.; Ye, F.; Kovesdy, C.P.; Brunori, G.; Burrowes, J.D.; Campbell, K.; Damster, S.; Fouque, D.; Friedman, A.N.; et al. Assessing Global Kidney Nutrition Care. Clin. J. Am. Soc. Nephrol. 2022, 17, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.H.; Chinna, K.; Gafor, A.H.A.; Morad, Z.; Ahmad, G.; Bavanandam, S.; Visvanathan, R.; Yahya, R.; Goh, B.L.; Bee, B.C.; et al. The state of nutrition care in outpatient hemodialysis settings in Malaysia: A nationwide survey. BMC Health Serv. Res. 2018, 18, 939. [Google Scholar] [CrossRef]
- Karupaiah, T.; Morad, Z. Perspectives on the nutritional management of renal disease in Asia: People, practice, and programs. J. Ren. Nutr. 2007, 17, 93–96. [Google Scholar] [CrossRef]
- Ismail, H.; Manaf, M.R.A.; Gafor, A.H.A.; Zaher, Z.M.M.; Ibrahim, A.I.N. Economic burden of ESRD to the Malaysian health care system. Kidney Int. Rep. 2019, 4, 1261–1270. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer. Adherence 2013, 7, 379–390. [Google Scholar] [CrossRef]
- Karupaiah, T.; Swee, C.S.; Abdullah, R. Developing a nutrition education package for Malaysian hemodialysis patients. J. Ren. Nutr. 2001, 11, 220–227. [Google Scholar] [CrossRef]
- Chee, W.S.S.; Karupaiah, T. Smart Eating for Chronic Kidney Disease-Slowing down Kidney Failure with Protein Counting; Universiti Kebangsaan Malaysia (UKM)-International Medical University (IMU)-Fresenius Kabi: Kuala Lumpur, Malaysia, 2009. [Google Scholar]
- Karupaiah, T.; Daud, Z.A.M.; Khor, B.H.; Sahathevan, S.; Sualeheen, A. Food Phosphate Guide for Chronic Kidney Disease: What Patients Need to Know, Learn, and Practice; Sanofi-Malaysian Society of Nephrology: Kuala Lumpur, Malaysia, 2019. [Google Scholar]
- Leung, S.; McCormick, B.; Wagner, J.; Biyani, M.; Lavoie, S.; Imtiaz, R.; Zimmerman, D. Meal phosphate variability does not support fixed dose phosphate binder schedules for patients treated with peritoneal dialysis: A prospective cohort study. BMC Nephrol. 2015, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Akiba, T.; Albert, J.M.; McCarthy, J.T.; Kerr, P.G.; Mendelssohn, D.C.; Jadoul, M. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2004, 44, 34–38. [Google Scholar] [CrossRef]
- Karduck, J.; Chapman-Novakofski, K. Results of the clinician apps survey, how clinicians working with patients with diabetes and obesity use mobile health apps. J. Nutr. Educ. Behav. 2018, 50, 62–69. [Google Scholar] [CrossRef]
- Prest, M. Mobile phone applications for kidney patients. J. Ren. Nutr. 2013, 23, 83–85. [Google Scholar] [CrossRef]
- Campbell, J.; Porter, J. Dietary mobile apps and their effect on nutritional indicators in chronic renal disease: A systematic review. Nephrology 2015, 20, 744–751. [Google Scholar] [CrossRef]
- Kosa, S.D.; Monize, J.; D’Souza, M.; Joshi, A.; Philip, K.; Reza, S.; Samra, S.; Serrago, B.; Thabane, L.; Gafni, A.; et al. Nutritional mobile applications for CKD patients: Systematic review. Kidney Int. Rep. 2019, 4, 399–407. [Google Scholar] [CrossRef]
- Imtiaz, R.; Atkinson, K.; Guerinet, J.; Wilson, K.; Leidecker, J.; Zimmerman, D. A pilot study of OkKidney, a phosphate counting application in patients on peritoneal dialysis. Perit. Dial. Int. 2017, 37, 613–618. [Google Scholar] [CrossRef]
- Farfan-Ruiz, A.C.; Czikk, D.; Leidecker, J.; Ramsay, T.; McCormick, B.; Wilson, K.; Zimmerman, D. Multidisciplinary Team Versus a ‘Phosphate Counting’ App for Serum Phosphate Control: A Randomized Controlled Trial. Kidney360 2020, 2, 290–297. [Google Scholar] [CrossRef]
- El Khoury, C.F.; Crutzen, R.; Schols, J.M.; Halfens, R.J.; Karavetian, M. A dietary mobile app for patients undergoing hemodialysis: Prospective pilot study to improve dietary intakes. J. Med. Internet Res. 2020, 22, e17817. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chang, Y.P.; Lin, S.C.; Lin, C.; Hsu, P.H.; Hsu, Y.J.; Wu, T.J. Effects of individualized dietary phosphate control program with a smartphone application in hemodialysis patients in Taiwan. Biol. Res. Nurse 2021, 23, 375–381. [Google Scholar] [CrossRef]
- Bobek, E.; Tversky, B. Creating visual explanations improves learning. Cogn. Res. Princ. Implic. 2016, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K. Management of hyperphosphatemia. Hemodial. Int. 2006, 10, 338–345. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Hanning, R.M. Dietary assessment and self-monitoring with nutrition applications for mobile devices. Can. J. Diet. Pract. Res. 2012, 73, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.O.; Ortman, C.; Almaani, S.; Lee, Y.H.; Jordan, K. Understanding the associations between modifying factors, individual health beliefs, and hemodialysis patients’ adherence to a low-phosphorus diet. J. Ren. Nutr. 2015, 25, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, H.A. A mobile app for hypertension management based on clinical practice guidelines: Development and deployment. JMIR mHealth uHealth 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease (CKD) Evidence-Based Nutrition Practice Guideline. Available online: https://www.andeal.org/topic.cfm?cat=3927&highlight=Chronic%20Kidney%20Disease%20Guideline%202010%20&home=1 (accessed on 14 September 2018).
- National Kidney Foundation. Dialysis Patients’ Bill of Rights and Responsibilities; National Kidney Foundation: New York, NY, USA, 2013. [Google Scholar]
- Khor, B.H.; Sualeheen, A.; Sahathevan, S.; Chinna, K.; Gafor, A.H.A.; Bavanandan, S.; Goh, B.L.; Morad, Z.; Daud, Z.A.M.; Khosla, P.; et al. Association of dietary patterns with serum phosphorus in maintenance haemodialysis patients: A cross-sectional study. Sci. Rep. 2020, 10, 12278. [Google Scholar] [CrossRef]
- Sualeheen, A.; Khor, B.H.; Balasubramanian, G.V.; Sahathevan, S.; Ali, M.S.M.; Narayanan, S.S.; Chinna, K.; Daud, Z.A.M.; Khosla, P.; Gafor, A.H.A.; et al. Habitual dietary patterns of patients on hemodialysis indicate nutritional risk. J. Ren. Nutr. 2020, 30, 322–332. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M.; Rizzo, M.A.; Caria, S.; Meola, M.; Bolasco, P. Phosphate control in dialysis. Int. J. Nephrol. Renov. Dis. 2013, 6, 193–205. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Finn, W.F.; Emmett, M.; Chertow, G.M.; Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin. Dial. 2011, 24, 41–49. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Etikan, I.; Musa, S.A.; Alkassim, R.S. Comparison of convenience sampling and purposive sampling. Am. J. Theor. Appl. Stat. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Bertini, E.; Gabrielli, S.; Kimani, S.; Catarci, T.; Santucci, G. Appropriating and assessing heuristics for mobile computing. In Proceedings of the Working Conference on Advanced Visual Interfaces, Venezia, Italy, 23–26 May 2006; pp. 119–126. [Google Scholar]
- Zhou, L.; Bao, J.; Setiawan, I.M.A.; Saptono, A.; Parmanto, B. The mHealth APP usability questionnaire (MAUQ): Development and validation study. JMIR mHealth uHealth 2019, 7, 11500. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Liu, M.L.; Lin, C.P. Integrating technology readiness into the expectation–confirmation model: An empirical study of mobile services. Cyberpsychol. Behav. Soc. Netw. 2013, 16, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Baharum, A.; Jaafar, A. User interface design: A study of expectation-confirmation theory. In Proceedings of the 5th International Conference on Computing and Informatic, Istanbul, Turkey, 11–13 August 2015. [Google Scholar]
- Lim, J.-H.; Chinna, K.; Khosla, P.; Karupaiah, T.; Daud, Z.A.M. Understanding How Nutrition Literacy Links to Dietary Adherence in Patients Undergoing Maintenance Hemodialysis: A Theoretical Exploration Using Partial Least Squares Structural Equation Modeling. Int. J. Environ. Res. Public Health 2020, 17, 7479. [Google Scholar] [CrossRef]
- Lambert, K.; Mullan, J.; Mansfield, K.; Owen, P. Should we recommend renal diet–related apps to our patients? An evaluation of the quality and health literacy demand of renal diet–related mobile applications. J. Ren. Nutr. 2017, 27, 430–438. [Google Scholar] [CrossRef]
- Pinto, L.C.S.; Andrade, M.C.; Chaves, R.O.; Lopes, L.L.B.; Maués, K.G.; Monteiro, A.M.; Nascimento, M.B.; Barros, C.A.V. Development and validation of an application for follow-up of patients undergoing dialysis: NefroPortátil. J. Ren. Nutr. 2020, 30, 51–57. [Google Scholar] [CrossRef]
- Elder, G.J.; Malik, A.; Lambert, K. Role of dietary phosphate restriction in chronic kidney disease. Nephrology 2018, 23, 1107–1115. [Google Scholar] [CrossRef]
- Welch, J.; Dowell, S.; Johnson, C.S. Feasibility of using a personal digital assistant to self-monitor diet and fluid intake: A pilot study. Nephrol. Nurs. J. 2007, 34, 43. [Google Scholar]
- Koprucki, M.; Piraino, B.; Bender, F.; Snetselaar, L.; Hall, B.; Stark, S.; Sevick, M.A. RCT of Personal Digital Assistant (PDA) supported dietary intervention to reduce sodium intake in PD. Am. J. Kidney Dis. 2010, 55, A72. [Google Scholar] [CrossRef]
- Stark, S.; Snetselaar, L.; Piraino, B.; Stone, R.A.; Kim, S.; Hall, B.; Burke, L.E.; Sevick, M.A. Personal digital assistant-based self-monitoring adherence rates in 2 dialysis dietary intervention pilot studies: BalanceWise-HD and BalanceWise-PD. J. Ren. Nutr. 2011, 21, 492–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cueto-Manzano, A.M.; Gallardo-Rincón, H.; Martínez-Ramírez, H.R.; Cortés-Sanabria, L.; Rojas-Campos, E.; Tapia-Conyer, R.; Martínez, P.; Cerrillos, I.; Andrade, J.; Medina, M. A pilot study of a mobile phone application to improve lifestyle and adherence of patients with kidney disease. J. Telemed. Telecare 2015, 21, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.; Siek, K.A.; Chaudry, B.; Jones, J.; Astroth, K.; Welch, J.L. An offline mobile nutrition monitoring intervention for varying-literacy patients receiving hemodialysis: A pilot study examining usage and usability. J. Am. Med. Inform. Assoc. 2012, 19, 705–712. [Google Scholar] [CrossRef]
- Welch, J.L.; Astroth, K.S.; Perkins, S.M.; Johnson, C.S.; Connelly, K.; Siek, K.A.; Jones, J.; Scott, L.L. Using a mobile application to self-monitor diet and fluid intake among adults receiving hemodialysis. Res. Nurs. Health 2013, 36, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Wong, C.P.; Lee, S.W.H. m-Health views and perception among Malaysian: Findings from a survey among individuals living in Selangor. Mhealth 2020, 6, 6–13. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Haskard, K.B.; Williams, S.L. Health beliefs, disease severity, and patient adherence: A meta-analysis. Med. Care 2007, 45, 521–528. [Google Scholar] [CrossRef]
- Weinstein, N.D.; Sandman, P.M.; Blalock, S.J. The precaution adoption process model. In The Wiley Encyclopedia of Health Psychology; Sweeny, K., Robbins, M.L., Cohen, L.M., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]

| Parameter | Mean ± SD | n (%) |
|---|---|---|
| Age | 48.1 ± 13.2 | |
| ꝉ HD vintage (month) | 72 (76) | |
| Gender | ||
| Male | 70 (50.4) | |
| Female | 69 (49.6) | |
| Ethnicity | ||
| Malay | 65 (46.8) | |
| Chinese | 50 (36.0) | |
| Indian | 22 (15.8) | |
| Others | 2 (1.4) | |
| Marital status | ||
| Married | 105 (75.5) | |
| Single | 29 (20.9) | |
| Divorced | 5 (3.6) | |
| Education level | ||
| Diploma/Degree/Higher | 57 (41.0) | |
| Secondary | 72 (51.8) | |
| Primary | 10 (7.2) | |
| Monthly income | ||
| <RM1000 | 68 (48.9) | |
| RM1001–RM3000 | 47 (33.8) | |
| RM3001–RM5000 | 13 (9.4) | |
| >RM5000 | 11 (7.9) | |
| Employment | ||
| Working | 50 (36.0) | |
| Not working | 89 (64.0) | |
| Sector (Dialysis center) | ||
| Government | 42 (30.2) | |
| Non-government | 68 (48.9) | |
| Private | 29 (20.9) | |
| Type of phosphate binder | ||
| Calcium carbonate | 124 (89.2) | |
| Sevelamer carbonate | 8 (5.8) | |
| Lanthanum carbonate | 5 (3.6) | |
| Not on phosphate binder | 2 (1.4) | |
| Operating System | ||
| Android | 130 (93.5) | |
| iPhone | 9 (6.5) | |
| Smartphone use during HD treatment | ||
| Yes | 124 (89.2) | |
| No | 15 (10.8) | |
| ꝉ If yes, mobile app use during HD (minute/session) | 120 (105) | |
| Type of mobile app use during HD * | ||
| Watch video | 85 (61.2) | |
| Social interaction | 78 (56.1) | |
| Games | 38 (27.3) | |
| Listen to music | 36 (25.9) | |
| Educational & information seeking | 25 (18.0) | |
| Previous nutrition apps use | ||
| Yes | 7 (5.0) | |
| No | 132 (95.0) | |
| Challenges of nutrition app use * | ||
| Features do not meet my expectation | 3 (2.2) | |
| Information is too general | 3 (2.2) | |
| No new information | 1 (0.7) | |
| Reason for not using a nutrition app * | ||
| I am not aware of the app in the app store | 102 (77.3) | |
| Features offered do not meet my expectation | 24 (18.2) | |
| Not interested in a nutrition app | 4 (3.0) | |
| I have to pay for it | 1 (0.8) | |
| Not enough phone storage | 1 (0.8) | |
| No. | Statement | n (%) | ||
|---|---|---|---|---|
| Agree | Neutral | Disagree | ||
| Ease of use a | 96 (69.2) | 26 (18.7) | 17 (12.1) | |
| 1 | The app was easy to use. | 97 (69.8) | 27 (19.4) | 15 (10.8) |
| 2 | It was easy for me to learn to use the app. | 99 (71.2) | 24 (17.3) | 16 (11.5) |
| 3 | The navigation was consistent when moving between screens. | 98 (70.5) | 23 (16.5) | 18 (13.0) |
| 4 | The interface of the app allowed me to use all the functions (such as entering information. Responding to reminders, viewing information) offered by the app. | 94 (67.6) | 29 (20.9) | 16 (11.5) |
| 5 | Whenever I made a mistake using the app, I could recover easily and quickly. | 93 (66.9) | 27 (19.4) | 19 (13.7) |
| Interface and satisfaction a | 96 (69.0) | 30 (21.7) | 13 (9.3) | |
| 6 | I like the interface of the app. | 96 (69.0) | 29 (21.0) | 14 (10.0) |
| 7 | The information in the app was well organized, so I could easily find the information I needed. | 96 (69.0) | 31 (22.3) | 12 (8.7) |
| 8 | The app adequately acknowledged and provided information to let me know the progress of my action. | 98 (70.5) | 28 (20.1) | 13 (9.4) |
| 9 | I feel comfortable using this app in social settings. | 98 (70.5) | 33 (23.7) | 8 (5.8) |
| 10 | The amount of time involved in using this app has been fitting for me. | 92 (66.2) | 30 (21.6) | 17 (12.2) |
| Usefulness a | 98 (70.1) | 25 (18.1) | 16 (11.8) | |
| 11 | I would use this app again. | 104 (74.8) | 24 (17.3) | 11 (7.9) |
| 12 | Overall, I am satisfied with this app. | 99 (71.2) | 28 (20.1) | 12 (8.7) |
| 13 | The app would be useful for my health care practice. | 103 (74.1) | 21 (15.1) | 15 (10.8) |
| 14 | The app improved my access to health care services. | 101 (72.7) | 21 (15.1) | 17 (12.2) |
| 15 | The app helped me manage my health effectively. | 97 (69.8) | 23 (16.5) | 19 (13.7) |
| 16 | This app has all the functions and capabilities I expected it to have. | 97 (69.8) | 21 (15.1) | 21 (15.1) |
| 17 | I could use the app when the Internet connection was poor or not available. | 78 (56.1) | 38 (27.3) | 23 (16.6) |
| 18 | This app provided an acceptable way to receive health care services, such as accessing educational materials, tracking my own activities, and performing self-assessment. | 101 (72.7) | 25 (18.0) | 13 (9.3) |
| Recommendation * | ||||
| 19 | I would recommend this app to my friend on dialysis. | 101 (72.7) | 27 (19.4) | 11 (7.9) |
| Feature | Utilize a | Likert Scale a | ||
|---|---|---|---|---|
| Agree | Neutral | Disagree | ||
| Information | ||||
| Measurement | 100 (71.9) | 96 (96.0) | 4 (4.0) | 0 (0.0) |
| Phosphate | 125 (89.9) | 119 (95.2) | 3 (2.4) | 3 (2.4) |
| Dialysis | 122 (87.8) | 115 (94.2) | 3 (2.5) | 4 (3.3) |
| Phosphate binder | 127 (91.4) | 120 (94.5) | 4 (3.1) | 3 (2.4) |
| Dietary phosphate | 127 (91.4) | 120 (94.5) | 4 (3.1) | 3 (2.4) |
| Lifestyle | 101 (72.7) | 93 (92.1) | 6 (5.9) | 2 (2.0) |
| Responsibility | 102 (73.4) | 96 (94.1) | 2 (2.0) | 4 (3.9) |
| Input | ||||
| Food/drink | 112 (80.6) | 105 (93.7) | 4 (3.6) | 3 (2.7) |
| Treatment | 45 (32.4) | 43 (95.6) | 1 (2.2) | 1 (2.2) |
| Blood test | 38 (27.3) | 37 (97.4) | 1 (2.6) | 0 (0.0) |
| Weight | 39 (28.1) | 37 (94.9) | 2 (5.1) | 0 (0.0) |
| Phosphate binder | 48 (34.5) | 47 (97.9) | 1 (2.1) | 0 (0.0) |
| Reminder | 36 (25.9) | 34 (94.4) | 2 (5.6) | 0 (0.0) |
| Exercise | 34 (24.5) | 33 (97.1) | 1 (2.9) | 0 (0.0) |
| Emotion | 31 (22.3) | 30 (96.8) | 1 (3.2) | 0 (0.0) |
| Phosphate calculator | 37 (26.6) | 36 (97.3) | 1 (2.7) | 0 (0.0) |
| Log | ||||
| Daily | 36 (25.9) | 35 (97.2) | 1 (2.8) | 0 (0.0) |
| Periodic | 31 (22.3) | 30 (96.8) | 1 (3.2) | 0 (0.0) |
| Feature-based | 30 (21.6) | 29 (96.7) | 1 (3.3) | 0 (0.0) |
| Setting | ||||
| Unit converter | 31 (22.3) | 30 (96.8) | 1 (3.2) | 0 (0.0) |
| Goal setting | 30 (21.6) | 29 (96.7) | 1 (3.3) | 0 (0.0) |
| FAQ | 34 (24.5) | 33 (97.1) | 1 (2.9) | 0 (0.0) |
| Feedback | 35 (25.2) | 33 (94.3) | 2 (5.7) | 0 (0.0) |
| Adjust font | 41 (29.5) | 40 (97.6) | 1 (2.4) | 0 (0.0) |
| Language option | 104 (74.8) | 104 (100.0) | 0 (0.0) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teong, L.-F.; Khor, B.-H.; Radion Purba, K.; Gafor, A.H.A.; Goh, B.-L.; Bee, B.-C.; Yahya, R.; Bavanandan, S.; Ng, H.-M.; Sahathevan, S.; et al. A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance. Healthcare 2022, 10, 535. https://doi.org/10.3390/healthcare10030535
Teong L-F, Khor B-H, Radion Purba K, Gafor AHA, Goh B-L, Bee B-C, Yahya R, Bavanandan S, Ng H-M, Sahathevan S, et al. A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance. Healthcare. 2022; 10(3):535. https://doi.org/10.3390/healthcare10030535
Chicago/Turabian StyleTeong, Lee-Fang, Ban-Hock Khor, Kristo Radion Purba, Abdul Halim Abdul Gafor, Bak-Leong Goh, Boon-Cheak Bee, Rosnawati Yahya, Sunita Bavanandan, Hi-Ming Ng, Sharmela Sahathevan, and et al. 2022. "A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance" Healthcare 10, no. 3: 535. https://doi.org/10.3390/healthcare10030535
APA StyleTeong, L.-F., Khor, B.-H., Radion Purba, K., Gafor, A. H. A., Goh, B.-L., Bee, B.-C., Yahya, R., Bavanandan, S., Ng, H.-M., Sahathevan, S., Narayanan, S. S., Daud, Z. A. M., Khosla, P., & Karupaiah, T. (2022). A Mobile App for Triangulating Strategies in Phosphate Education Targeting Patients with Chronic Kidney Disease in Malaysia: Development, Validation, and Patient Acceptance. Healthcare, 10(3), 535. https://doi.org/10.3390/healthcare10030535










