Paroxysmal Sustained Ventricular Tachycardia with Cardiac Arrest and Myocardial Infarction in 29-Year-Old Man Addicted to Medical Marijuana—It Never Rains but It Pours
Abstract
:1. Introduction
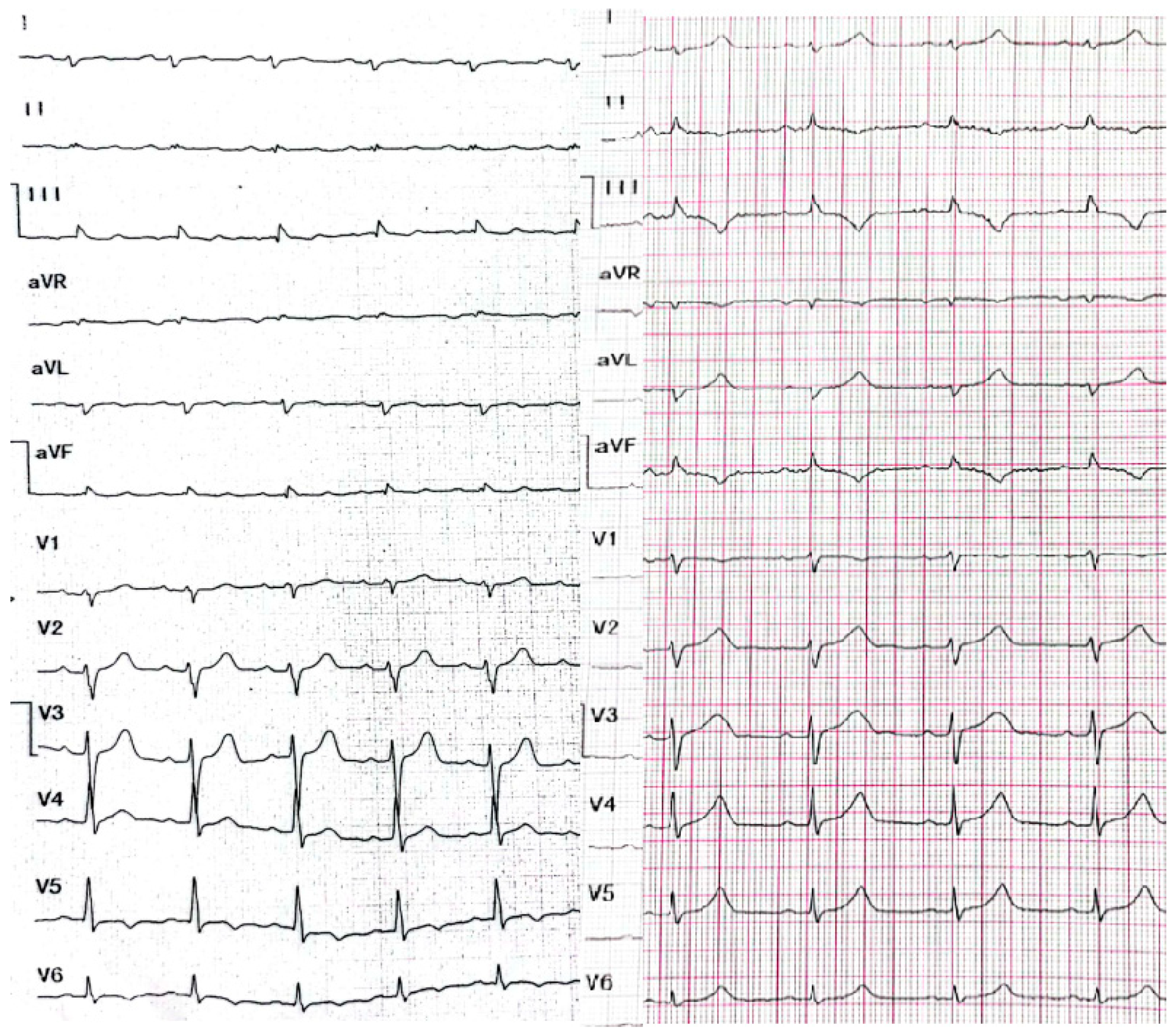
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slawek, D.E.; Curtis, S.A.; Arnsten, J.H.; Cunningham, C.O. Clinical Approaches to Cannabis: A Narrative Review. Med. Clin. N. Am. 2022, 106, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Bougrine, R.; Boutaybi, M.; Boulouiz, S.; Elouafi, N.; Bazid, Z. Severe Myocardial Infarction Caused by Excessive Cannabis Consumption. Cureus 2021, 13, E14643. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.; Abadir, S.; Kooshkabadi, M.; Yusuf, S.O.; Khanna, R.; Collura, B.; Anais Hichard, M. Recreational Marijuana Use and Coronary Artery Dissection: A Case Series. Cureus 2022, 14, E21778. [Google Scholar] [CrossRef]
- Chelikam, N.; Vyas, V.; Dondapati, L.; Iskander, B.; Patel, G.; Jain, S.; Singla, T.; Bombaywala, A.; Zarrate, D.; Debnath, N.; et al. Epidemiology, Burden, and Association of Substance Abuse Amongst Patients with Cardiovascular Disorders: National Cross-Sectional Survey Study. Cureus 2022, 14, e27016. [Google Scholar] [CrossRef]
- Harding, B.N.; Austin, T.R.; Floyd, J.S.; Smith, B.M.; Szklo, M.; Heckbert, S.R. Self-reported marijuana use and cardiac arrhythmias (from the Multiethnic Study of Atherosclerosis). Am. J. Cardiol. 2022, 177, 48–52. [Google Scholar] [CrossRef]
- Gordon, A.J.; Conley, J.W.; Gordon, J.M. Medical consequences of marijuana use: A review of current literature. Curr. Psychiatry Rep. 2013, 15, 419. [Google Scholar] [CrossRef] [Green Version]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Wilson, M.C.; Crowley, C.T.; Ling, W.; Petry, N.M.; et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [Green Version]
- McDowell, M. Marijuana. In Clinical Textbook of Addictive Disorders; Guilford Press: New York, NY, USA, 2005; p. 169. [Google Scholar]
- Jastrzębski, M.; Sasaki, K.; Kukla, P.; Fijorek, K.; Stec, S.; Czarnecka, D. The ventricular tachycardia score: A novel approach to electrocardiographic diagnosis of ventricular tachycardia. Europace 2016, 18, 578–584. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, J.; Gianni, C.; Trivedi, C.; Della Rocca, D.G.; Bassiouny, M.; Canpolat, U.; Tapia, A.C.; Burkhardt, J.D.; Sanchez, J.E. Simple electrocardiographic criteria for rapid identification of wide QRS complex tachycardia: The new limb lead algorithm. Heart Rhythm 2020, 17, 431–438. [Google Scholar] [CrossRef]
- Magnani, J.W.; Dec, G.W. Myocarditis: Current trends in diagnosis and treatment. Circulation 2006, 113, 876–890. [Google Scholar] [CrossRef]
- Vermes, E.; Carbone, I.; Friedrich, M.G.; Merchant, N. Patterns of myocardial late enhancement: Typical and atypical features. Arch. Cardiovasc. Dis. 2012, 105, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fang, L.; Chen, W.; Zhu, Y.; Lin, X.; Wang, Y.; Li, X.; Wang, Q.; Liu, Z. Identification of characteristics of overt myocarditis in adult patients with idiopathic inflammatory myopathies. Cardiovasc. Diagn. Ther. 2020, 10, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castro, C.E.; Alkhateeb, H.; Elfar, A.; Saifuddin, F.; Abbas, A.; Siddiqui, T. Recurrent myopericarditis as a complication of Marijuana use. Am. J. Case Rep. 2014, 15, 60–62. [Google Scholar] [PubMed] [Green Version]
- Trusz-Gluza, M. Rhythm and conduction disturbances. In Szczeklik’s Internal Medicine 2022; Medycyna Praktyczna: Krakow, Poland, 2022; pp. 233–302. [Google Scholar]
- Tournebize, J.; Gibaja, V.; Puskarczyk, E.; Popovic, B.; Kahn, J.P. Myocarditis associated with cannabis use in a 15-year-old boy: A rare case report. Int. J. Cardiol. 2016, 203, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Nappe, T.M.; Hoyte, C.O. Pediatric Death Due to Myocarditis after Exposure to Cannabis. Clin. Pract. Cases Emerg. Med. 2017, 1, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Kariyanna, P.T.; Jayarangaiah, A.; Singh, N.; Song, T.; Soroka, S.; Amarnani, A.; Ray, J.; McFarlane, S.I. Marijuana Induced Myocarditis: A New Entity of Toxic Myocarditis. Am. J. Med. Case Rep. 2018, 6, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, G.E.D.; Izquierdo, D.A.; Boettcher, B.T.; Pagel, P.S. Chronic Marijuana and Synthetic Cannabinoid-Induced Toxic Myocarditis and End-Stage Cardiomyopathy: Management with Mechanical Circulatory Support as a Bridge-to-Transplantation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2508–2512. [Google Scholar] [CrossRef]
- Tso, M.; Kushneriuk, D.J.; Bree, T.L.; Nosib, S.S. Incidental Acute ST Elevation Due to Cannabis-Induced Myocarditis after a Mechanical Fall. CJC Open 2021, 3, 1303–1306. [Google Scholar] [CrossRef]
- Alirezaei, T.; Mohammadi, M.K.A.; Irilouzadian, R.; Zarinparsa, H. Marijuana-induced myocarditis in a 24-year-old man. Arch. Clin. Cases 2022, 9, 69–74. [Google Scholar] [CrossRef]
- Seif El Dahan, K.; Machtoub, D.; Massoud, G.; Nasser, S.A.; Hamam, B.; Kobeissy, F.; Zouein, F.A.; Eid, A.H. Cannabinoids and Myocardial Ischemia: Novel insights, Updated Mechanisms, and Implications for Myocardial Infarction. Curr. Med. Chem. 2022, 29, 1990–2010. [Google Scholar] [CrossRef]
- Patel, K.H.; Kariyanna, P.T.; Jayarangaiah, A.; Khondakar, N.; Abduraimova, M.; McFarlane, S.I. Myocardial Infarction Secondary to Marijuana-Induced Coronary Vasospasm. Am. J. Med. Case Rep. 2020, 8, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Weresa, J.; Pędzińska-Betiuk, A.; Mińczuk, K.; Malinowska, B.; Schlicker, E. Why Do Marijuana and Synthetic Cannabimimetics Induce Acute Myocardial Infarction in Healthy Young People? Cells 2022, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.D.; Williams, C.; Ma, T.M. Cardiac Ischemia Associated with Marijuana Use in an Adolescent. Cureus 2020, 12, E9661. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.L.; Dhore-Patil, A.; Lavie, C.J. Early-Onset Cardiovascular Disease from Cocaine, Amphetamines, Alcohol, and Marijuana. Can. J. Cardiol. 2022, 38, 1342–1351. [Google Scholar] [CrossRef]
- Aissaoui, H.; Boulouiz, S.; El-Azrak, M.; Bouchlarhem, A.; Elouafi, N.; Bazid, Z. Cannabis-induced myocardial infarction in a 27-year-old man: Case report. Ann. Med. Surg. 2022, 80, 104054. [Google Scholar] [CrossRef]
- Ladha, K.S.; Mistry, N.; Wijeysundera, D.N.; Clarke, H.; Verma, S.; Hare, G.M.T.; Mazer, C.D. Recent cannabis use and myocardial infarction in young adults: A cross-sectional study. CMAJ 2021, 193, E1377–E1384. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar]
- Sadowski, M.; Rekść, Ł.; Stępień, A.; Kuchinka, J.; Zieliński, P.; Grabski, P.; Ręba, P.; Barańska, E. Myocardial infarction with non-obstructive coronary arteries. Med. Stud. 2018, 34, 332–336. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar]
- Szumowski, Ł. Ordinance of the Polish Minister of Health of August 29, 2019 on medical examinations of applicants for the authorization to drive vehicles and drivers. Pol. J. Laws 2019, 1, 1659. [Google Scholar]
- Hallinan, C.M.; Bonomo, Y.A. The Rise and Rise of Medicinal Cannabis, What Now? Medicinal Cannabis Prescribing in Australia 2017-2022. Int. J. Environ. Res. Public Health. 2022, 19, 9853. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiliński, J.; Skwarek, A.; Chrzan, I.; Zeliaś, A.; Borek, R.; Dykla, D.E.; Bober-Fotopoulos, M.; Dudek, D. Paroxysmal Sustained Ventricular Tachycardia with Cardiac Arrest and Myocardial Infarction in 29-Year-Old Man Addicted to Medical Marijuana—It Never Rains but It Pours. Healthcare 2022, 10, 2024. https://doi.org/10.3390/healthcare10102024
Wiliński J, Skwarek A, Chrzan I, Zeliaś A, Borek R, Dykla DE, Bober-Fotopoulos M, Dudek D. Paroxysmal Sustained Ventricular Tachycardia with Cardiac Arrest and Myocardial Infarction in 29-Year-Old Man Addicted to Medical Marijuana—It Never Rains but It Pours. Healthcare. 2022; 10(10):2024. https://doi.org/10.3390/healthcare10102024
Chicago/Turabian StyleWiliński, Jerzy, Anna Skwarek, Iwona Chrzan, Aleksander Zeliaś, Radosław Borek, Dominika Elżbieta Dykla, Maria Bober-Fotopoulos, and Dariusz Dudek. 2022. "Paroxysmal Sustained Ventricular Tachycardia with Cardiac Arrest and Myocardial Infarction in 29-Year-Old Man Addicted to Medical Marijuana—It Never Rains but It Pours" Healthcare 10, no. 10: 2024. https://doi.org/10.3390/healthcare10102024
APA StyleWiliński, J., Skwarek, A., Chrzan, I., Zeliaś, A., Borek, R., Dykla, D. E., Bober-Fotopoulos, M., & Dudek, D. (2022). Paroxysmal Sustained Ventricular Tachycardia with Cardiac Arrest and Myocardial Infarction in 29-Year-Old Man Addicted to Medical Marijuana—It Never Rains but It Pours. Healthcare, 10(10), 2024. https://doi.org/10.3390/healthcare10102024





