Enhanced Patient-Centricity: How the Biopharmaceutical Industry Is Optimizing Patient Care through AI/ML/DL
Abstract
1. Introduction
2. Patient-Centricity
3. Adoptions
3.1. Disease Diagnoses
3.2. Treatment Patterns
3.3. Disease Management
4. Data Volume
5. Patient Health Information Protection
6. Use-Case Examples
6.1. AI Adoptions
6.2. ML for Fibromyalgia and Pain
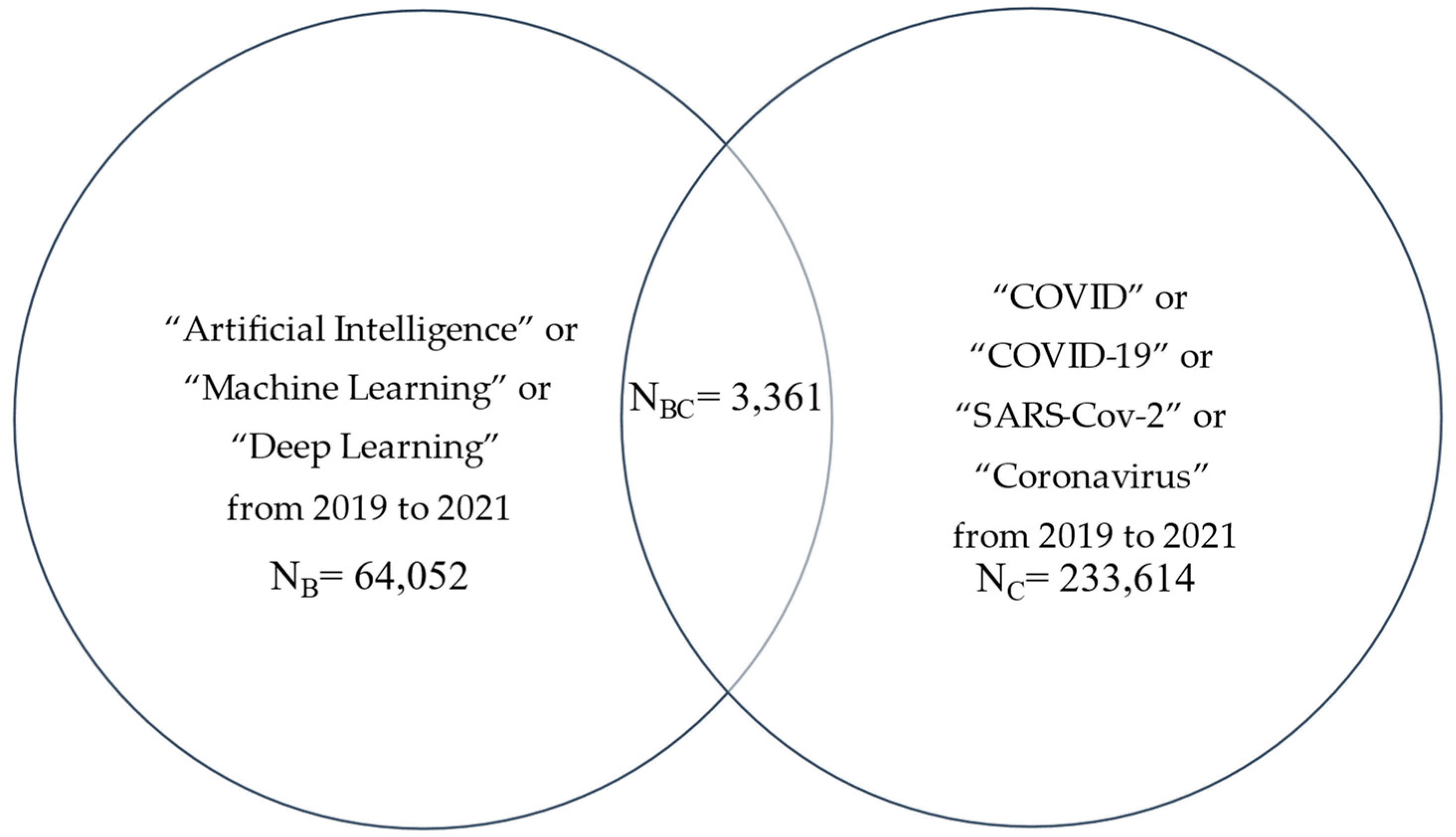
7. AI and COVID-19
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Haskett, C.; Faircloth, B.; Roper, S. Artificial Intelligence in Life Sciences: The Formula for Pharma Success Across the Drug Lifecycle. 2018. Available online: https://www.lek.com/insights/ei/artificial-intelligence-life-sciences-formula-pharma-success-across-drug-lifecycle (accessed on 6 October 2022).
- Turing, A.M.I. Computing machinery and intelligence. Mind LIX 1950, 236, 433–460. [Google Scholar] [CrossRef]
- McCarthy, J.; Minsky, M.I.; Rochester, N.; Shannon, C.E. A proposal for the Dartmouth Summer Research Project on artificial intelligence. AI Mag. 2006, 27, 12. [Google Scholar]
- Solomonoff, R.J. An Inductive Inference Machine. Preprint from 1957 IRE Convention Record, Section on Information Theory. Available online: http://www.raysolomonoff.com/publications/An%20Inductive%20Inference%20Machine1957.pdf (accessed on 6 October 2022).
- Solomonoff, R.J. A formal theory of inductive inference. Part I. Inf. Control 1964, 7, 1–22. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Sejnowski, T.J. The Deep Learning Revolution; MIT Press: Cambridge, MA, USA; London, UK, 2018. [Google Scholar]
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Pearson: Boston, MA, USA, 2020. [Google Scholar]
- Zou, K.H.; Salem, L.A.; Ray, A. (Eds.) Real-World Evidence in a Patient-Centric Digital Era; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Zou, K.H.; Li, J.Z.; Imperato, J.; Potkar, C.N.; Sethi, N.; Edwards, J.; Ray, A. Harnessing real-world data for regulatory use and applying innovative applications. J. Multidiscip. Healthc. 2020, 13, 671–679. [Google Scholar] [CrossRef]
- Zou, K.H.; Li, J.Z.; Salem, L.A.; Imperato, J.; Edwards, J.; Ray, A. Harnessing real-world evidence to reduce the burden of noncommunicable disease: Health information technology and innovation to generate insights. Health Serv. Outcomes Res. Methodol. 2021, 21, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, D.; Cappelleri, J.C.; Emir, B.; Zou, K.H. Statistical Topics in Health Economics and Outcomes Research; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- U.S. Food & Drug Administration. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML) Based Software as a Medical Device. Available online: https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf (accessed on 6 October 2022).
- European Medicines Agency. Draft Guideline on Computerised Systems and Electronic Data in Clinical Trials. 2021. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/draft-guideline-computerised-systems-electronic-data-clinical-trials_en.pdf (accessed on 6 October 2022).
- U.S. Food & Drug Administration. Clinical Decision Support Software. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-decision-support-software (accessed on 6 October 2022).
- HealthIT. Common Data Model Harmonization. The Office of the National Coordinator for Health Information Technology (ONC). Available online: https://www.healthit.gov/topic/scientific-initiatives/pcor/common-data-model-harmonization-cdm (accessed on 6 October 2022).
- U.S. Food & Drug Administration. Artificial Intelligence and Machine Learning in Software as a Medical Device. 2021. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device (accessed on 6 October 2022).
- U.S. Food & Drug Administration. Advancing Oncology Decentralized Trials: Modernizing Evidence Generation. Available online: https://www.fda.gov/about-fda/oncology-center-excellence/advancing-oncology-decentralized-trials (accessed on 6 October 2022).
- European Union. Digital Therapeutics (DTx). Available online: https://edps.europa.eu/press-publications/publications/techsonar/digital-therapeutics-dtx_en (accessed on 6 October 2022).
- GDPR. Complete Guide to GDPR Compliance. Available online: https://gdpr.eu (accessed on 6 October 2022).
- HHS. Health Information Privacy. Available online: https://www.hhs.gov/hipaa/index.html (accessed on 6 October 2022).
- National Institute on Aging. Pragmatic Clinical Trials: Testing Treatments in the Real World. 2017. Available online: https://www.nia.nih.gov/research/blog/2017/06/pragmatic-clinical-trials-testing-treatments-real-world (accessed on 6 October 2022).
- HHS. What Is PHI? Available online: https://www.hhs.gov/answers/hipaa/what-is-phi/index.html (accessed on 6 October 2022).
- Congressional Budget Office. Research and Development in the Pharmaceutical Industry. 2021. Available online: https://www.cbo.gov/publication/57126 (accessed on 6 October 2022).
- National Cancer Institute. Randomized Clinical Trial. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/randomized-clinical-trial (accessed on 6 October 2022).
- U.S. Food & Drug Administration. Real-World Evidence. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 6 October 2022).
- U.S. Department of Health and Human Services. Social Determinants of Health. Available online: https://health.gov/healthypeople/priority-areas/social-determinants-health (accessed on 6 October 2022).
- Yeoman, G.; Furlong, P.; Seres, M.; Binder, H.; Chung, H.; Garzya, V.; Jones, R.R. Defining patient centricity with patients for patients and caregivers: A collaborative endeavour. BMJ Innov. 2017, 3, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Zou, K.H.; Bushmakin, A.G.; Alvir, J.M.J.; Alemayehu, D.; Symonds, T. Patient-Reported Outcomes; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Congress. US H.R.1865—Further Consolidated Appropriations Act, 2020. 2020. Available online: https://www.congress.gov/bill/116th-congress/house-bill/1865/text (accessed on 6 October 2022).
- National Academies. Building Data Capacity for Patient-Centered Outcomes Research: An Agenda for 2021 to 2030. 2021–2022. Available online: https://www.nationalacademies.org/our-work/building-data-capacity-for-patient-centered-outcomes-research-an-agenda-for-2021-to-2030 (accessed on 6 October 2022).
- Mongan, J.; Moy, L.; Kahn, C.E., Jr. Checklist for artificial intelligence in medical imaging (CLAIM): A guide for authors and reviewers. Radiol. Artif. Intell. 2020, 25, e200029. [Google Scholar] [CrossRef]
- Radiological Society of North America. Special Report Lays Out Best Practices for Handling Bias in Radiology AI: Regulatory Challenges, Translational Gaps Hinder Machine Learning Implementation. 2022. Available online: https://www.rsna.org/news/2022/august/Handling-AI-Bias (accessed on 6 October 2022).
- Rouzrokh, P.; Khosravi, B.; Faghani, S.; Moassefi, M.; Vera Garcia, D.V.; Singh, Y.; Zhang, K.; Conte, G.M.; Erickson, B.J. Mitigating Bias in Radiology Machine Learning: 1. Data Handling. Radiol. Artif. Intell. 2022, 4, e210290. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center for Biotechnology Information. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 6 October 2022).
- Hie, B.; Zhong, E.D.; Berger, B.; Bryson, B. Learning the language of viral evolution and escape. Science 2021, 371, 284–288. [Google Scholar] [CrossRef]
- Laino, M.E.; Ammirabile, A.; Lofino, L.; Mannelli, L.; Fiz, F.; Francone, M.; Chiti, A.; Saba, L.; Orlandi, M.A.; Savevski, V. Artificial intelligence applied to pancreatic imaging: A narrative review. Healthcare 2022, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.N.; Monaghan, T.F.; Weiss, J.P. Development and validation of a machine learning algorithm for predicting response to anticholinergic medications for overactive bladder syndrome. Obstet. Gynecol. 2020, 135, 483. [Google Scholar] [CrossRef]
- Goldstein, I.; Goren, A.; Liebert, R.; Tang, W.Y.; Hassan, T.A. National Health and Wellness Survey exploratory cluster analysis of males 40–70 years old focused on erectile dysfunction and associated risk factors across the USA, Italy, Brazil and China. Int. J. Clin. Pract. 2019, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Panch, T. Using AI to Amplify Care for Patients with Chronic Disease. AJMC. 2019. Available online: https://www.ajmc.com/contributor/trishan-panch/2019/01/using-ai-to-amplify-care-for-patients-with-chronic-disease (accessed on 6 October 2022).
- Demanuele, C.; Lokker, C.; Jhaveri, K.; Georgiev, P.; Sezgin, E.; Geoghegan, C.; Zou, K.H.; Izmailova, E.; McCarthy, M. Considerations for conducting Bring Your Own “Device” (BYOD) Clinical Studies. Digit. Biomark. 2022, 6, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Gay, V.; Alturki, R.; AlGhamdi, M.J. Towards understanding the usability attributes of AI-enabled eHealth mobile applications. J. Healthc. Eng. 2021, 2021, 5313027. [Google Scholar] [CrossRef]
- Schwartz, R.; Vassilev, A.; AGreene, K.K.; Perine, L.; Burt, A.; Hall, P. Towards a Standard for Identifying and Managing Bias in Artificial Intelligence; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022.
- U.S. Food & Drug Administration. Clinical Trial Diversity. 2022. Available online: https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity (accessed on 6 October 2022).
- U.S. Food & Drug Administration. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials: Guidance for Industry; Draft Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations (accessed on 6 October 2022).
- Vasey, B.; Nagendran, M.; Campbell, B.; Clifton, D.A.; Collins, G.S.; Denaxas, S.; Denniston, A.K.; Faes, L.; Geerts, B.; Ibrahim, M.; et al. DECIDE-AI expert group. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat. Med. 2022, 28, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Crossnohere, N.L.; Elsaid, M.; Paskett, J.; Bose-Brill, S.; Bridges, J.F.P. Guidelines for Artificial Intelligence in Medicine: Literature Review and Content Analysis of Frameworks. J. Med. Internet Res. 2022, 24, e36823. [Google Scholar] [CrossRef]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef] [PubMed]
- Lawton, G. Using Small Data Sets for Machine Learning Models Sees Growth. TechTarget 2019. Available online: https://searchenterpriseai.techtarget.com/feature/Using-small-data-sets-for-machine-learning-models-sees-growth (accessed on 6 October 2022).
- Feng, R.; Zheng, X.; Gao, T.; Chen, J.; Wang, W.; Chen, D.Z.; Wu, J. Interactive few-shot learning: Limited supervision, better medical image segmentation. IEEE Trans. Med. Imaging 2021, 40, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, A.; Metaxas, D.N.; Liu, D. Greedy auto-augmentation for n-shot learning using deep neural networks. Neural Netw. 2021, 135, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Biswas, S. Generalized Zero-Shot Cross-Modal Retrieval. IEEE Trans. Image Process. 2019, 28, 5953–5962. [Google Scholar] [CrossRef] [PubMed]
- Deloitte. Getting Real with Real-World Evidence (RWE) 2017 RWE Benchmark Survey. 2017. Available online: https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/real-world-evidence-benchmarking-survey.html (accessed on 6 October 2022).
- Davis, B.; Morgan, J.; Shah, S. The Future of Real-World Evidence Biopharma Companies Focus on End-to-End, AI-Driven, Internally Developed Solutions. 2018. Available online: https://www2.deloitte.com/us/en/insights/industry/life-sciences/2018-real-world-evidence-benchmarking.html?id=gx:2sm:3tw:4RWE_LSHC18::6Life_Sciences_and_Healthcare:20180711095200:Global&linkId=54041116 (accessed on 6 October 2022).
- Morgan, J.; Feghali, K.; Shah, S.; Miranda, W. RWE Focus Is Shifting to R&D, Early Investments Begin to Pay Off. 2020. Available online: https://www2.deloitte.com/us/en/insights/industry/health-care/real-world-evidence-study.html?id=us:2sm:3li:4di_gl:5eng:6di (accessed on 6 October 2022).
- Morgan, J.; Feghali, K.; Chang, C.; Miranda, W. Real-World Evidence’s Evolution into a True End-to-End Capability. Available online: https://www2.deloitte.com/us/en/insights/industry/health-care/real-world-evidence-study.html?id=us:2em:3na:4diUS175115:5awa:6di:MMDDYY:author&pkid=1008576 (accessed on 6 October 2022).
- The Professional Society for Health Economics and Outcomes Research. ISPOR 2022–2023 top 10 HEOR Trends Report. Available online: https://www.ispor.org/heor-resources/about-heor/top-10-heor-trends (accessed on 6 October 2022).
- European Commission. European Health Data Space. Available online: https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space_en (accessed on 6 October 2022).
- The White House. FACT SHEET: United States and European Commission Announce Trans-Atlantic Data Privacy Framework. 2022. Available online: https://www.whitehouse.gov/briefing-room/statements-releases/2022/03/25/fact-sheet-united-states-and-european-commission-announce-trans-atlantic-data-privacy-framework (accessed on 6 October 2022).
- European Data Protection Board. Statement 01/2022 on the Announcement of an Agreement in Principle on a New Trans-Atlantic Data Privacy Framework. 2022. Available online: https://edpb.europa.eu/our-work-tools/our-documents/statements/statement-012022-announcement-agreement-principle-new-trans_en (accessed on 6 October 2022).
- Digital Medicine Society (DiMe). DiMe’s Library of Digital Endpoints. 2022. Available online: https://www.dimesociety.org/communication-education/library-of-digital-endpoints (accessed on 6 October 2022).
- FDA Voices. Leveraging Real World Evidence in Regulatory Submissions of Medical Devices. 2021. Available online: https://www.fda.gov/news-events/fda-voices/leveraging-real-world-evidence-regulatory-submissions-medical-devices (accessed on 6 October 2022).
- U.S. Food and Drug Administration. Examples of Real-World Evidence (RWE) Used in Medical Device Regulatory Decisions: Selected Examples with File Summaries, Details on Real-World Data Source, Populations, and Descriptions of Use. Center for Devices and Radiological Health. Selected Examples with File Summaries, Details on Real-World Data Source, Populations, and Descriptions of Use. 2021. Available online: https://www.fda.gov/media/146258/download (accessed on 6 October 2022).
- AbbVie. ChemBeads: Improving Artificial Intelligence through Human Ingenuity. 2019. Available online: https://stories.abbvie.com/stories/chembeads-improving-artificial-intelligence-through-human-ingenuity.htm (accessed on 6 October 2022).
- Amgen. AI & Data Science: Opening Up Vast New Frontiers in Drug Discovery and Development. Available online: https://www.amgen.com/science/research-and-development-strategy/ai-and-data-science (accessed on 6 October 2022).
- AstraZeneca. Data Science & Artificial Intelligence: Unlocking New Science Insights. Available online: https://www.astrazeneca.com/r-d/data-science-and-ai.html (accessed on 6 October 2022).
- MIT Media Lab. GSK Manufacturing Initiative. Available online: https://www.media.mit.edu/projects/gsk-manufacturing-initiative/overview (accessed on 6 October 2022).
- Johnson & Johnson. Can Artificial Intelligence Change How We Discover Drugs? 2018. Available online: https://www.jnj.com/latest-news/how-artificial-intelligence-is-helping-Janssen-discover-new-drugs (accessed on 6 October 2022).
- Merck. Merck Announces the Launch of the Merck Digital Sciences Studio to Help Healthcare Startups Quickly Bring Their Innovations to Market. 2022. Available online: https://www.merck.com/news/merck-announces-the-launch-of-the-merck-digital-sciences-studio-to-help-healthcare-startups-quickly-bring-their-innovations-to-market (accessed on 6 October 2022).
- Novartis. AI-Powered Diagnostic Tool to Aid in the Early Detection of Leprosy. 2020. Available online: https://www.novartisfoundation.org/news/ai-powered-diagnostic-tool-aid-early-detection-leprosy (accessed on 6 October 2022).
- Pfizer. CytoReason Announces Expanded Collaboration Deal with Pfizer to Deliver AI for Drug Discovery and Development. 2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/cytoreason-announces-expanded-collaboration-deal-pfizer (accessed on 6 October 2022).
- Roche. Roche Announces the Release of its Newest Artificial Intelligence (AI) Based Digital Pathology Algorithms to Aid Pathologists in Evaluation of Breast Cancer Markers, Ki-67, ER and PR. Available online: https://diagnostics.roche.com/global/en/news-listing/2021/roche-announces-release-of-its-newest-ai-based-digital-pathology-algorithms-to-aid-pathologists-in-evaluation-breast-cancer-markers-ki67-er-pr.html (accessed on 6 October 2022).
- Massachusetts Institute of Technology. MIT-Takeda Program Launches; Research Projects Will Harness the Power of Artificial Intelligence to Positively Impact Human Health. Available online: https://news.mit.edu/2020/mit-takeda-program-launches-research-ai-and-human-health-0618 (accessed on 6 October 2022).
- Kaitin, K.I. Artificial intelligence and patient-centric approaches to advance pharmaceutical Innovation. Clin. Ther. 2019, 41, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Bhhatarai, B.; Walters, W.P.; Hop, C.E.C.A.; Lanza, G.; Ekins, S. Opportunities and challenges using artificial intelligence in ADME/Tox. Nat. Mater. 2019, 18, 418–422. [Google Scholar] [CrossRef]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug. Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Roberts, R.A.; Lal-Nag, M.; Chen, X.; Huang, R.; Tong, W. AI-based language models powering drug discovery and development. Drug. Discov. Today 2021, 26, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug. Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.W.; Pujol, J.; Deus, J.; Harrison, B.J.; Monfort, J.; Wager, T.D. Towards a neurophysiological signature for fibromyalgia. Pain 2017, 158, 34–47. [Google Scholar]
- Alter, B.J.; Anderson, N.P.; Gillman, A.G.; Yin, Q.; Jeong, J.H.; Wasan, A.D. Hierarchical clustering by patient-reported pain distribution alone identifies distinct chronic pain subgroups differing by pain intensity, quality, and clinical outcomes. PLoS ONE 2021, 16, e0254862. [Google Scholar]
- Minerbi, A.; Gonzalez, E.; Brereton, N.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered serum bile acid profile in fibromyalgia is associated with specific gut microbiome changes and symptom severity. Pain 2022. [Google Scholar] [CrossRef]
- Andrés-Rodríguez, L.; Borràs, X.; Feliu-Soler, A.; Pérez-Aranda, A.; Rozadilla-Sacanell, A.; Arranz, B.; Montero-Marin, J.; García-Campayo, J.; Angarita-Osorio, N.; Maes, M.; et al. Machine learning to understand the immune-inflammatory pathways in fibromyalgia. Int. J. Mol. Sci. 2019, 20, 4231. [Google Scholar] [CrossRef]
- Orrù, G.; Gemignani, A.; Ciacchini, R.; Bazzichi, L.; Conversano, C. Machine learning increases diagnosticity in psychometric evaluation of alexithymia in fibromyalgia. Front. Med. 2020, 6, 319. [Google Scholar] [CrossRef]
- Alexander, J., Jr.; Edwards, R.A.; Brodsky, M.; Manca, L.; Grugni, R.; Savoldelli, A.; Bonfanti, G.; Emir, B.; Whalen, E.; Watt, S.; et al. Using time series analysis approaches for improved prediction of pain outcomes in subgroups of patients with painful diabetic peripheral neuropathy. PLoS ONE 2018, 13, e0207120, Erratum in PLoS ONE 2019, 14, e0212959. [Google Scholar] [CrossRef] [PubMed]
- AI2 Allen Institute for AI. CORD-19: COVID-19 Open Research Dataset. 2022. Available online: https://github.com/allenai/cord19 (accessed on 6 October 2022).
- Hassan, T.A.; Saenz, J.E.; Li, J.Z.; Ducinskiene, D.; Imeprato, J.; Zou, K.H. A Confluence of Acute and Chronic Diseases: Risk Factors Among Covid-19 Patients. Significance 2020. Available online: https://www.significancemagazine.com/science/671-a-confluence-of-acute-and-chronic-diseases-risk-factors-among-covid-19-patients (accessed on 6 October 2022).
- Zou, K.H.; Li, J.Z.; Hassan, T.A.; Imeprato, J.; Saenz, J.E.; Ducinskiene, D. The Role of Data Science and Risk Assessments During the COVID-19 Pandemic. CIO Applications. Available online: https://www.cioapplications.com/cxoinsights/the-roles-of-data-science-and-risk-assessments-during-the-covid19-pandemic-nid-5981.html (accessed on 6 October 2022).
- Hassan, T.A.; Sáenz, J.E.; Ducinskiene, D.; Cook, J.P.; Imperato, J.S.; Zou, K.H. New Strategies to Improve Patient Adherence to Medications for Noncommunicable Diseases During and After the COVID-19 Era Identified via a Literature Review. J. Multidiscip. Healthc. 2021, 14, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Alam, F.; Qadir, J.; Qolomany, B.; Khan, I.; Khan, T.; Suleman, M.; Said, N.; Hassan, S.Z.; Gul, A.; et al. Global user-level perception of COVID-19 contact tracing applications: Data-driven approach using natural language processing. JMIR Form. Res. 2022, 6, e36238. [Google Scholar] [CrossRef] [PubMed]
- Babukarthik, R.G.; Adiga, V.A.K.; Sambasivam, G.; Chandramohan, D.; Amudhavel, J. Prediction of COVID-19 using genetic deep Learning Convolutional Neural Network (GDCNN). IEEE Access 2020, 8, 177647–177666. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Cicioğlu, M.; Çalhan, A. Real-time internet of medical things framework for early detection of Covid-19. Neural Comput. Appl. 2022, 1–14. [Google Scholar] [CrossRef]
- Mohanty, S.; Rashid, M.H.A.; Mridul, M.; Mohanty, C.; Swayamsiddha, S. Application of artificial intelligence in COVID-19 drug repurposing. Diabetes Metab. Syndr. 2020, 14, 1027–1031. [Google Scholar] [CrossRef]
- Hosny, A.; Aerts, H.J.W.L. Artificial intelligence for global health. Science 2019, 366, 955–956. [Google Scholar]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Jassar, S.; Adams, S.J.; Zarzeczny, A.; Burbridge, B.E. The future of artificial intelligence in medicine: Medical-legal considerations for health leaders. Healthc. Manag. Forum 2022, 35, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Babicsara, B.; Evgenouand, G.; Cohen, I.G. Beware explanations from AI in health care: The benefits of explainable artificial intelligence are not what they appear. Science 2021, 373, 284–286. [Google Scholar]
- Ng, M.Y.; Kapur, S.; Blizinsky, K.D.; Hernandez-Boussard, T. The AI life cycle: A holistic approach to creating ethical AI for health decisions. Nat. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- The White House. The White House. Available online: https://www.whitehouse.gov/ostp/ai-bill-of-rights (accessed on 6 October 2022).


| Abbreviation | Terminology | Source | Reference |
|---|---|---|---|
| AI | Artificial Intelligence | FDA | [13] |
| BYOD | Bring Your Own Device | EMA | [14] |
| CDS | Clinical Decision Support | FDA | [15] |
| CDM | Common Data Model | National Coordinator for Health Information Technology (HealthIT.gov, accessed on 6 October 2022) | [16] |
| DL | Deep Learning | FDA | [17] |
| DTC | Decentralized Clinical Trial | FDA | [18] |
| DTx | Digital Therapeutics | EU | [19] |
| GDPR | General Data Protection Regulation | GDPR.EU | [20] |
| HIPAA | The Health Insurance Portability and Accountability Act of 1996 | U.S. Department of Health and Health Services (HHS) | [21] |
| ML | Machine Learning | FDA | [17] |
| PCT | Pragmatic Clinical Trial | National Institute of Aging | [22] |
| PHI | Protected Health Information | HHS.gov | [23] |
| R&D | Research and Development | Congressional Budget Office | [24] |
| RCT | Randomized Controlled Trial | National Cancer Institute | [25] |
| RWD | Real-World Data | FDA | [26] |
| RWE | Real-World Evidence | FDA | [26] |
| SDOH | Social Determinants of Health | HHS | [27] |
| Example | Organization | Purpose | Project | Reference |
|---|---|---|---|---|
| 1 | AbbVie | Compound Screening | “ChemBeads: Improving Artificial Intelligence Through Human Ingenuty.” | [64] |
| 2 | Amgen | Drug Discovery and Development | “AI & Data Science: Opening Up Vast New Frontiers in Drug Discovery and Development.” | [65] |
| 3 | AstraZeneca | Drug Discovery and Delivery | “Data Science & Artificial Intelligence: Unlocking New Science Insights.” | [66] |
| 5 | GSK (with Massachusetts Institute of Technology; MIT) | Manufacturing | “GSK Manufacturing Initiative.” | [67] |
| 6 | Johnson & Johnson | Drug Discovery | “Can Artificial Intelligence Change How We Discover Drugs?” | [68] |
| 7 | Merck | Drug Discovery and Development | “Merck Announces the Launch of the Merck Digital Sciences Studio to Help Healthcare Startups Quickly Bring their Innovations to Market.” | [69] |
| 8 | Novartis | Disease Diagnosis | “AI-powered Diagnostic Tool to Aid in the Early Detection of Leprosy.” | [70] |
| 8 | Pfizer (with CytoReason) | Drug Discovery and Development | “CytoReason Announces Expanded Collaboration Deal with Pfizer to Deliver AI for Drug Discovery and Development.” | [71] |
| 9 | Roche | Biomarker Evaluation | “Roche Announces the Release of Its Newest Artificial Intelligence (AI) Based Digital Pathology Algorithms to Aid Pathologists in Evaluation of Breast Cancer Markers, Ki-67, ER and PR.” | [72] |
| 10 | Takeda (with MIT) | Human Health and Drug Development | “MIT-Takeda Program Launches: Research Projects Will Harness the Power of Artificial Intelligence to Positively Impact Human Health.” | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, K.H.; Li, J.Z. Enhanced Patient-Centricity: How the Biopharmaceutical Industry Is Optimizing Patient Care through AI/ML/DL. Healthcare 2022, 10, 1997. https://doi.org/10.3390/healthcare10101997
Zou KH, Li JZ. Enhanced Patient-Centricity: How the Biopharmaceutical Industry Is Optimizing Patient Care through AI/ML/DL. Healthcare. 2022; 10(10):1997. https://doi.org/10.3390/healthcare10101997
Chicago/Turabian StyleZou, Kelly H., and Jim Z. Li. 2022. "Enhanced Patient-Centricity: How the Biopharmaceutical Industry Is Optimizing Patient Care through AI/ML/DL" Healthcare 10, no. 10: 1997. https://doi.org/10.3390/healthcare10101997
APA StyleZou, K. H., & Li, J. Z. (2022). Enhanced Patient-Centricity: How the Biopharmaceutical Industry Is Optimizing Patient Care through AI/ML/DL. Healthcare, 10(10), 1997. https://doi.org/10.3390/healthcare10101997








