Sociodemographic and Clinical Characteristics Associated with Improvements in Quality of Life for Participants with Opioid Use Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Measures
2.3. Participants
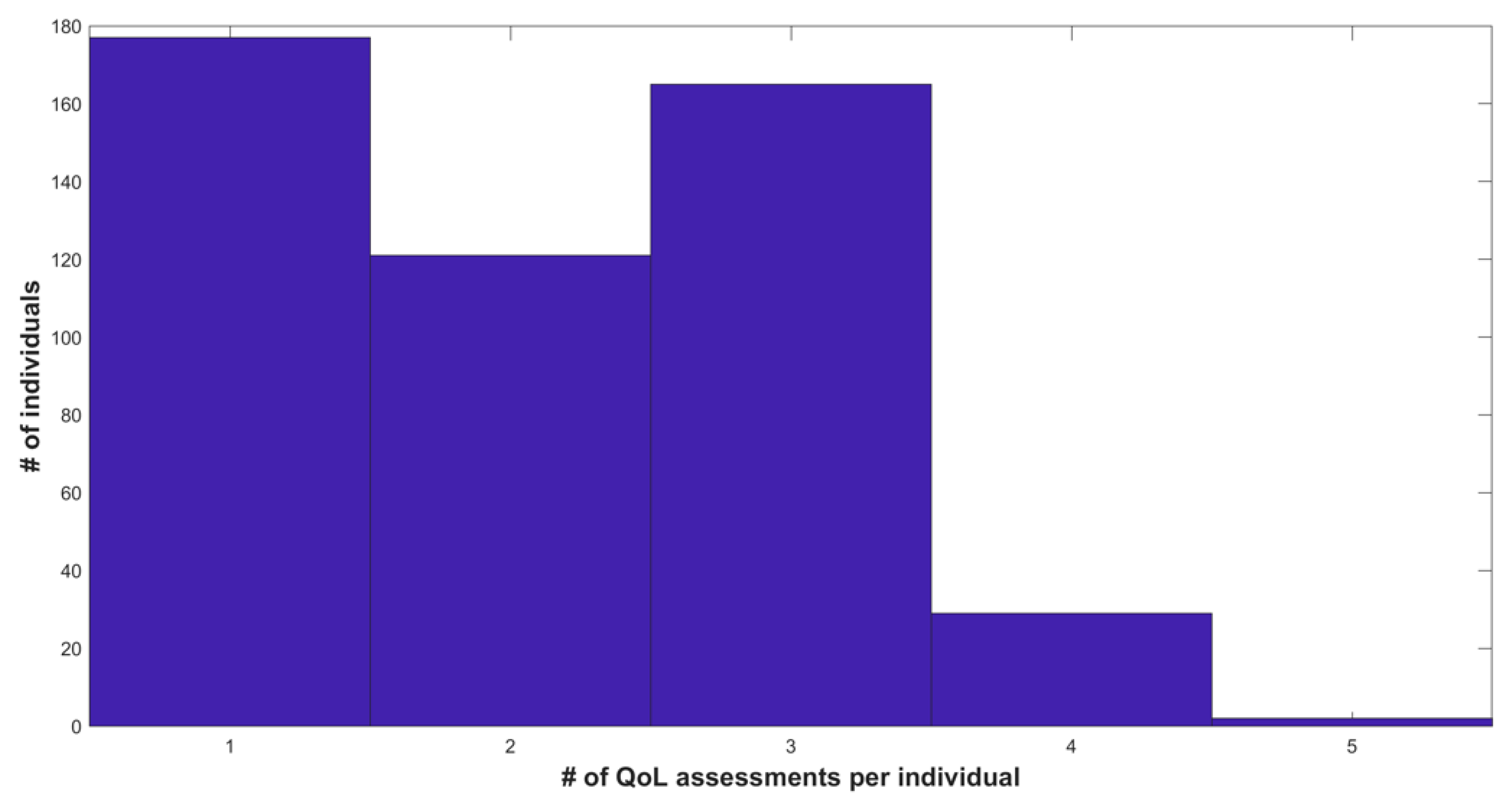
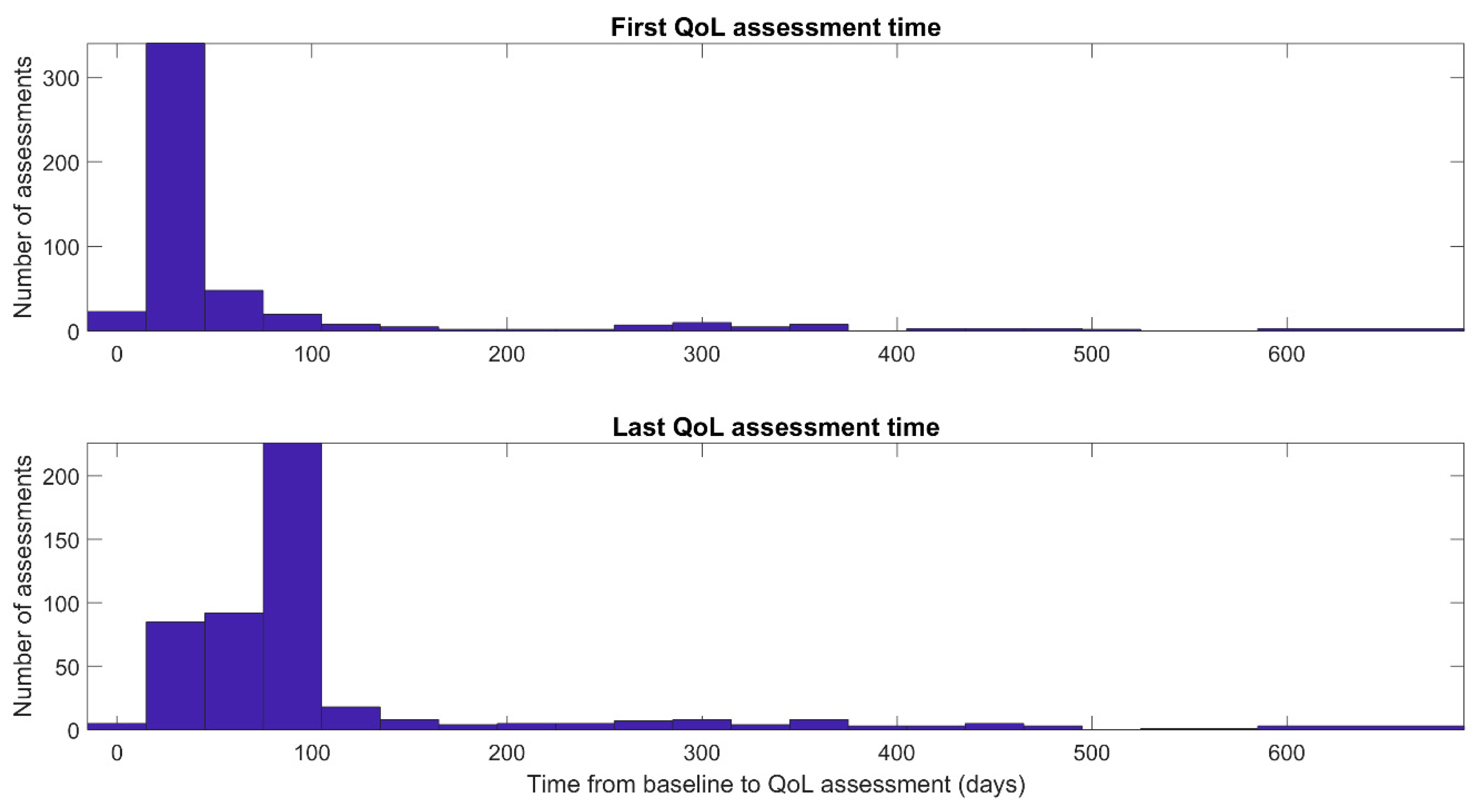
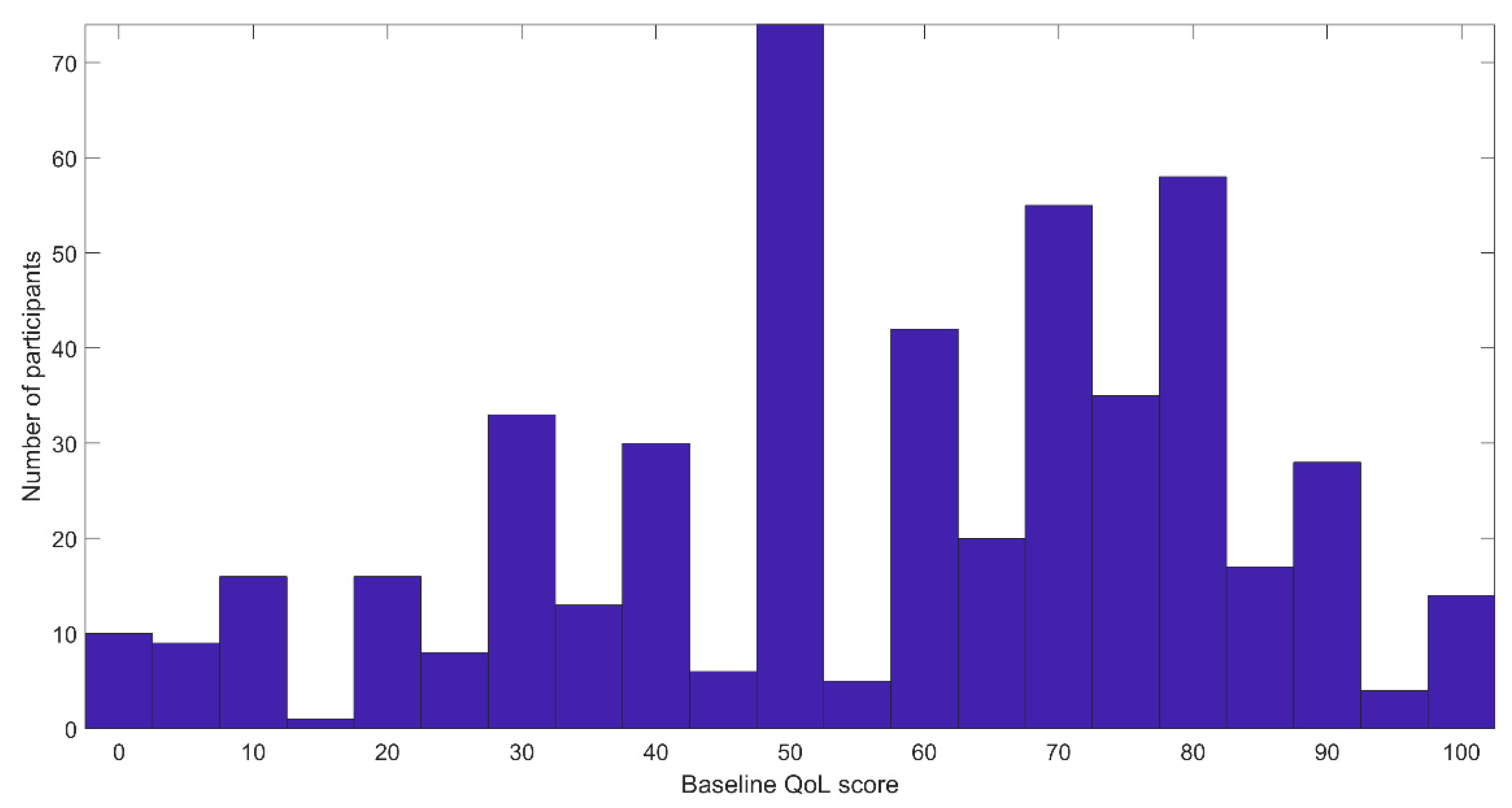
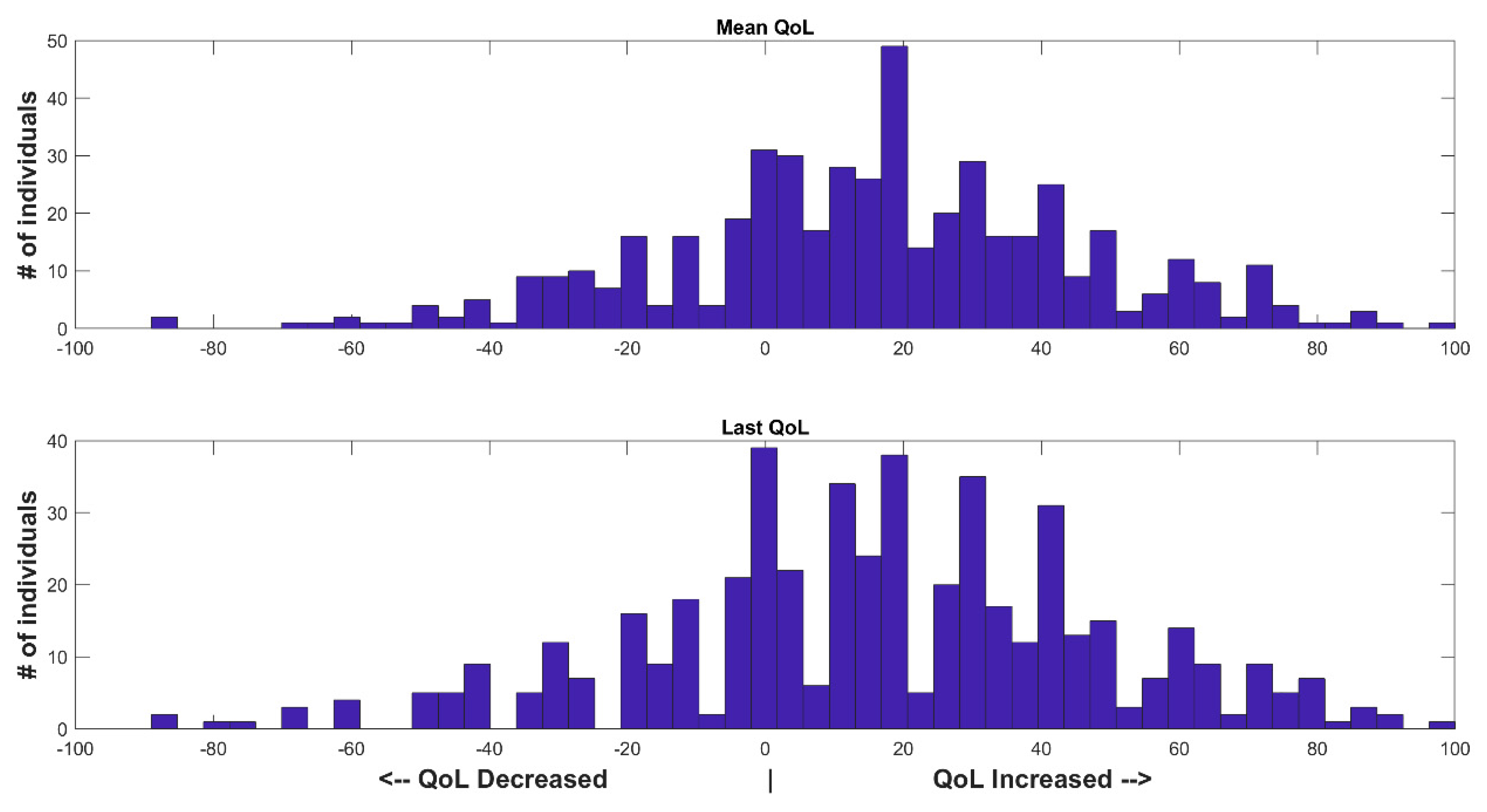
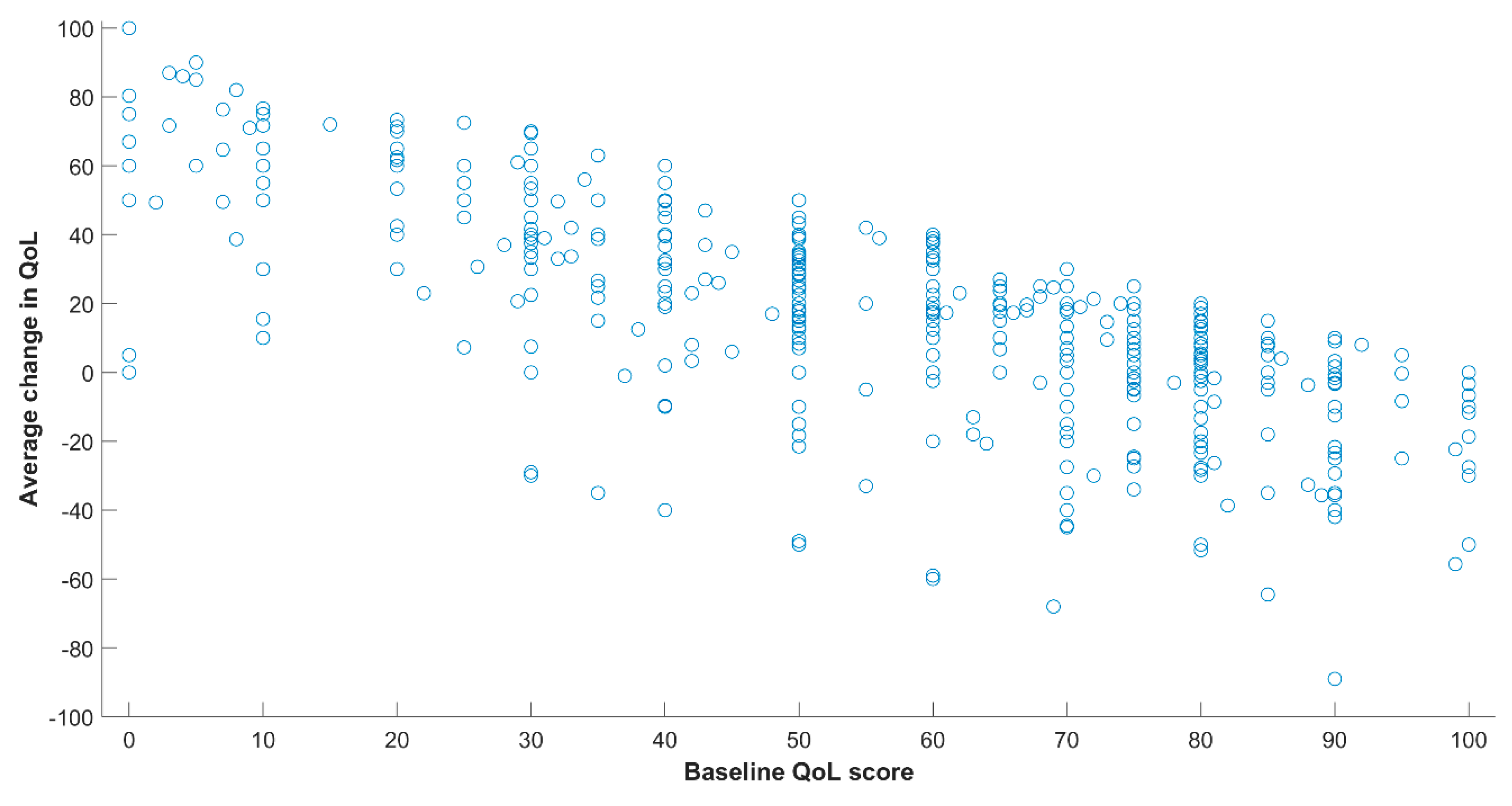
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A.; Group, W. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Saitz, R.; Larson, M.J.; LaBelle, C.; Richardson, J.; Samet, J.H. The Case for Chronic Disease Management for Addiction. J. Addict. Med. 2008, 2, 55–65. [Google Scholar] [CrossRef]
- Coleman, K.; Austin, B.T.; Brach, C.; Wagner, E.H. Evidence On The Chronic Care Model In The New Millennium. Health Aff. 2009, 28, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.Y.; Glasgow, R.E.; Dickinson, L.M.; Froshaug, D.B.; Fernald, D.H.; Balasubramanian, B.A.; Green, L.A. The Chronic Care Model and Relationships to Participant Health Status and Health-Related Quality of Life. Am. J. Prev. Med. 2008, 35, S398–S406. [Google Scholar] [CrossRef]
- McLellan, A.T.; Lewis, D.C.; O’Brien, C.P.; Kleber, H.D. Drug Dependence, a Chronic Medical Illness: Implications for Treatment, Insurance, and Outcomes Evaluation. JAMA 2000, 284, 1689–1695. [Google Scholar] [CrossRef]
- De Maeyer, J.; Vanderplasschen, W.; Broekaert, E. Quality of life among opiate-dependent individuals: A review of the literature. Int. J. Drug Policy 2010, 21, 364–380. [Google Scholar] [CrossRef]
- SAMHSA. Medications for Opioid Use Disorder. Treatment Protocol Tip (TIP) Series 63; Publication No. PEP20-02-02-006; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2020.
- Cavaiola, A.A.; Fulmer, B.A.; Stout, D. The Impact of Social Support and Attachment Style on Quality of Life and Readiness to Change in a Sample of Individuals Receiving Medication-Assisted Treatment for Opioid Dependence. Subst. Abus. 2015, 36, 183–191. [Google Scholar] [CrossRef]
- Wasserman, D.A.; Stewart, A.L.; Delucchi, K.L. Social support and abstinence from opiates and cocaine during opioid maintenance treatment. Drug Alcohol Depend. 2001, 65, 65–75. [Google Scholar] [CrossRef]
- Jessica De, M.; Wouter, V.; Jan, L.; van Chijs, N.; Bernard, S.; Eric, B. Current quality of life and its determinants among opiate-dependent individuals five years after starting methadone treatment. Qual. Life Res. 2011, 20, 139–150. [Google Scholar] [CrossRef]
- De Maeyer, J.; Vanderplasschen, W.; Broekaert, E. Exploratory Study on Drug Users’ Perspectives on Quality of Life: More than Health-Related Quality of Life? Soc. Indic. Res. 2008, 90, 107–126. [Google Scholar] [CrossRef]
- De Maeyer, J.; Vanderplasschen, W.; Camfield, L.; Vanheule, S.; Sabbe, B.; Broekaert, E. A good quality of life under the influence of methadone: A qualitative study among opiate-dependent individuals. Int. J. Nurs. Stud. 2011, 48, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- De Maeyer, J.; van Nieuwenhuizen, C.; Bongers, I.L.; Broekaert, E.; Vanderplasschen, W. Profiles of quality of life in opiate-dependent individuals after starting methadone treatment: A latent class analysis. Int. J. Drug Policy 2013, 24, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.; Erga, A.H.; Hagen, K.P.; Nesvåg, S.M.; McKay, J.R.; Lundervold, A.J.; Walderhaug, E. One-year sobriety improves satisfaction with life, executive functions and psychological distress among participants with polysubstance use disorder. J. Subst. Abus. Treat. 2017, 76, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.-U.; Apelt, S.M.; Soyka, M.; Gastpar, M.; Backmund, M.; Gölz, J.; Kraus, M.R.; Tretter, F.; Schäfer, M.; Siegert, J.; et al. Feasibility and outcome of substitution treatment of heroin-dependent participants in specialized substitution centers and primary care facilities in Germany: A naturalistic study in 2694 participants. Drug Alcohol Depend. 2008, 95, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Giacomuzzi, S.M.; Riemer, Y.; Ertl, M.; Kemmler, G.; Rossler, H.; Hinterhuber, H.; Kurz, M. Buprenorphine versus methadone maintenance treatment in an ambulant setting: A health-related quality of life assessment. Addiction 2003, 98, 693–702. [Google Scholar] [CrossRef]
- Nosyk, B.; Guh, D.P.; Sun, H.; Oviedo-Joekes, E.; Brissette, S.; Marsh, D.C.; Schechter, M.T.; Anis, A.H. Health related quality of life trajectories of participants in opioid substitution treatment. Drug Alcohol Depend. 2011, 118, 259–264. [Google Scholar] [CrossRef]
- Nosyk, B.; Bray, J.W.; Wittenberg, E.; Aden, B.; Eggman, A.A.; Weiss, R.D.; Potter, J.; Ang, A.; Hser, Y.I.; Ling, W.; et al. Short term health-related quality of life improvement during opioid agonist treatment. Drug Alcohol Depend. 2015, 157, 121–128. [Google Scholar] [CrossRef]
- Astals, M.; Domingo-Salvany, A.; Buenaventura, C.C.; Tato, J.; Vazquez, J.M.; Martín-Santos, R.; Torrens, M. Impact of Substance Dependence and Dual Diagnosis on the Quality of Life of Heroin Users Seeking Treatment. Subst. Use Misuse 2008, 43, 612–632. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, G.; Babayan, K.; Fontes, M.; Burg, M.M. Pain and Psychology-A Reciprocal Relationship. Ochsner J. 2017, 17, 173–180. [Google Scholar]
- Strada, L.; Vanderplasschen, W.; Buchholz, A.; Schulte, B.; Muller, A.E.; Verthein, U.; Reimer, J. Measuring quality of life in opioid-dependent people: A systematic review of assessment instruments. Qual. Life Res. 2017, 26, 3187–3200. [Google Scholar] [CrossRef]
- Papadimitriou, G. The “Biopsychosocial Model”: 40 years of application in Psychiatry. Psychiatriki 2017, 28, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Rani Das, P.; Murthy, P.S.; Diwan, C.; Patil, A.A.; Jagtap, B. Quality of life in psychiatric disorders. Trends Biomed. Res. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- González-Blanch, C.; Hernández-de-Hita, F.; Muñoz-Navarro, R.; Ruíz-Rodríguez, P.; Medrano, L.A.; Cano-Vindel, A. The association between different domains of quality of life and symptoms in primary care participants with emotional disorders. Sci. Rep. 2018, 8, 11180. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, K.M.; Sterling, R.C.; Van Bockstaele, E.J. Psychological and physiological stress negatively impacts early engagement and retention of opioid-dependent individuals on methadone maintenance. J. Subst. Abus. Treat. 2014, 48, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. Chronic Stress, Drug Use, and Vulnerability to Addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105–130. [Google Scholar] [CrossRef]
- Bray, J.W.; Aden, B.; Eggman, A.A.; Hellerstein, L.; Wittenberg, E.; Nosyk, B.; Stribling, J.C.; Schackman, B.R. Quality of life as an outcome of opioid use disorder treatment: A systematic review. J. Subst. Abus. Treat. 2017, 76, 88–93. [Google Scholar] [CrossRef]
- Kelly, J.F.; Greene, M.C.; Bergman, B.G. Beyond Abstinence: Changes in Indices of Quality of Life with Time in Recovery in a Nationally Representative Sample of U.S. Adults. Alcohol. Clin. Exp. Res. 2018, 42, 770–780. [Google Scholar] [CrossRef]
- Fei, J.T.B.; Yee, A.; Habil, M.H.B.; Danaee, M. Effectiveness of Methadone Maintenance Therapy and Improvement in Quality of Life Following a Decade of Implementation. J. Subst. Abus. Treat. 2016, 69, 50–56. [Google Scholar] [CrossRef]
- Krebs, E.; Kerr, T.; Wood, E.; Nosyk, B. Characterizing long-term health related quality of life trajectories of individuals with opioid use disorder. J. Subst. Abus. Treat. 2016, 67, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.E.; Skurtveit, S.; Clausen, T. Building abstinent networks is an important resource in improving quality of life. Drug Alcohol Depend. 2017, 180, 431–438. [Google Scholar] [CrossRef]
- Kapoor, A.; Kohli, K.; Kapoor, A.; Jose, N.A. Improvement in quality of life with buprenorphine in opioid dependence. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 689–694. [Google Scholar] [CrossRef]
- Wittenberg, E.; Bray, J.W.; Aden, B.; Gebremariam, A.; Nosyk, B.; Schackman, B.R. Measuring benefits of opioid misuse treatment for economic evaluation: Health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction 2016, 111, 675–684. [Google Scholar] [CrossRef]
- Cranmer, H.; Ronquest, N.A.; Barnes, A.; Nadipelli, V.R.; Akehurst, R. Health-Related Quality of Life in Opioid use Disorder Measured by Utilities: A Systematic Literature Review. Value Health 2016, 19, A387. [Google Scholar] [CrossRef][Green Version]
- Langabeer, J.R.; Champagne-Langabeer, T.; Yatsco, A.J.; O’Neal, M.M.; Cardenas-Turanzas, M.; Prater, S.; Luber, S.; Stotts, A.; Fadial, T.; Khraish, G.; et al. Feasibility and outcomes from an integrated bridge treatment program for opioid use disorder. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12417. [Google Scholar] [CrossRef]
- Langabeer, J.; Champagne-Langabeer, T.; Luber, S.D.; Prater, S.J.; Stotts, A.; Kirages, K.; Yatsco, A.; Chambers, K.A. Outreach to people who survive opioid overdose: Linkage and retention in treatment. J. Subst. Abus. Treat. 2020, 111, 11–15. [Google Scholar] [CrossRef]
- United States Census, Population Demographics. Annual Resident Population Estimates for Metropolitan and Micropolitan Statistical Areas and Their Geographic Components. 2021. Available online: https://www.census.gov/programs-surveys/popest/technical-documentation/research/evaluation-estimates/2020-evaluation-estimates/2010s-totals-metro-and-micro-statistical-areas.html (accessed on 16 November 2021).
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Benjamini, Y.; Cohen, R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics 2017, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.W.; Larson, M.J. Quality of Life Assessments by Adult Substance Abusers Receiving Publicly Funded Treatment in Massachusetts. Am. J. Drug Alcohol Abus. 2003, 29, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Picci, R.L.; Oliva, F.; Zuffranieri, M.; Vizzuso, P.; Ostacoli, L.; Sodano, A.J.; Furlan, P.M. Quality of life, alcohol detoxification and relapse: Is quality of life a predictor of relapse or only a secondary outcome measure? Qual. Life Res. 2014, 23, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Laudet, A.B.; Becker, J.B.; White, W.L. Don’t Wanna Go Through That Madness No More: Quality of Life Satisfaction as Predictor of Sustained Remission from Illicit Drug Misuse. Subst. Use Misuse 2009, 44, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.; Ryan, D.A.; Jeng, P.J.; McCollister, K.E.; Leff, J.A.; Lee, J.D.; Nunes, E.V.; Novo, P.; Rotrosen, J.; Schackman, B.R.; et al. Health-related quality of life and opioid use disorder pharmacotherapy: A secondary analysis of a clinical trial. Drug Alcohol Depend. 2020, 215, 108221. [Google Scholar] [CrossRef] [PubMed]
- Laudet, A.B. The case for considering quality of life in addiction research and clinical practice. Addict. Sci. Clin. Pract. 2011, 6, 44–55. [Google Scholar] [PubMed]
- Moudatsou, M.; Stavropoulou, A.; Philalithis, A.; Koukouli, S. The Role of Empathy in Health and Social Care Professionals. Healthcare 2020, 8, 26. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Common Comorbidities with Substance Use Disorders: Part 1: The Connection between Substance Use and Mental Health. Available online: https://www.drugabuse.gov/publications/research-reports/common-comorbidities-substance-use-disorders/part-1-connection-between-substance-use-disorders-mental-illness (accessed on 16 October 2021).
- Serrano-Aguilar, P.; Ramallo-Fariña, Y.; Trujillo-Martín, M.D.M.; Muñoz-Navarro, S.R.; Perestelo-Perez, L.; Cuevas-Castresana, C.D.L. The relationship among Mental Health Status (GHQ-12), Health Related Quality of Life (EQ-5D) and Health-State Utilities in a general population. Epidemiol. Psichiatr. Sci. 2009, 18, 229–239. [Google Scholar] [CrossRef]
- Degnan, A.; Berry, K.; Humphrey, C.; Bucci, S. The relationship between stigma and subjective quality of life in psychosis: A systematic review and meta-analysis. Clin. Psychol. Rev. 2021, 85, 102003. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, R.J.; Gourishankar, A.A.; Chambers, A.K.; Giri, A.S.; Madu, A.R.; Champagne-Langabeer, A.T. Disparities Between US Opioid Overdose Deaths and Treatment Capacity: A Geospatial and Descriptive Analysis. J. Addict. Med. 2019, 13, 476–482. [Google Scholar] [CrossRef]
- Berchick, E.; Barnett, J.; Upton, R. Health Insurance Coverage in the United States: 2018; P60-267(RV); United States Census Bureau: Washington, DC, USA, 2019.
- Velez, F.F.; Colman, S.; Kauffman, L.; Ruetsch, C.; Anastassopoulos, K.; Maricich, Y.A. Comparison of Healthcare Resource Utilization Between Patients Who Engaged or Did Not Engage with a Prescription Digital Therapeutic for Opioid Use Disorder. Clinicoecon. Outcomes Res. 2021, 13, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Maricich, Y.A.; Bickel, W.K.; Marsch, L.A.; Gatchalian, K.; Botbyl, J.; Luderer, H.F. Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder. Curr. Med. Res. Opin. 2021, 37, 167–173. [Google Scholar] [CrossRef]
- Velez, F.F.; Colman, S.; Kauffman, L.; Ruetsch, C.; Anastassopoulos, K. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 69–76. [Google Scholar] [CrossRef]
- Rowan, K.; McAlpine, D.D.; Blewett, L.A. Access And Cost Barriers to Mental Health Care, By Insurance Status, 1999–2010. Health Aff. 2013, 32, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Zibell, J.; Howard, J.; Duhart Clarke, S.; Ferrell, A.; Karon, S. Non-Fatal Opioid Overdose and Associated Health Outcomes: Final Summary Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2019.
- Darke, S.; Torok, M.; McKetin, R.; Kaye, S.; Ross, J. Patterns of psychological distress related to regular methamphetamine and opioid use. Addict. Res. Theory 2011, 19, 121–127. [Google Scholar] [CrossRef]
- Bakos-Block, C.; Langabeer, J.R.; Yatsco, A.; Cardenas-Turanzas, M.; Champagne-Langabeer, T. Prevalence of Mental Health Disorders among Individuals Enrolled in an Emergency Response Program for Treatment of Opioid Use Disorder. Subst. Abus. 2020, 14, 1178221820981998. [Google Scholar] [CrossRef] [PubMed]
- Weisner, C.; Thomas Ray, G.; Mertens, J.R.; Satre, D.D.; Moore, C. Short-term alcohol and drug treatment outcomes predict long-term outcome. Drug Alcohol Depend. 2003, 71, 281–294. [Google Scholar] [CrossRef]
| Characteristic | N |
|---|---|
| Total | 494 (100) |
| Age, mean (sd) | 36.1 (9.9) |
| Gender † | |
| Male | 279 (56) |
| Female | 210 (43) |
| Race * | |
| White | 428 (87) |
| Black or African American | 60 (12) |
| Asian | 5 (1) |
| Native/Hawaiian or Other Pacific Islander | 4 (0.81) |
| Other/Did not provide | 7 (1.4) |
| Insurance | |
| Commercial | 68 (13.8) |
| Medicare | 14 (2.8) |
| Medicaid | 41 (8.3) |
| Uninsured/Unknown | 371 (75.1) |
| Housing status | |
| Own/Rent | 191 (38.7) |
| Live with family or friend | 207 (41.9) |
| Homeless | 49 (9.9) |
| Other | 47 (9.5) |
| A veteran | 14 (2.8) |
| Description | BH-Adjusted p-Value | Mean QoL Change among Answering No | Mean QoL Change among Answering Yes |
|---|---|---|---|
| Have you relapsed since joining the program? | e−5 | 22.0 | 7.5 |
| Are you currently attending LCDC or professional counseling? | 0.001 | 5.5 | 19.0 |
| Substance use history (nicotine/tobacco) | 0.007 | 10.9 | 21.6 |
| Substance use history (marijuana) | 0.007 | 10.3 | 21.2 |
| Substance use history (benzodiazepines) | 0.007 | 11.4 | 21.8 |
| Substance use history (alcohol) | 0.007 | 10.3 | 21.1 |
| Have you had any experiences you would consider to be traumatic? | 0.007 | 6.2 | 25.7 |
| Arrested for? (shoplifting or vandalism) | 0.01 | 14.4 | 28.2 |
| Status of legal problems (past) | 0.02 | 13.2 | 23.6 |
| Substance use history (non-Rx opiates) | 0.03 | 11.5 | 20.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottlieb, A.; Bakos-Block, C.; Langabeer, J.R.; Champagne-Langabeer, T. Sociodemographic and Clinical Characteristics Associated with Improvements in Quality of Life for Participants with Opioid Use Disorder. Healthcare 2022, 10, 167. https://doi.org/10.3390/healthcare10010167
Gottlieb A, Bakos-Block C, Langabeer JR, Champagne-Langabeer T. Sociodemographic and Clinical Characteristics Associated with Improvements in Quality of Life for Participants with Opioid Use Disorder. Healthcare. 2022; 10(1):167. https://doi.org/10.3390/healthcare10010167
Chicago/Turabian StyleGottlieb, Assaf, Christine Bakos-Block, James R. Langabeer, and Tiffany Champagne-Langabeer. 2022. "Sociodemographic and Clinical Characteristics Associated with Improvements in Quality of Life for Participants with Opioid Use Disorder" Healthcare 10, no. 1: 167. https://doi.org/10.3390/healthcare10010167
APA StyleGottlieb, A., Bakos-Block, C., Langabeer, J. R., & Champagne-Langabeer, T. (2022). Sociodemographic and Clinical Characteristics Associated with Improvements in Quality of Life for Participants with Opioid Use Disorder. Healthcare, 10(1), 167. https://doi.org/10.3390/healthcare10010167






