Effects of Different Scan Projections on the Quantitative Ultrasound-Based Evaluation of Hepatic Steatosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. US Examination
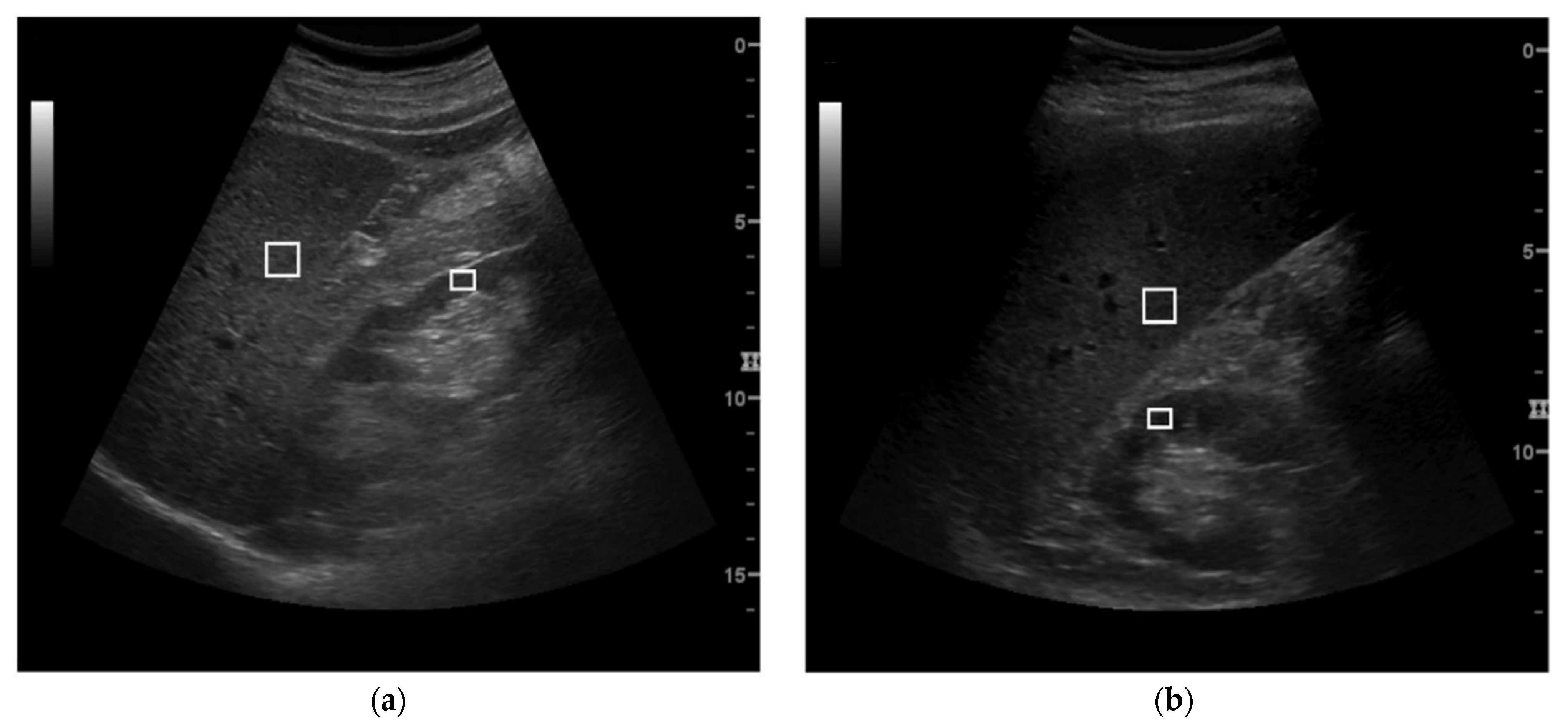
2.3. US Parameters Calculation
2.4. Steato-Score Assessment
2.5. Qualitative US Evaluations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marchesini, G. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Howe, L.; Jones, H.; Higgins, J.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [Green Version]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis—New insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef]
- Lim, S.; Taskinen, M.-R.; Borén, J. Crosstalk between nonalcoholic fatty liver disease and cardiometabolic syndrome. Obes. Rev. 2019, 20, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Askling, J.; Hultcrantz, R.; Kechagias, S. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sao, R.; Aronow, W.S. Association of non-alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch. Med. Sci. 2018, 14, 1233–1244. [Google Scholar] [CrossRef]
- Cetindağlı, I.; Kara, M.; Tanoglu, A.; Ozalper, V.; Aribal, S.; Hancerli, Y.; Unal, M.; Ozarı, O.; Hira, S.; Kaplan, M.; et al. Evaluation of endothelial dysfunction in patients with nonalcoholic fatty liver disease: Association of selenoprotein P with carotid intima-media thickness and endothelium-dependent vasodilation. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Ruffini, R.; Agnoletti, D.; Magnani, E.; Pagliarani, G.; Comandini, G.; Praticò, A.; Borghi, C.; Benetos, A.; Pazzi, P. Increased arterial stiffness in nonalcoholic fatty liver disease: The Cardio-GOOSE study. J. Hypertens. 2010, 28, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Merriman, R.B.; Ferrell, L.D.; Patti, M.G.; Weston, S.R.; Pabst, M.S.; Aouizerat, B.E.; Bass, N.M. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology 2006, 44, 874–880. [Google Scholar] [CrossRef]
- Hannah, W.N.; Harrison, S.A. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2016, 64, 2234–2243. [Google Scholar] [CrossRef]
- Bohte, A.E.; Van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2010, 21, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Cowin, G.J.; Jonsson, J.R.; Bauer, J.; Ash, S.; Ali, A.; Osland, E.; Purdie, D.M.; Clouston, A.; Powell, E.; Galloway, G. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J. Magn. Reson. Imaging 2008, 28, 937–945. [Google Scholar] [CrossRef]
- Khov, N. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 6821–6825. [Google Scholar] [CrossRef]
- Riley, T.R.; Mendoza, A.; Bruno, M.A.; Iii, T.R.R. Bedside Ultrasound Can Predict Nonalcoholic Fatty Liver Disease in the Hands of Clinicians Using a Prototype Image. Dig. Dis. Sci. 2006, 51, 982–985. [Google Scholar] [CrossRef]
- Ballestri, S.; Lonardo, A.; Romagnoli, D.; Carulli, L.; Losi, L.; Day, C.P.; Loria, P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012, 32, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Prinster, A.; Annuzzi, G.; Liuzzi, R.; Giacco, R.; Medagli, C.; Cremone, M.; Clemente, G.; Maurea, S.; Riccardi, G.; et al. Sonographic hepatic-renal ratio as indicator of hepatic steatosis: Comparison with 1H magnetic resonance spectroscopy. Metab. Clin. Exp. 2009, 58, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-F.; Yan, H.-M.; He, W.-Y.; Li, X.-M.; Li, C.-L.; Yao, X.-Z.; Li, R.-K.; Zeng, M.-S.; Gao, X. Standardized Ultrasound Hepatic/Renal Ratio and Hepatic Attenuation Rate to Quantify Liver Fat Content: An Improvement Method. Obesity 2012, 20, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ding, F.; Chen, T.; Xia, L.-H.; Qian, J.; Lv, G.-Y. Ultrasound hepatic/renal ratio and hepatic attenuation rate for quantifying liver fat content. World J. Gastroenterol. 2014, 20, 17985–17992. [Google Scholar] [CrossRef] [PubMed]
- Di Lascio, N.; Avigo, C.; Salvati, A.; Martini, N.; Ragucci, M.; Monti, S.; Prinster, A.; Chiappino, D.; Mancini, M.; D’Elia, D.; et al. Steato-Score: Non-Invasive Quantitative Assessment of Liver Fat by Ultrasound Imaging. Ultrasound Med. Biol. 2018, 44, 1585–1596. [Google Scholar] [CrossRef]
- Qayyum, A.; Chen, D.M.; Breiman, R.S.; Westphalen, A.C.; Yeh, B.M.; Jones, K.D.; Lu, Y.; Coakley, F.V.; Callen, P.W. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: Which modality is best? Clin. Imaging 2009, 33, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Almeida, A.D.M. Fatty liver disease in severe obese patients: Diagnostic value of abdominal ultrasound. World J. Gastroenterol. 2008, 14, 1415–1418. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-Y.; Lee, J.Y.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S.; Kim, K.G.; Lee, J.M.; Han, J.K.; Choi, B.I. Hepatic Steatosis: Assessment with Acoustic Structure Quantification of US Imaging. Radiology 2016, 278, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmulewitz, A.; Teefey, S.A.; Robinson, B.S. Factors affecting image quality and diagnostic efficacy in abdominal sonography: A prospective study of 140 patients. J. Clin. Ultrasound 1993, 21, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Brahee, D.D.; Ogedegbe, C.; Hassler, C.; Nyirenda, T.; Hazelwood, V.; Morchel, H.; Patel, R.S.; Feldman, J. Body Mass Index and Abdominal Ultrasound Image Quality. J. Diagn. Med. Sonogr. 2013, 29, 66–72. [Google Scholar] [CrossRef]
- Hayashi, T.; Saitoh, S.; Takahashi, J.; Tsuji, Y.; Ikeda, K.; Kobayashi, M.; Kawamura, Y.; Fujii, T.; Inoue, M.; Miyati, T.; et al. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatol. Res. 2017, 47, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [Green Version]

| Sample Size | Percentage of Males (%) | Age (Years) | BMI (kg/m2) | |
|---|---|---|---|---|
| Entire population | 214 | 55.6 | 53.8 ± 12.9 | 28.1 ± 5.5 |
| BMI classes | ||||
| BMI < 25 kg/m2 | 65 | 52.3 | 52.9 ± 13.3 | 22.4 ± 1.8 |
| BMI ≥ 25 kg/m2 | 149 | 57 | 54.3 ± 12.8 | 30.6 ± 4.6 |
| Steatosis classes by qualitative US | ||||
| Absent (class 0) | 42 | 40.5 | 57.1 ± 12.6 | 23.6 ± 2.9 |
| Mild (class 1) | 26 | 42.3 | 55.2 ± 12.6 | 25.4 ± 3.2 |
| Moderate (class 2) | 57 | 63.1 | 52.9 ± 13.4 | 27.9 ± 5.2 |
| Severe (class 3) | 80 | 61.2 | 52.0 ± 13.2 | 31.5 ± 5.2 |
| Bias | SD | Upper Limit | Lower Limit | |
|---|---|---|---|---|
| HR | 0.06 | 0.50 | 1.05 | −0.92 |
| Steato-score | 0.35 | 2.69 | 5.62 | −4.92 |
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Bias | SD | Upper Limit | Lower Limit | Bias | SD | Upper Limit | Lower Limit | |
| HR | 0.07 | 0.39 | 0.85 | −0.70 | 0.08 | 0.55 | 1.14 | −0.93 |
| Steato-score | 0.37 | 2.12 | 4.53 | −3.78 | 0.33 | 2.93 | 6.08 | −5.41 |
| Bias | SD | Upper Limit | Lower Limit | R | |
|---|---|---|---|---|---|
| S0 | |||||
| HR | 0.12 * | 0.27 | 0.65 | −0.41 | 0.23 (p = 0.15) |
| Steato-score | 0.64 * | 1.45 | 3.49 | −2.19 | 0.14 (p = 0.39) |
| S1 | |||||
| HR | 0.11 * | 0.28 | 0.67 | −0.44 | 0.28 (p = 0.18) |
| Steato-score | 0.61 * | 1.51 | 3.59 | −2.35 | 0.09 (p = 0.69) |
| S2 | |||||
| HR | 0.05 | 0.55 | 1.13 | −1.03 | −0.14 (p = 0.32) |
| Steato-score | 0.27 | 2.95 | 6.06 | −5.51 | −0.14 (p = 0.31) |
| S3 | |||||
| HR | 0.05 | 0.62 | 1.16 | −1.05 | 0.10 (p = 0.40) |
| Steato-score | 0.13 | 3.32 | 6.65 | −6.38 | −0.28 (p = 0.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, L.; Salvati, A.; Bonino, F.; Brunetto, M.R.; Faita, F. Effects of Different Scan Projections on the Quantitative Ultrasound-Based Evaluation of Hepatic Steatosis. Healthcare 2022, 10, 374. https://doi.org/10.3390/healthcare10020374
De Rosa L, Salvati A, Bonino F, Brunetto MR, Faita F. Effects of Different Scan Projections on the Quantitative Ultrasound-Based Evaluation of Hepatic Steatosis. Healthcare. 2022; 10(2):374. https://doi.org/10.3390/healthcare10020374
Chicago/Turabian StyleDe Rosa, Laura, Antonio Salvati, Ferruccio Bonino, Maurizia Rossana Brunetto, and Francesco Faita. 2022. "Effects of Different Scan Projections on the Quantitative Ultrasound-Based Evaluation of Hepatic Steatosis" Healthcare 10, no. 2: 374. https://doi.org/10.3390/healthcare10020374
APA StyleDe Rosa, L., Salvati, A., Bonino, F., Brunetto, M. R., & Faita, F. (2022). Effects of Different Scan Projections on the Quantitative Ultrasound-Based Evaluation of Hepatic Steatosis. Healthcare, 10(2), 374. https://doi.org/10.3390/healthcare10020374










