Parental Reminder Strategies and the Cost Implication for Improved Immunisation Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
Objectives
- To determine the effective parental strategy for improving coverage of childhood immunisation;
- To determine the effective parental strategy for enhancing the timeliness of childhood immunisation;
- To determine the cost associated with delivering parental strategies for improved immunisation uptake.
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy and Selection Process
2.4. Study Selection
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias in Individual Studies
| Author | Year | Eligibility | Randomised Allocation | Concealed Allocation | Similarity at Baseline | Blinding of Participants | Blinding of Therapist | Blinding of Assessor | Dropout | Intention to Treat | Group Comparison | PMVD | Total Score (10) | Internal Validity (8) | SUBSCALE (2) | Interpretation | Decision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagar et al. | [7] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| Mekonnen et al. | [9] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 9 | 7 | 2 | Excellent | Accepted |
| Wallace et al. | [12] | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Ye s | Yes | 6 | 4 | 2 | Good | Accepted |
| Niederhauser et al. | [26] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | 6 | 4 | 2 | Good | Accepted |
| Busso et al. | [27] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | 4 | 2 | Good | Accepted |
| Kempe et al. | [28] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 | 3 | 2 | Fair | Accepted |
| Bangure et al. | [29] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | 6 | 5 | 1 | Good | Accepted |
| Brown et al. | [30] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 | 4 | 2 | Good | Accepted |
| Gibson et al. | [31] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 | 7 | 1 | Good | Accepted |
| Seth et al. | [32] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| O’Grady et al. | [33] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 | 6 | 2 | Good | Accepted |
| Domek et al. | [34] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 8 | 7 | 1 | Good | Accepted |
| Menzies et al. | [35] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| Brownstone | [36] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | 3 | 2 | Fair | Accepted |
| Siddiqi et al. | [37] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| Kagucia | [38] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| Kazi et al. | [39] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 6 | 2 | Good | Accepted |
| Ekhaguere et al. | [40] | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| Dissieka et al. | [41] | Yes | Yes | Yes | Yes | No | No | No | No | No | Yes | Yes | 5 | 3 | 2 | Fair | Accepted |
| Hofstetter et al. | [42] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 | 5 | 2 | Good | Accepted |
| No | Author | Year | Preliminary | Introduction | Design | Sampling | Data Collection | Ethical Matter | Results | Discussion | Total Score | % Score | Decision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Oladepo | [13] | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 30 | 75 | Accepted |
| 2 | Yunusa | [15] | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 3 | 29 | 73 | Accepted |
| 3 | Uddin et al. | [43] | 4 | 3 | 4 | 4 | 4 | 4 | 3 | 4 | 31 | 78 | Accepted |
| 4 | Ibraheem et al. | [44] | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 4 | 29 | 73 | Accepted |
2.8. Certainty of Evidence
2.9. Data Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias
3.4. Findings from Meta-Analysis
3.4.1. Coverage of Immunisation
3.4.2. Timeliness of Immunisation
3.5. Narrative Synthesis
3.5.1. Coverage of Immunisation
3.5.2. Timeliness of Immunisation
3.6. Cost Implication
3.7. Certainty of Evidence
4. Discussion
5. Implication
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Author (Year) | Participants | Sample Size | Design | Intervention | Control | Vaccine type | Country | Dose | Instrument | Results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq. | Course | |||||||||||
| 1 | Niederhauser et al. [26] | Mothers | 42 | 2-arm RCT | SMS reminder n = 19 | Usual/standard care n = 23 | DTaP, hepatitis B, Hib, PCV and polio | USA | 2 SMS sent at 4 and 2 weeks to the due date | Seven months | Vaccination records | Immunisation coverage at seven months was 7 (41.2%) in the intervention group compared to 15 (65.2%) in the control group. This indicates that 58.8% and 34.8% were not up to date with immunisation at seven months. |
| 2 | Kempe et al. [28] | Mother–infant pair | 18,235 | RCT | Collaborative centralized reminder/recall approach (n = 9049) | Practice-based reminder/recall approach n = 9186 | DPT, Poliovirus, measles, Hib, Hepatitis B, Varicella and PCV. | United States | Autodialled/mail protocol (2 calls or four mails followed by two postcards) Mail only (1 letter and three postcards every six weeks) | 6 Months | Immunisation record | Intervention improves coverage by 26.9% for the collaborative centralized/recall approach treatment group compared to 21.7% for practice-based (p < 0.001). |
| 3 | Hofstetter et al. [39] | Mother–child pair | 2054 | RCT | A. scheduling + appointment SMS reminder (n = 686) B. appointment SMS reminder only n = 686 | Usual care n = 682 | MMR | United States | Three schedules plus one SMS | 16 months | 1. Hospital vaccination record 2. Vaccine cards | The intervention led to timely uptake of MMR vaccination 686(61.1%) vs. 682 (55.1%) (RRR 1.11 95% CI 1.01–1.21). For the SMS and control arm, respectively. |
| 4 | Bangure et al. [29] | Mothers | 304 | 2-arm RCT | SMS reminder n = 152 | Usual health talk n = 152 | OPV, PCV and Pentavalent | Zimbabwe | Three times per visit on days 7, 3 and 1 before each scheduled | 14 weeks | Immunisation register | On the 14th week, the intervention resulted in 82% of respondents receiving timely immunisation compared to only 8% in the control group. While at the 14th week, vaccination coverage was 95% and 75% for the intervention and control groups, respectively. |
| 5 | Busso et al. [27] | Mothers | 13,000 | RCT | Community outreach with patients list requiring immunisation | Community outreach without patients list | BCG, Pentavalent, Poliovirus and MMR | Guatemala | Once a month | 6 Months | Hospital records | Immunisation coverage increased by 4.6% among the intervention group The direct cost per child in the study was USD 0.11 per child for 6 months. |
| 6 | Brown et al. [30] | Mothers | 595 | RCT | A. Telephone call: reminder/recall n = 148 B. PHCIPT n = 150 C. Combination of A and B n = 147 | C. Usual care practice n = 150 | OPV, DPT, Hepatitis B, Measles and Yellow fever | Nigeria | A. Two reminder calls (on days 2 and 1 before due date) and recall four times B. Two days of one-off training for RI providers | 12 months | American Immunisation Registry Association (AIRA) questionnaire | Coverage at the of the study was 98.6% for the voice call reminder or recall group compared to the control with coverage of 57.3%. |
| 7 | Uddin et al. [43] | Mothers | 1030 | Cluster quasi-experimental | Mobile SMS in rural areas n = 520 | Mobile SMS in urban areas n = 518 | BCG, Pentavalent and MR | Bangladesh | Three messages One a day to the scheduled visit, in the morning of the opening hour of the clinic and two hours to the close of the clinic | 12 months | Immunisation register and mother’s recall | RURAL COVERAGE The intervention increased coverage in rural areas from 58.9% to 76.8%, difference +18.8% (95% CI 5.7–31.9) URBAN COVERAGE Urban coverage improved from 40.7% to 57.1% with a difference of +16.5% (95% CI 3.9–29.0). |
| 8 | Gibson et al. [31] | Mother | 1600 | 4-arm cluster RCT | A. SMS-only n = 388 B. SMS+ 75 KES for each pentavalent received n = 446 C. SMS+ 200 KES for each pentavalent received n = 406 | Usual care practice n = 360 | BCG, OPV, Pentavalent and Measles | Kenya | SMS reminder on days 3 and 1 before the immunisation due date | 12 months | Immunisation register | Full immunisation coverage at 12 months of age for SMS-only group, 296 (82%) of 360 control group compared to intervention participants, 332 (86%) of 388. RR = 1.04 (95% CI 0.97–1.12). SMS plus 75 KES intervention group had achieved 86% compared to the control group that had 82%, control participants group. Intervention comparing higher incentive 200 KES in addition to SMS leads to 90% 364 of 406 participants achieving immunisation coverage compared to the control group that had 82%, participants group. SMS plus 75 KES on the timeliness of immunisation is 70%; RR 1.37 (95% CI 1.18–1.59) compared to the control group of 50% timely coverage. SMS plus 200 KES on the timeliness of immunisation is 72%; RR 1.42 (95% CI 1.23–1.65) compared to the control group of 50% timely coverage. |
| 9 | Nagar et al. [7] | Mothers | 198 | 3-arm RCT | A. Pendant with Voice Call arm (P + V). n = 75 B. Pendant only n = 61 | Near Field Communication (NFC) stickers placed on the child immunisation card. n = 62 | DTP | India | Voice call a day to due date and after the due date for no show | 180 days from birth | Immunisation records and patient recall | DPT3 completion within 2 months. Control NFC sticker 74.2%, pendant only 67.2% and pendant +voice call 69.3. DPT Shots within 180 days 69.4, 57.4 and 58.7% for pendant with voice call, pendant only and NFC stickers, respectively. |
| 10 | Seth et al. [32] | Mothers | 549 | 3-arm RCT | A. Automated SMS-only (n = 188) B. Automated SMS with airtime for each received scheduled vaccine n = 179 | Immunisation record cards (n = 182) | OPV, Rotavirus, Pentavalent, PCV, IPV and MR | India | One (1) automated SMS sent before the scheduled due date | 12 months | Immunisation records | Timeliness of immunisation dropped in the SMS-only participants by 24.7% (60 out of 243) when compared to the standard treatment group, 31.3% (76 of 243) Vaccination coverage for the control and intervention groups was 40.1% (Inter Quartile Range: 30.8–69.2%), and 50.0% (Inter Quartile Range: 30.8–76.9%), respectively. The incentive-linked group achieved immunisation coverage of 50.0% interquartile range (IQR: 30.8–76.9%) compared to the control group of 41.7% (IQR: 23.1–69.2%). 40.8% of the SMS and incentive intervention group completed immunisation on time compared to 31.3% of the control group. |
| 11 | O’Grady et al. [33] | Mother | 196 | 3-arm RCT | A. Simple SMS. n = 64 B. educational SMS ± additional support group ESMS±S n = 65 | Usual care practices n= 67 | HepB-DTPa_Hib_IPV, PCV and Rotavirus | Australia | SMS sent 2 and 1 weeks before the due date and after two weeks if the child is still not vaccinated | Eight months | Questionnaire and immunisation record | Immunisation coverage rose to 70.3% of 45/64 in the SMS intervention group compared to 67.2% 45/67 in the control arm. At the end of the intervention, infants in the intervention group utilizing SMS-delivered educational messages had more vaccine coverage (83.1%) compared to the control group that received standard care 45/67 (67.2%). |
| 12 | Kazi et al. [39] | Caregiver | 300 | 2-arm RCT | SMS reminder n = 150 | Standard care practice n = 150 | DPT-Hep-B-Hib vaccine OPV | Pakistan | Four SMS reminders sent on the week of the scheduled appointment | 18 weeks | Record | 14 weeks scheduled visit was 47 (31.3%) for the intervention group vs. 39 (26.0%) for the control group, p = 0.31). It is therefore not statistically significant. |
| 13 | Ekhaguere et al. [40] | Mothers | 600 | 2-arm RCT | Voice call message and SMS n = 171 | Standard care n = 140 | Pentavalent, OPV, Rota vaccine, PCV, Measles and Yellow fever. | Nigeria | Two SMS or voice call at day two and one to the scheduled immunisation | 12 month | Record | All scheduled immunisations collectively led to (57% vs. 47%, RR 1.13, 95% CI 1.02 to 1.26; p = 0.01) within 1 week of the recommended date for the intervention compared to the control group. The cost of delivering the SMS was USD 0.0075 and USD 0.015 for SMS and voice call, respectively. |
| 14 | Dissieka et al. [41] | Mothers | 1596 | 2-arm RCT | Voice call and SMS n= 484 | Standard care n = 302 | Pentavalent, MMR and Yellow fever immunisations | Côte d’Ivoire | One SMS reminder and call and two recalls | 9 month | Records | Immunisation coverage at 9 month were 484 (60.7%) for the intervention group compared to 302 (37.8%) for the control with the adjusted odds ratio of 4.52 (2.84–7.20). |
| 15 | Domek et al. [34] | Mothers | 720 | RCT | SMS sent in local languages n = 329 | The usual practice of care using child card n = 333 | Pentavalent, PCV, IPV, OPV, Rotavirus | Guatemala | 3 SMS at 3, 2 and 1 day to the due date | Eight months | Electronic immunisation record | Timeliness at Visit 3 was found to be 112 (34.0%) of 329 for experimental group and 90 (27.0%) of 333 in the control group, p = 0.05. Both intervention and control groups had a high rate of immunisation coverage (89.1% vs. 89.2%) for intention to treat analysis but were not statistically different. |
| 16 | Wallace et al. [12] | Mothers | 3616 | Cluster RCT | A. Home base records (HBR) only. N = 1290 B. Home base record + stickers. n = 711 | Routine care practice n = 1615 | DPT | Indonesia | A. HBR provided whenever, missing, damaged or destroyed and information transferred to the new one. B. In addition to A above, stickers are placed indicating due date for each immunisation visit. | 7 months | HBR + sticker group for DTPcv3 vaccination completion rate (77%) compared with the control group (81%) (RR = 0.97, 95% CI:0.90, 1.04), neither HBR-only group for DTPcv3 (74%) compared with the control group (RR = 0.94,95% CI:0.87, 1.02). HBR + sticker vs. control (77 vs. 78%) (RR = 0.99, 95% CI: 0.98, 1.09), HBR-only vs. control 74% (RR = 0.96, 95% CI: 0.88, 1.05) However, children in the HBR + sticker group were 50% more likely to. | |
| 17 | Menzies et al. [35] | Mothers | 1594 | Four arm RCT | A. SMS-only. (n = 398) B. Personalized calendar (PC) only. n = 398 C. PC+ SMS. n = 404 | Usual care practice n= 394 | DPT, Polio, Hib, Rotavirus, Pnuemococcal, MMR and Varicella | Australia | 2 SMS at 14 and 2 days to the due date | Ten months | Excel spreadsheet information extracted from immunisation register | Results on the 6th-month timely immunisation show 310 (78%) RR 1.05 (95% CI 0.97–1.14) SMS reminder intervention group had their children immunised on time compared to 291 (74%) of the control arm. |
| 18 | Brownstone et al. [36] | Mothers | 951 | 3-arm RCT | Model 1: higher incentive (baseline amount NGN 3000 + NGN 1000 a reminder call) n = 345 | Model 2: more minor incentive (baseline amount NGN 2000 + NGN 1000 plus a reminder call). n = 606 | BCG, Pentavalent, PCV and Measles | Nigeria | One call or SMS before the scheduled date and an additional increase in incentive | Four months | Child health record book | Results from the intervention show that increasing monetary intervention was effective in resulting in 90.1% coverage of measles vaccine compared to 86.1% recorded in the control group. |
| 19 | Siddiqi et al. [37] | Caregiver | 1445 | 3-arm RCT | A. Alma Sana Bracelet Group n = 482 B. Star Bracelet Group n = 482 | Usual care practice n = 481 | Pentavalent and Measles | Pakistan | 6 punctures at the appropriate age or 6 crescent punctures before completion of vaccination. One per visit | 12 months | Hospital records | Coverage of Penta 3 at 12 month is 84.3, 85.4, 83% for Alma Sana Bracelet, Star Bracelet and control group, respectively. While measles 1 coverage at 12 months was 72.0, 70.5 and 68.5% for Alma Sana Bracelet, Star Bracelet and control group, respectively. |
| 20 | Mekonnen et al. [9] | Mothers | 426 | 2-arm RCT | Reminder SMS n = 213 | Usual care practice n = 213 | BCG, OPV, Rotavirus, Pentavalent, PCV, Rotavirus, Measles and Inactivated Polio | Ethiopia | 1 SMS sent a day to the due date | 12 months | Hospital record | Timely vaccination was 213 (63.3%) and 213 (39.9%) for experimental and control group, respectively p < 0.001; risk ratio 1.59, 95% lower CI: 1.35. While coverage was 82% of 176/213 compared to 70.9% of 151/213, respectively; p = 0.002; Risk Ratio 1.17, 95% lower CI 1.07) compared to those in the control group. |
| 21 | Kagucia et al. [38] | Caregivers | 537 | 3-arm RCT | A. SMS reminder n = 146 B. SMS reminder+ monitory incentive (150KES) n = 149 | Usual care practice n = 160 | Measles vaccine | Kenya | 2 SMS sent 3 and 1 day to the due date | 3 months | Checklist and questionnaire | SMS intervention yielded 146 (78%) timely completion against 160(68%) timely coverage in the control arm with adjusted RR 1.13; 95% CI 0.99 1.30; p = 0.070. |
| 22 | Ibraheem et al. [44] | Mothers | 540 | 4-arm Quasi-experimental | A. SMS reminder n = 136 B. Phone call reminder n = 133 C. SMS health education n = 133 | Routine care practice n = 136 | Pentavalent, PCV, OPV, IPV, Measles and Yellow fever | Nigeria | 1 SMS a day to the due date | Five months | Immunisation register | 86 (63.7%) received timely the 9th-month immunisation schedule while the 45 (36.6) vaccines timely for the intervention and control arm, respectively, p < 0.001. Completion of immunisation at 9 months was higher among the SMS reminder group (99.3%) compared with the control group, with percentage coverage of 90.4%. Immunisation fact messages sent through SMS improved immunisation timeliness in the intervention group compared to the control group of 97% and 90.4%, respectively. SMS conveyed health education messages yield 99.2% immunisation coverage compared to 90.4% in the standard care group. Coverage for phone call was 99.2% compared to control with coverage of 90.4%. |
| 23 | Oladepo et al. [13] | Mother–infant pair | 3139 | 2-arm quasi experimental | SMS on immunisation health education n = 1479 | Flyers on nutrition and growth monitoring n = 1499 | BCG, Pentavalent, OPV, HBV, IPV, Measles and Yellow fever | Nigeria | Immunisation education messages are sent three times a week | Ten month | Immunisation register | SMS education messages led to a 76.0 vs. 73.3% completion rate in the control group with standard care. |
| 24 | Yunusa et al. [15] | Mothers | 541 | 2-arm Quasi experimental | Mobile phone call reminder n = 271 | Routine care practice with child health card n = 270 | Pentavalent | Nigeria | Two calls on days 3 and 1 and call if the client fails to show up | Four month | Immunisation register | The coverage rate in the experimental group was 59.4% compared to the control group of 34.1% after four months of intervention. |
| Trivial | Small | Moderate | Large | Very Large | Nearly Perfect | Perfect | |
|---|---|---|---|---|---|---|---|
| Correlation | 0.0 | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | 1 |
| Difference in means | 0.0 | 0.2 | 0.6 | 1.2 | 2.0 | 4.0 | Infinite |
| Frequency difference | 0 | 10 | 30 | 50 | 70 | 90 | 100 |
| Relative risk | 1.0 | 1.2 | 1.9 | 3.0 | 5.7 | 19 | Infinite |
| Odd ratio | 1.0 | 1.5 | 3.5 | 9.0 | 32 | 360 | Infinite |
Appendix B. Funnel Plots
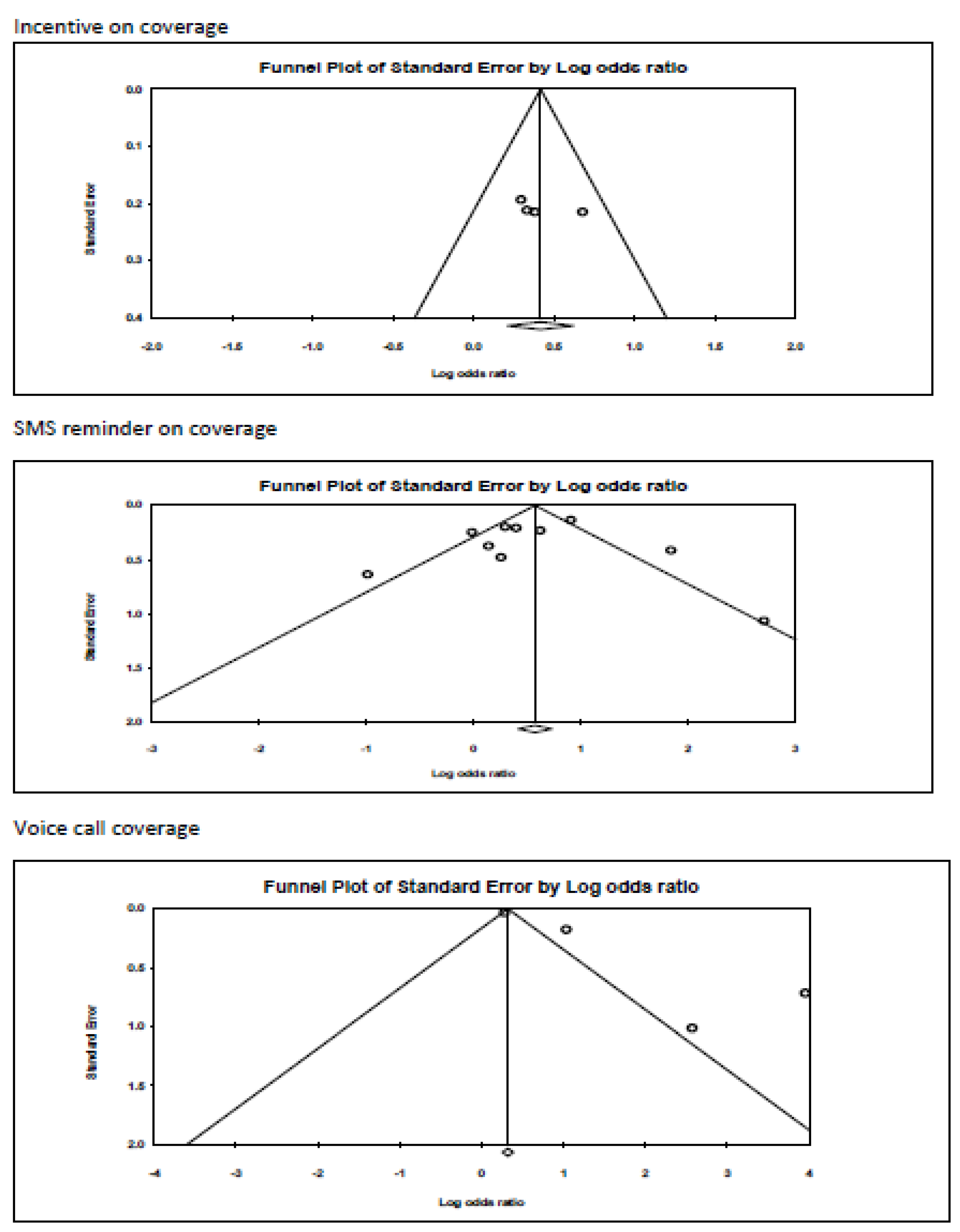
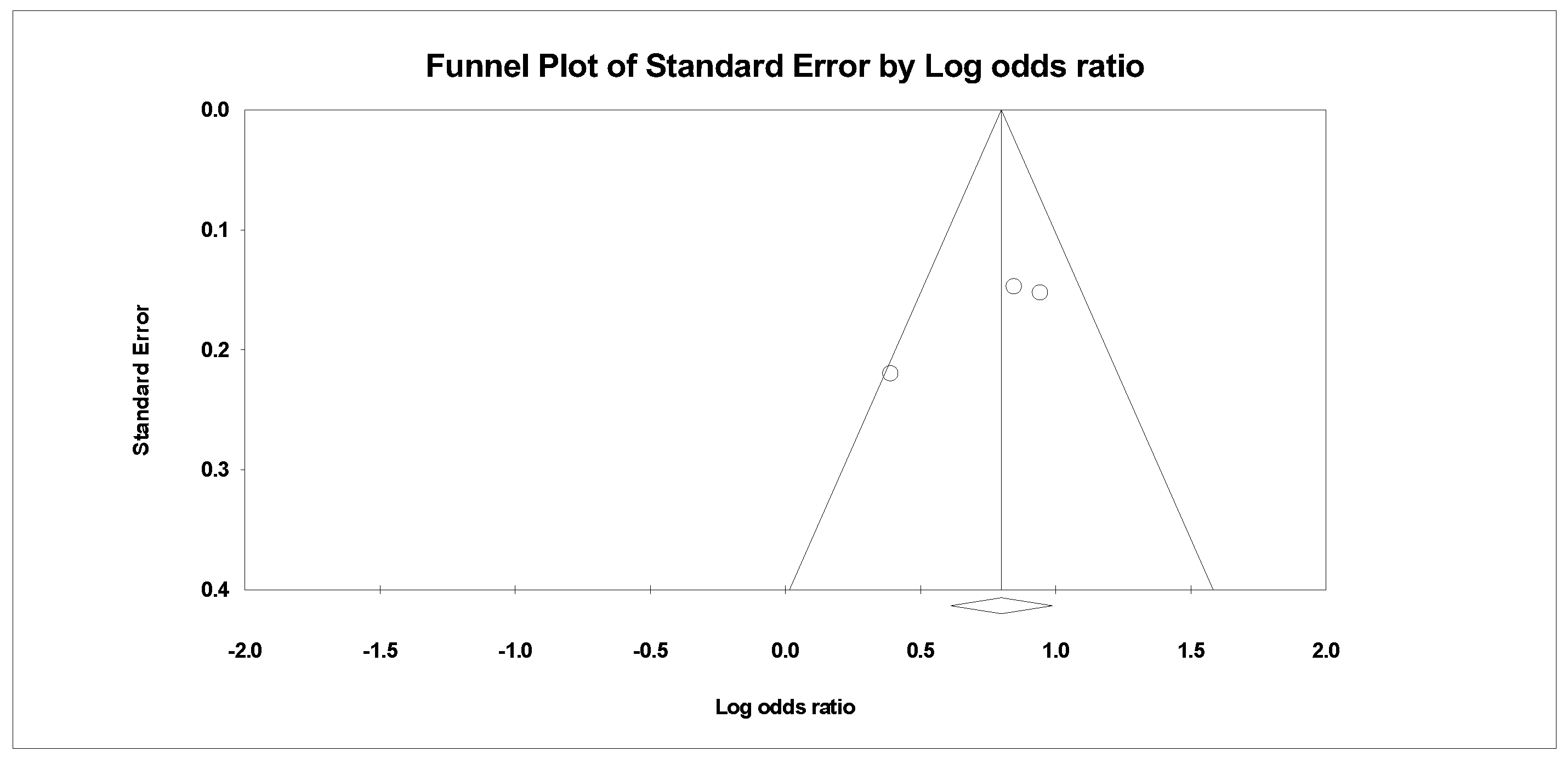
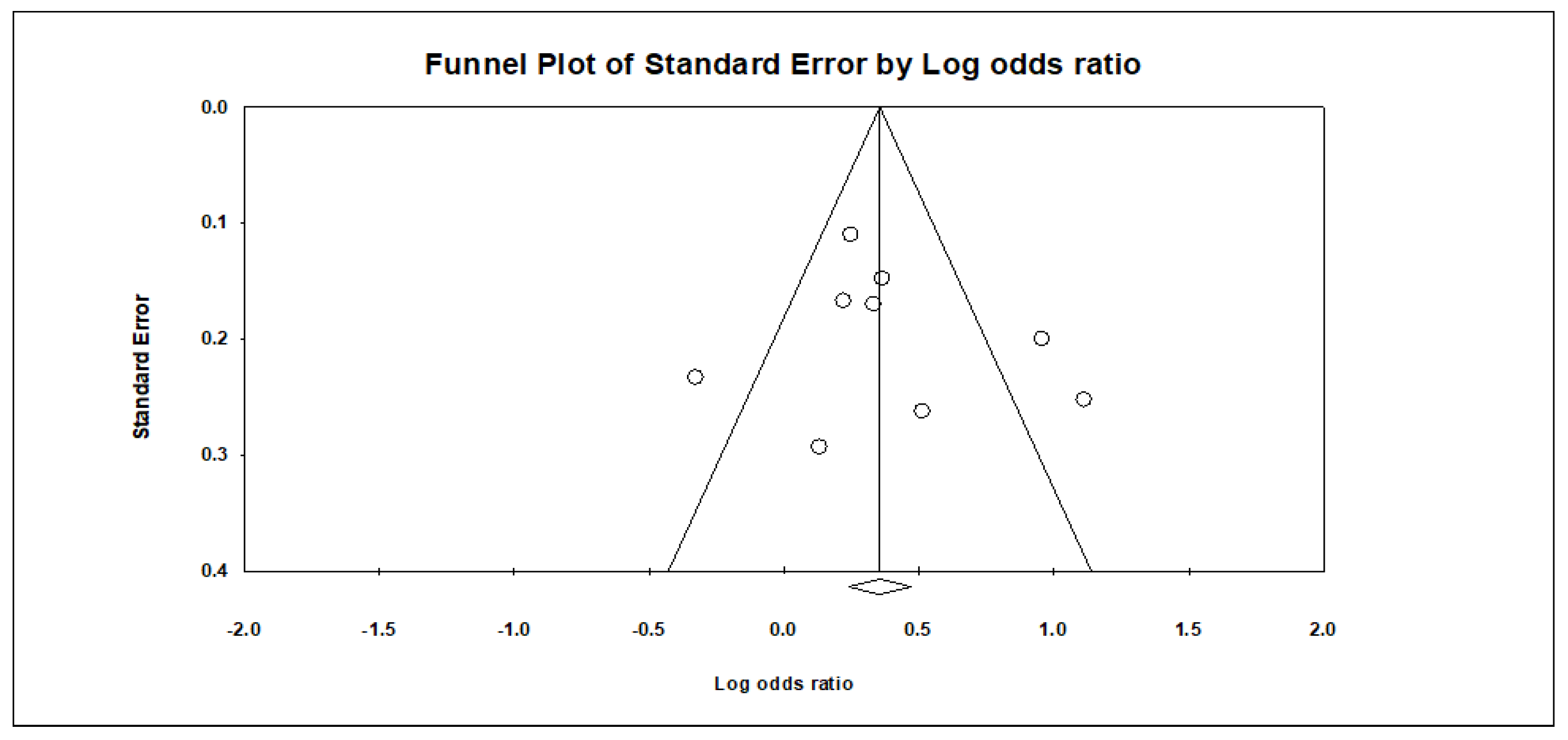
References
- How Do Vaccines Work? Available online: https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAjw2rmWBhB4EiwAiJ0mtYOYzi0LnpL7VoX8xBEskri-Gg-l-oCgWzh2oV5EmyjlETgMZUbWKRoCMZYQAvD_BwE (accessed on 14 July 2022).
- Understanding How Vaccines Work. Available online: https://www.cdc.gov/vaccines/hcp/conversations/understanding-vacc-work.html (accessed on 14 July 2022).
- Das, J.K.; Salam, R.A.; Arshad, A.; Lassi, Z.S.; Bhutta, Z.A. Systematic Review and Meta-Analysis of Interventions to Improve Access and Coverage of Adolescent Immunizations. J. Adolesc. Health 2016, 59, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lwembe, S.; Green, S.A.; Tanna, N.; Connor, J.; Valler, C.; Barnes, R. A Qualitative Evaluation to Explore the Suitability, Feasibility and Acceptability of Using a ‘Celebration Card’ Intervention in Primary Care to Improve the Uptake of Childhood Vaccinations. BMC Fam. Pract. 2016, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W.A.; Ahmed, R. Simply Put: Vaccination Saves Lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef]
- Nagar, R.; Venkat, P.; Stone, L.D.; Engel, K.A.; Sadda, P.; Shahnawaz, M. A Cluster Randomized Trial to Determine the Effectiveness of a Novel, Digital Pendant and Voice Reminder Platform on Increasing Infant Immunization Adherence in Rural Udaipur, India. Vaccine 2018, 36, 6567–6577. [Google Scholar] [CrossRef]
- Use of Appropriate Digital Technologies for Public Health. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_20-en.pdf (accessed on 27 July 2022).
- Mekonnen, Z.A.; Gelaye, K.A.; Were, M.; Tilahun, B. Effect of Mobile Phone Text Message Reminders on the Completion and Timely Receipt of Routine Childhood Vaccinations: Superiority Randomized Controlled Trial in Northwest Ethiopia. JMIR MHealth UHealth 2021, 9, e27603. [Google Scholar] [CrossRef]
- IVAC Identifies Solutions to Nigeria Childhood Mortality Crisis. Available online: https://www.gavi.org/news/media-room/ivac-identifies-solutions-nigeria-childhoodmortality-crisis (accessed on 18 July 2022).
- Garcia-Dia, M.J.; Fitzpatrick, J.J.; Madigan, E.A.; Peabody, J.W. Using Text Reminder to Improve Childhood Immunization Adherence in the Philippines. Comput. Inform. Nurs. 2017, 35, 212–218. [Google Scholar] [CrossRef]
- Wallace, A.S.; Peetosutan, K.; Untung, A.; Ricardo, M.; Yosephine, P.; Wannemuehler, K.; Brown, D.W.; McFarland, D.A.; Orenstein, W.A.; Rosenberg, E.S.; et al. Home-Based Records and Vaccination Appointment Stickers as Parental Reminders to Reduce Vaccination Dropout in Indonesia: A Cluster-Randomized Controlled Trial. Vaccine 2019, 37, 6814–6823. [Google Scholar] [CrossRef]
- Oladepo, O.; Dipeolu, I.O.; Oladunni, O. Outcome of Reminder Text Messages Intervention on Completion of Routine Immunization in Rural Areas, Nigeria. Health Promot. Int. 2021, 36, 765–773. [Google Scholar] [CrossRef]
- Oyo-Ita, A.; Wiysonge, C.S.; Oringanje, C.; Nwachukwu, C.E.; Oduwole, O.; Meremikwu, M.M. Interventions for Improving Coverage of Childhood Immunisation in Low- and Middle-Income Countries. Cochrane Database Syst. Rev. 2016, 7, CD008145. [Google Scholar] [CrossRef]
- Yunusa, U.; Ibrahim, A.H.; Ladan, M.A.; Gomaa, H.E.M. Effect of Mobile Phone Text Message and Call Reminders in the Completeness of Pentavalent Vaccines in Kano State, Nigeria. J. Pediatr. Nurs. 2022, 64, e77–e83. [Google Scholar] [CrossRef]
- Harvey, H.; Reissland, N.; Mason, J. Parental Reminder, Recall and Educational Interventions to Improve Early Childhood Immunisation Uptake: A Systematic Review and Meta-Analysis. Vaccine 2015, 33, 2862–2880. [Google Scholar] [CrossRef]
- Lukusa, L.A.; Ndze, V.N.; Mbeye, N.M.; Wiysonge, C.S. A Systematic Review and Meta-Analysis of the Effects of Educating Parents on the Benefits and Schedules of Childhood Vaccinations in Low and Middle-Income Countries. Hum. Vaccines Immunother. 2018, 14, 2058–2068. [Google Scholar] [CrossRef]
- Eze, P.; Lawani, L.O.; Acharya, Y. Short Message Service (SMS) Reminders for Childhood Immunisation in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. BMJ Glob. Health 2021, 6, e005035. [Google Scholar] [CrossRef]
- Selçuk, A.A. A Guide for Systematic Reviews: PRISMA. Turk. Arch. Otorhinolaryngol. 2019, 57, 57–58. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A Step by Step Guide for Conducting a Systematic Review and Meta-Analysis with Simulation Data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Matos, A.P.; Pegorari, M.S. How to Classify Clinical Trials Using the PEDro Scale? J. Lasers Med. Sci. 2020, 11, 1–2. [Google Scholar] [CrossRef]
- Summary of Measurement Properties of the PEDro Scale. PEDro. Available online: https://pedro.org.au/english/summary-of-measurement-properties-of-the-pedro-scale/ (accessed on 26 August 2022).
- Salihu, D.; Wong, E.M.L.; Bello, U.M.; Kwan, R.Y.C. Effects of Dance Intervention on Agitation and Cognitive Functioning of People Living with Dementia in Institutional Care Facilities: Systematic Review. Geriatr. Nurs. 2021, 42, 1332–1340. [Google Scholar] [CrossRef]
- Crowe Critical Appraisal Tool (CCAT) Form (v1.4). Available online: https://conchra.com.au/wp-content/uploads/2015/12/CCAT-form-v1.4.pdf (accessed on 26 July 2022).
- Niederhauser, V.; Johnson, M.; Tavakoli, A.S. Vaccines4Kids: Assessing the Impact of Text Message Reminders on Immunization Rates in Infants. Vaccine 2015, 33, 2984–2989. [Google Scholar] [CrossRef]
- Busso, M.; Cristia, J.; Humpage, S. Did You Get Your Shots? Experimental Evidence on the Role of Reminders. J. Health Econ. 2015, 44, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; Saville, A.W.; Beaty, B.; Dickinson, L.M.; Gurfinkel, D.; Eisert, S.; Roth, H.; Herrero, D.; Trefren, L.; Herlihy, R. Centralized Reminder/Recall to Increase Immunization Rates in Young Children: How Much Bang for the Buck? Acad. Pediatr. 2017, 17, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bangure, D.; Chirundu, D.; Gombe, N.; Marufu, T.; Mandozana, G.; Tshimanga, M.; Takundwa, L. Effectiveness of Short Message Services Reminder on Childhood Immunization Programme in Kadoma, Zimbabwe—A Randomized Controlled Trial, 2013. BMC Public Health 2015, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.B.; Oluwatosin, O.A.; Akinyemi, J.O.; Adeyemo, A.A. Effects of Community Health Nurse-Led Intervention on Childhood Routine Immunization Completion in Primary Health Care Centers in Ibadan, Nigeria. J. Community Health 2016, 41, 265–273. [Google Scholar] [CrossRef]
- Gibson, D.G.; Ochieng, B.; Kagucia, E.W.; Were, J.; Hayford, K.; Moulton, L.H.; Levine, O.S.; Odhiambo, F.; O’Brien, K.L.; Feikin, D.R. Mobile Phone-Delivered Reminders and Incentives to Improve Childhood Immunisation Coverage and Timeliness in Kenya (M-SIMU): A Cluster Randomised Controlled Trial. Lancet Glob. Health 2017, 5, e428–e438. [Google Scholar] [CrossRef]
- Seth, R.; Akinboyo, I.; Chhabra, A.; Qaiyum, Y.; Shet, A.; Gupte, N.; Jain, A.K.; Jain, S.K. Mobile Phone Incentives for Childhood Immunizations in Rural India. Pediatrics 2018, 141, 2058–2068. [Google Scholar] [CrossRef]
- O’Grady, K.-A.F.; Kaus, M.; Jones, L.; Boddy, G.; Rablin, S.; Roberts, J.; Arnold, D.; Parfitt, S.; Johnston, R.; Hall, K.K.; et al. SMS Reminders to Improve the Uptake and Timeliness of the Primary Immunisation Series in Infants: A Multi-Centre Randomised Controlled Trial. Commun. Dis. Intell. 2022, 46, 1–19. [Google Scholar] [CrossRef]
- Domek, G.J.; Contreras-Roldan, I.L.; Bull, S.; O’Leary, S.T.; Bolaños Ventura, G.A.; Bronsert, M.; Kempe, A.; Asturias, E.J. Text Message Reminders to Improve Infant Immunization in Guatemala: A Randomized Clinical Trial. Vaccine 2019, 37, 6192–6200. [Google Scholar] [CrossRef]
- Menzies, R.; Heron, L.; Lampard, J.; McMillan, M.; Joseph, T.; Chan, J.; Storken, A.; Marshall, H. A Randomised Controlled Trial of SMS Messaging and Calendar Reminders to Improve Vaccination Timeliness in Infants. Vaccine 2020, 38, 3137–3142. [Google Scholar] [CrossRef]
- Brownstone, S.; Connor, A.; Stein, D. Improving Measles Vaccine Uptake Rates in Nigeria: An RCT Evaluating the Impact of Incentive Sizes and Reminder Calls on Vaccine Uptake. PLoS ONE 2020, 15, e0233149. [Google Scholar] [CrossRef]
- Siddiqi, D.A.; Ali, R.F.; Munir, M.; Shah, M.T.; Khan, A.J.; Chandir, S. Effect of Vaccine Reminder and Tracker Bracelets on Routine Childhood Immunization Coverage and Timeliness in Urban Pakistan (2017-18): A Randomized Controlled Trial. BMC Public Health 2020, 20, 1086. [Google Scholar] [CrossRef]
- Kagucia, E.W.; Ochieng, B.; Were, J.; Hayford, K.; Obor, D.; O’Brien, K.L.; Gibson, D.G. Impact of Mobile Phone Delivered Reminders and Unconditional Incentives on Measles-Containing Vaccine Timeliness and Coverage: A Randomised Controlled Trial in Western Kenya. BMJ Glob. Health 2021, 6, e003357. [Google Scholar] [CrossRef]
- Kazi, A.M.; Ali, M.; Zubair, K.; Kalimuddin, H.; Kazi, A.N.; Iqbal, S.P.; Collet, J.-P.; Ali, S.A. Effect of Mobile Phone Text Message Reminders on Routine Immunization Uptake in Pakistan: Randomized Controlled Trial. JMIR Public Health Surveill. 2018, 4, e20. [Google Scholar] [CrossRef]
- Ekhaguere, O.A.; Oluwafemi, R.O.; Badejoko, B.; Oyeneyin, L.O.; Butali, A.; Lowenthal, E.D.; Steenhoff, A.P. Automated Phone Call and Text Reminders for Childhood Immunisations (Primm): A Randomised Controlled Trial in Nigeria. BMJ Glob. Health 2019, 4, e001232. [Google Scholar] [CrossRef]
- Dissieka, R.; Soohoo, M.; Janmohamed, A.; Doledec, D. Providing Mothers with Mobile Phone Message Reminders Increases Childhood Immunisation and Vitamin A Supplementation Coverage in Côte d’Ivoire: A Randomised Controlled Trial. J. Public Health Afr. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Hofstetter, A.M.; DuRivage, N.; Vargas, C.Y.; Camargo, S.; Vawdrey, D.K.; Fisher, A.; Stockwell, M.S. Text Message Reminders for Timely Routine MMR Vaccination: A Randomized Controlled Trial. Vaccine 2015, 33, 5741–5746. [Google Scholar] [CrossRef]
- Uddin, M.J.; Shamsuzzaman, M.; Horng, L.; Labrique, A.; Vasudevan, L.; Zeller, K.; Chowdhury, M.; Larson, C.P.; Bishai, D.; Alam, N. Use of Mobile Phones for Improving Vaccination Coverage among Children Living in Rural Hard-to-Reach Areas and Urban Streets of Bangladesh. Vaccine 2016, 34, 276–283. [Google Scholar] [CrossRef]
- Ibraheem, R.; Akintola, M.; Abdulkadir, M.; Ameen, H.; Bolarinwa, O.; Adeboye, M. Effects of Call Reminders, Short Message Services (SMS) Reminders, and SMS Immunization Facts on Childhood Routine Vaccination Timing and Completion in Ilorin, Nigeria. Afr. Health Sci. 2021, 21, 951–959. [Google Scholar] [CrossRef]
- Schäfer, T.; Schwarz, M.A. The Meaningfulness of Effect Sizes in Psychological Research: Differences between Sub-Disciplines and the Impact of Potential Biases. Front. Psychol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Introduction to Systematic Review and Meta-Analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Chutiyami, M.; Wyver, S.; Amin, J. Are Parent-Held Child Health Records a Valuable Health Intervention? A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Pinkbook. CDC.gov. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/strat.html (accessed on 26 August 2022).
- Frascella, B.; Oradini-Alacreu, A.; Balzarini, F.; Signorelli, C.; Lopalco, P.L.; Odone, A. Effectiveness of email-based reminders to increase vaccine uptake: A systematic review. Vaccine 2020, 38, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, F.; Frascella, B.; Oradini-Alacreu, A.; Gaetti, G.; Lopalco, P.L.; Edelstein, M.; Azzopardi-Muscat, N.; Signorelli, C.; Odone, A. Does the use of personal electronic health records increase vaccine uptake? A systematic review. Vaccine 2020, 38, 5966–5978. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.; Mazerolle, L.; Possingham, H.P.; Tam, K.-P.; Biggs, D. A Methodological Guide for Translating Study Instruments in Cross-cultural Research: Adapting the ‘Connectedness to Nature’ Scale into Chinese. Methods Ecol. Evol. 2020, 11, 1379–1387. [Google Scholar] [CrossRef]
- Galadima, A.N.; Zulkefli, N.A.M.; Said, S.M.; Ahmad, N. Factors Influencing Childhood Immunisation Uptake in Africa: A Systematic Review. BMC Public Health 2021, 21, 1475. [Google Scholar] [CrossRef]
- Arlinghaus, K.R.; Johnston, C.A. Advocating for Behavior Change with Education. Am. J. Lifestyle Med. 2018, 12, 113–116. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Brown, E.; Devereux, S.; Fairhead, C.; Holeman, I. Using Mobile Phones to Improve Vaccination Uptake in 21 Low- and Middle-Income Countries: Systematic Review. JMIR MHealth UHealth 2017, 5, e148. [Google Scholar] [CrossRef]
- Dumit, E.M.; Novillo-Ortiz, D.; Contreras, M.; Velandia, M.; Danovaro-Holliday, M.C. The Use of EHealth with Immunizations: An Overview of Systematic Reviews. Vaccine 2018, 36, 7923–7928. [Google Scholar] [CrossRef]
- Munk, C.; Portnoy, A.; Suharlim, C.; Clarke-Deelder, E.; Brenzel, L.; Resch, S.C.; Menzies, N.A. Systematic Review of the Costs and Effectiveness of Interventions to Increase Infant Vaccination Coverage in Low- and Middle-Income Countries. BMC Health Serv. Res. 2019, 19, 741. [Google Scholar] [CrossRef]



| No. | Variable | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|
| 1 | Population | Parent of children | Assess immunisation other than in children less than five years |
| 2 | Intervention | Parental reminder strategies for immunisation | Intervention not targeting parents or caregivers of children less than five years |
| 3 | Comparator | Usual or standard care practice | |
| 4 | Outcome | Immunisation coverage, timeliness and cost of interventions | Outcomes other than coverage, timeliness and cost of interventions |
| 5 | Study design | Randomised or quasi-experimental studies | Survey, pilot study, non-peer review articles such as thesis |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Incentive | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Incentive on coverage of childhood immunisation | ||||||||||||
| 3 | Randomised trials | Serious a | Not serious | Not serious | Not serious | None | 1379/2884 (47.8%) | 1508/2884 (52.3%) | OR 1.518 (1.238 to 1.862) | 102 more per 1000 (from 53 more to 148 more) | ⨁⨁⨁◯ Moderate | |
| SMS Reminder on coverage of childhood immunisation | ||||||||||||
| 10 | Randomised trials | Serious a | Very serious c | Not serious | Not serious d | None | 2683/5334 (50.3%) | 2651/5334 (49.7%) | OR 1.671 (1.169 to 2.390) | 126 more per 1000 (from 39 more to 206 more) | ⨁◯◯◯ Very low | |
| Voice call on coverage of childhood immunisation | ||||||||||||
| 4 | Randomised trials | Serious a | Serious c | Not serious | Not serious | Publication bias strongly suspected e strong association | 9597/19339 (49.6%) | 9742/19339 (50.4%) | OR 4.752 (1.846 to 12.231) | 325 more per 1000 (from 148 more to 422 more) | ⨁⨁◯◯ Low | |
| SMS health education on coverage of childhood immunisation | ||||||||||||
| 2 | Randomised trials | Not serious | Serious f | Not serious | Serious d | None | 1612/3247 (49.6%) | 1635/3247 (50.4%) | OR 3.158 (0.301 to 33.121) | 259 more per 1000 (from 270 fewer to 468 more) | ⨁⨁◯◯ Low | |
| Voice call and SMS reminder on coverage of childhood immunisation | ||||||||||||
| 2 | Randomised trials | Serious a | Serious g | Not serious | Not serious | Strong association | 601/1043 (57.6%) | 442/1043 (42.4%) | OR 2.025 (1.211 to 3.389) | 174 more per 1000 (from 47 more to 290 more) | ⨁⨁⨁◯ Moderate | |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Parental Strategies | Standard Care | Relative (95% CI) | Absolute (95% CI) | ||
| Incentive on timeliness of childhood immunisation | ||||||||||||
| 3 | Randomised trials | Serious a | Serious b | Not serious | Not serious | Strong association | 1031/1933 (53.3%) | 902/1933 (46.7%) | OR 2.151 (1.613 to 2.867) | 186 more per 1000 (from 119 more to 248 more) | ⨁⨁⨁◯ Moderate | |
| SMS reminder on timeliness of childhood immunisation | ||||||||||||
| 9 | Randomised trials | Serious b | Serious b | Not serious | Not serious | Publication bias strongly suspected c | 2636/5248 (50.2%) | 2612/5248 (49.8%) | OR 1.472 (1.164 to 1.863) | 96 more per 1000 (from 38 more to 151 more) | ⨁◯◯◯ Very low | |
| Health education on timeliness of childhood immunisation | ||||||||||||
| 2 | Randomised trials | Serious a | Not serious | Not serious | Not serious | Strong association | 198/401 (49.4%) | 203/401 (50.6%) | OR 2.711 (1.387 to 5.299) | 229 more per 1000 (from 81 more to 338 more) | ⨁⨁⨁⨁ High | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dathini, H.; Sharoni, S.K.A.; Robert, K.T. Parental Reminder Strategies and the Cost Implication for Improved Immunisation Outcomes: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1996. https://doi.org/10.3390/healthcare10101996
Dathini H, Sharoni SKA, Robert KT. Parental Reminder Strategies and the Cost Implication for Improved Immunisation Outcomes: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(10):1996. https://doi.org/10.3390/healthcare10101996
Chicago/Turabian StyleDathini, Hamina, Siti Khuzaimah Ahmad Sharoni, and Kever Teriyla Robert. 2022. "Parental Reminder Strategies and the Cost Implication for Improved Immunisation Outcomes: A Systematic Review and Meta-Analysis" Healthcare 10, no. 10: 1996. https://doi.org/10.3390/healthcare10101996
APA StyleDathini, H., Sharoni, S. K. A., & Robert, K. T. (2022). Parental Reminder Strategies and the Cost Implication for Improved Immunisation Outcomes: A Systematic Review and Meta-Analysis. Healthcare, 10(10), 1996. https://doi.org/10.3390/healthcare10101996





