Modeling Uncertainty in Fracture Age Estimation from Pediatric Wrist Radiographs
Abstract
:1. Introduction
- This is the first-ever attempt to tackle the issue of estimating pediatric fracture age using AI. Hence, we propose a standard, as well as guidelines for other researchers to follow;
- By utilizing the Monte-Carlo dropout method, which treats NN as a Gaussian sampling process, we are able to estimate the uncertainty of the proposed system decisions, unveiling prediction certainty to increase trustworthiness;
- We propose a novel system based on a CNN combining different features obtained from the medical reports/cases to estimate fracture age. The system is general-purpose, and we believe that its design can also be utilized in other research fields as well.
Related Work
2. Materials and Methods
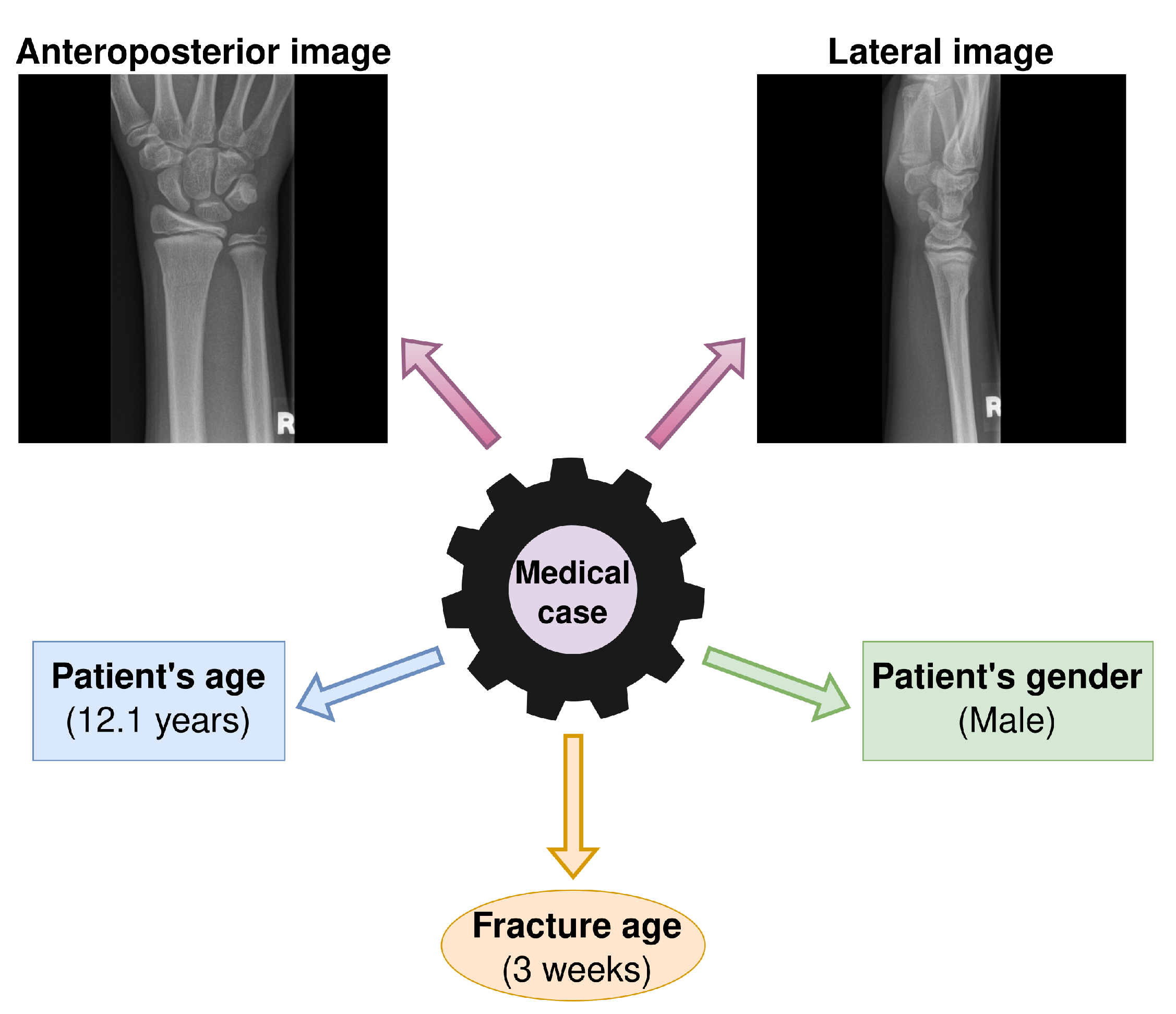
2.1. Dataset and Task Formulation
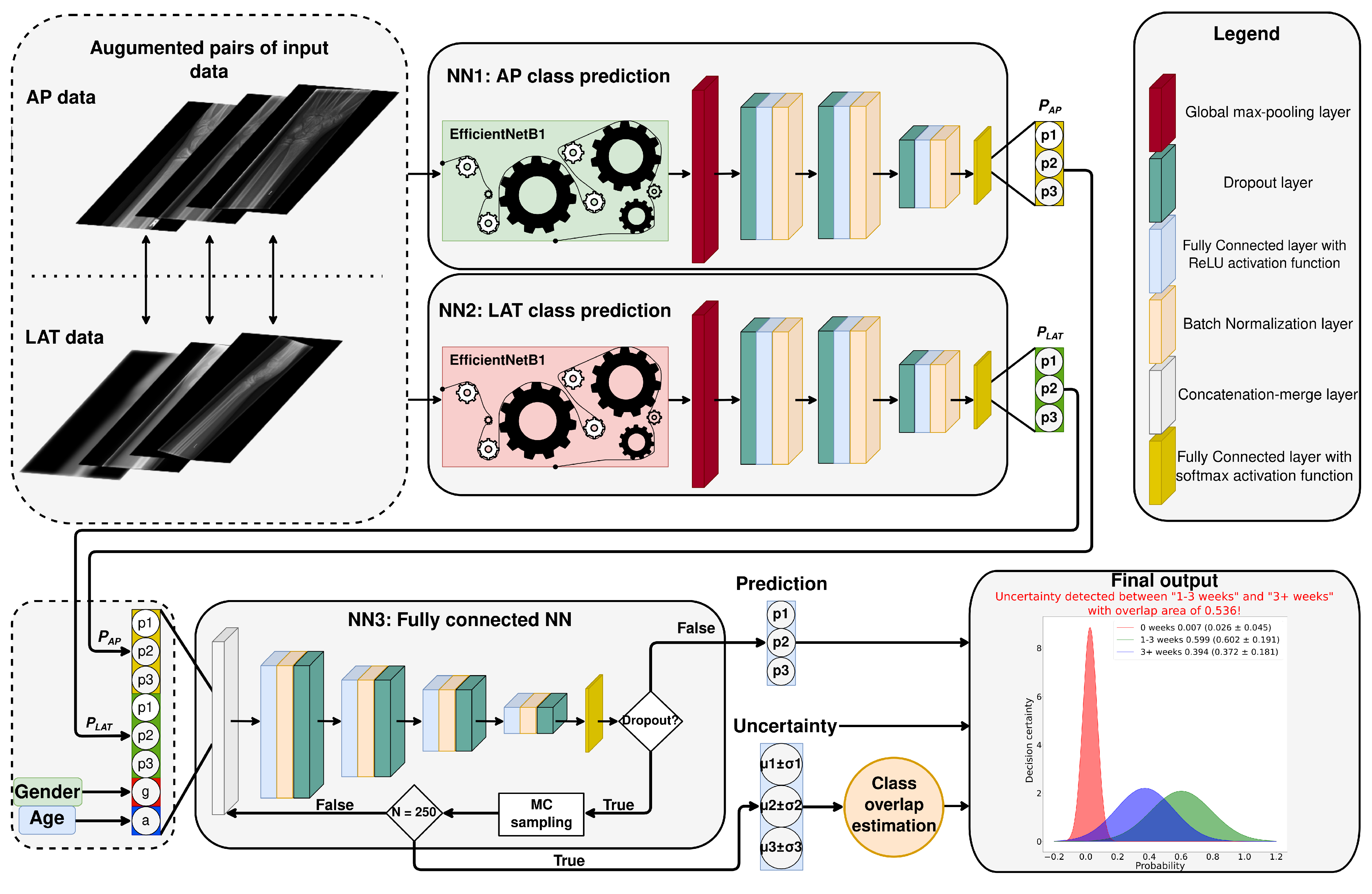
2.2. Proposed System Overview
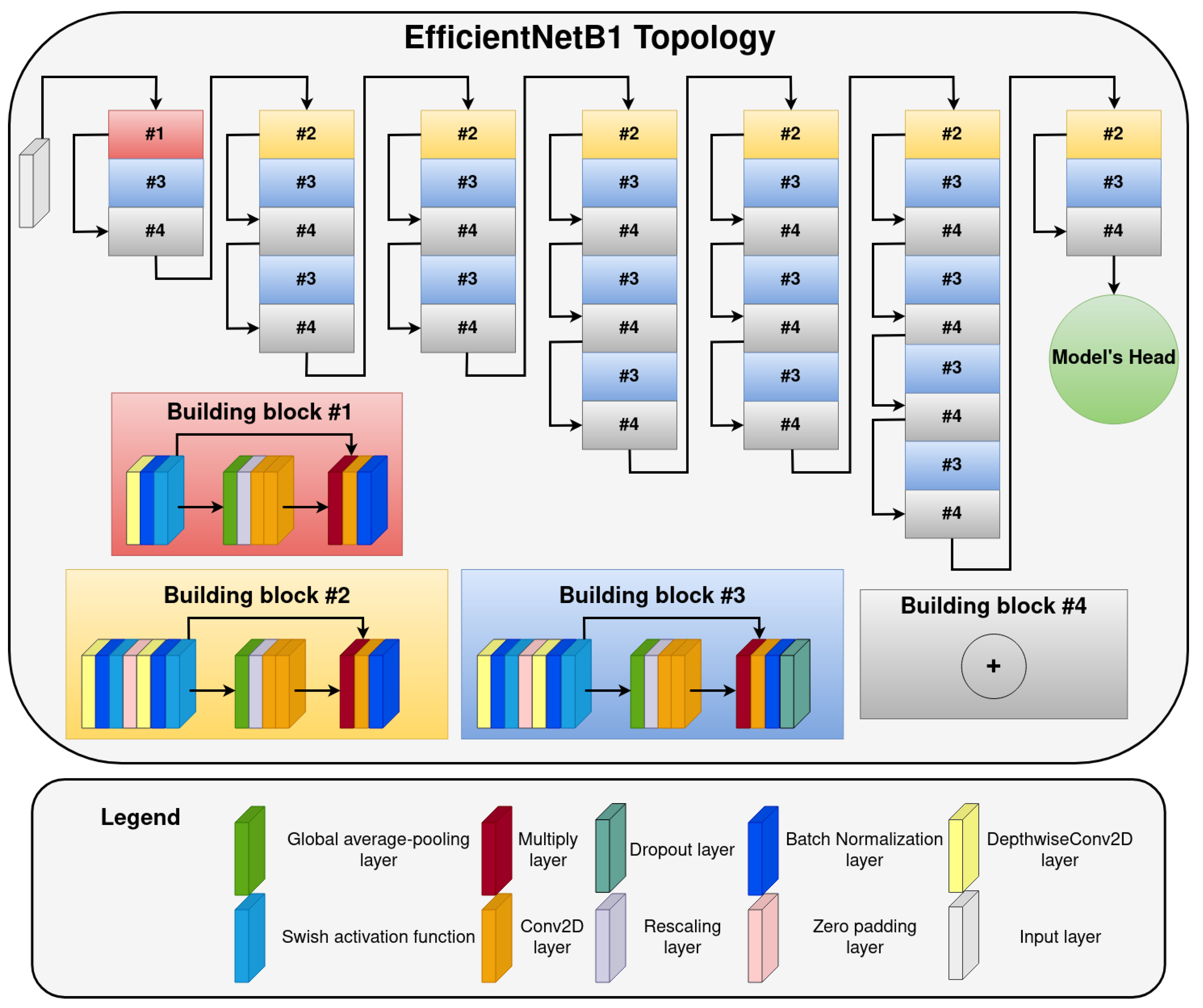
- The first part of the proposed system has two components—NNs (NN1 and NN2) predicting fracture age based on lateral () or anteroposterior () input images. The NNs are using EfficientNetB1 (current state-of-the-art NN architecture for classification) as a feature extractor with a custom-developed fully-connected NN head on top of it. The EfficientNetB1 topology (depicted on Figure 4) was the same as that proposed in the original paper (image input size is pixels) [29], while the fully-connected NN head architecture can be seen in Figure 3. The number of neurons in each dense layer of the NNs head is 1024, 1024, 512, and 3, respectively. The dropout rate in the dropout layers was set to . The output of each of the two NNs predicts fracture age based on the image it receives as the input. In order to determine if the EfficientNetB1 is the best performing network for our system, we have compared it with other popular deep learning architectures: VGG19 [30], ResNet101 [31], InceptionV3 [32], and Xception [33]. As it can be seen in Appendix B, the EfficientNetB1 was the best performing model of all tested models, and that is why we chose it for our system;
- The second part of the proposed system is a fully connected NN (NN3) that takes as the input a vector (size ) created from the outputs of NN1 and NN2, and patient’s gender (g) and age (a). The topology of the NN3 can also be seen in Figure 3. It is constructed from four fully connected layers with 512, 256, 128, and 64 layers, respectively. Dropout rate in the dropout layers was the same as for the first part of the proposed system (). The output of NN3 is a final prediction of the fracture age from the assembly of features. Also, we have employed a decision uncertainty estimation algorithm, which we discuss in the following subsection.
2.2.1. Uncertainty Estimation Algorithm
2.2.2. The Proposed System Training
3. Results and Discussion
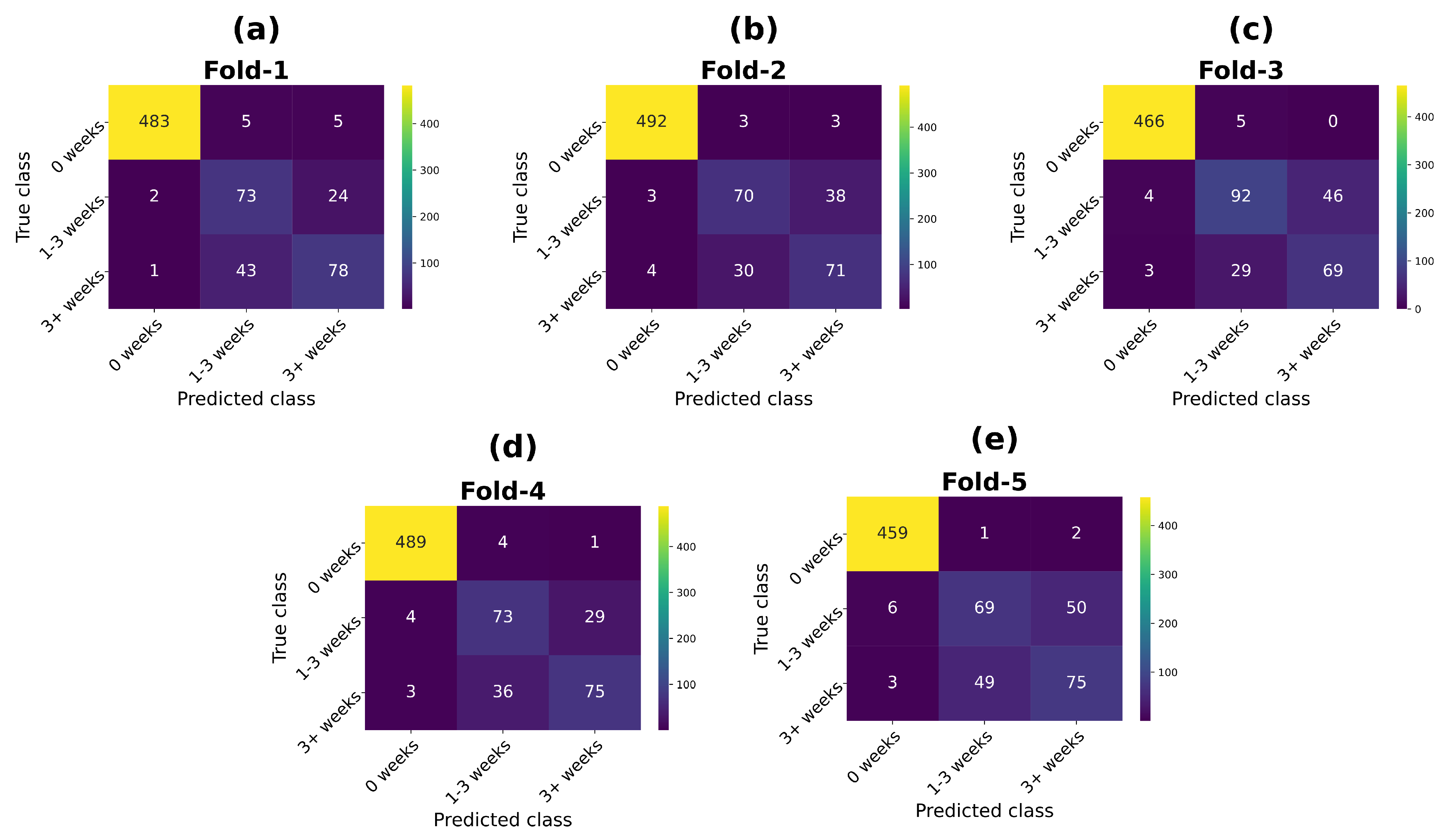
3.1. Proposed System Evaluation Results
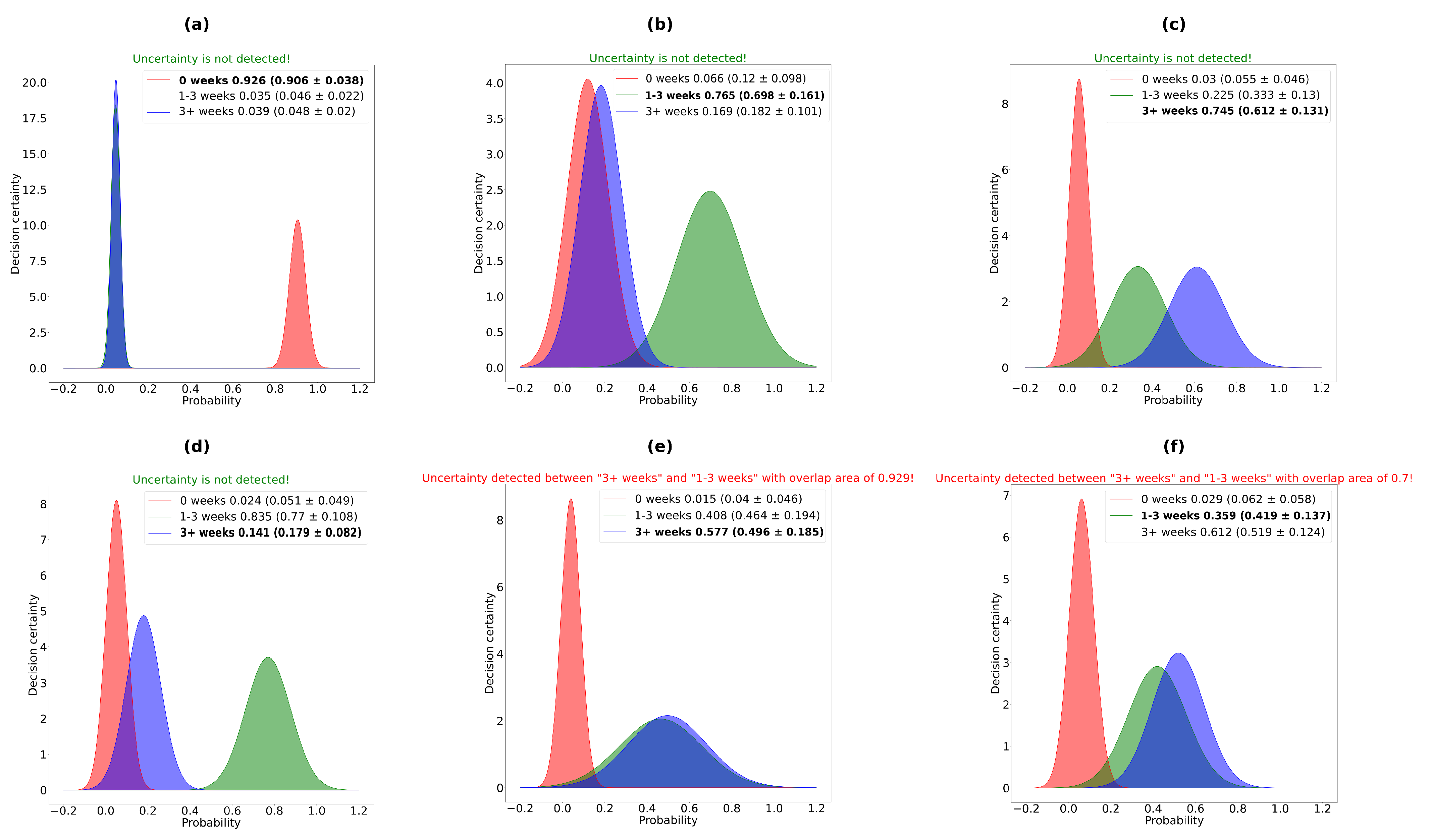
3.2. Uncertainty Estimation Results
- Both NNs and the proposed system have higher uncertainty on the erroneous predictions than on the correct ones. This is the desired behavior because we want the system to be confident (have the lowest uncertainty) when it is correct and be very uncertain when it makes an erroneous prediction. Also, it is necessary to observe that the biggest difference between overall uncertainty (mean ± stdev) is for the proposed system (), which indicates that the system, from this point of view, behaves better than its components;
- For the correct prediction, the best result was obtained by the NN1 (AP input) with an average uncertainty of . The worse result was obtained by the proposed system (). In other words, the proposed system was more uncertain in its correct decision than any of its components, although it obtained the highest F1-score and accuracy (Table 3). This phenomenon, we believe, is due to more inputs/information that the proposed system takes into account;
- For the erroneous predictions, the proposed system obtained the best results (highest average uncertainty (). Analogous to the case of correct predictions, we want the system to be as unsure as possible when making erroneous predictions.
3.3. Results Summary
- The fusion of the input data in the system results in increased model accuracy, compared independently to any of its components;
- The amount of uncertainty of the proposed system is greater than the amount of uncertainty of its individual components;
- The uncertainty in the incorrect predictions of the proposed system is higher than the uncertainty in its correct prediction, which is the desirable system behavior;
- Uncertainty estimation can help with the output interpretability and can enhance the system usability, especially in cases where the data is poor and very skewed (as was the case with fracture age estimation). Including the uncertainty in the proposed system’s decision increased its average accuracy to ∼90.6%, which can serve as a benchmark point for similar research.
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. McNemar’s Test Evaluation of the Proposed System and Its Components
| Fold-1 | NN1 | NN2 | NN3 | NN3 + UNC |
|---|---|---|---|---|
| NN1 | / | 0.045 | 0.008 | 0.000 |
| NN2 | 0.045 | / | 0.000 | 0.000 |
| NN3 | 0.008 | 0.000 | / | 0.000 |
| NN3 + UNC | 0.000 | 0.000 | 0.000 | / |
| Fold-2 | NN1 | NN2 | NN3 | NN3 + UNC |
|---|---|---|---|---|
| NN1 | / | 0.002 | 0.359 | 0.000 |
| NN2 | 0.002 | / | 0.000 | 0.000 |
| NN3 | 0.359 | 0.000 | / | 0.000 |
| NN3 + UNC | 0.000 | 0.000 | 0.000 | / |
| Fold-3 | NN1 | NN2 | NN3 | NN3 + UNC |
|---|---|---|---|---|
| NN1 | / | 0.861 | 0.026 | 0.000 |
| NN2 | 0.861 | / | 0.020 | 0.000 |
| NN3 | 0.026 | 0.020 | / | 0.000 |
| NN3 + UNC | 0.000 | 0.000 | 0.000 | / |
| Fold-4 | NN1 | NN2 | NN3 | NN3 + UNC |
|---|---|---|---|---|
| NN1 | / | 0.040 | 0.155 | 0.000 |
| NN2 | 0.040 | / | 0.000 | 0.000 |
| NN3 | 0.155 | 0.000 | / | 0.000 |
| NN3 + UNC | 0.000 | 0.000 | 0.000 | / |
| Fold-5 | NN1 | NN2 | NN3 | NN3 + UNC |
|---|---|---|---|---|
| NN1 | / | 0.702 | 0.141 | 0.000 |
| NN2 | 0.702 | / | 0.002 | 0.000 |
| NN3 | 0.141 | 0.002 | / | 0.000 |
| NN3 + UNC | 0.000 | 0.000 | 0.000 | / |
Appendix B. Models Comparison on AP and LAT Data Images
| Model | Learning Rate | Batch Size |
|---|---|---|
| VGG19 | 32 | |
| ResNet101 | 32 | |
| InceptionV3 | 32 | |
| Xception | 32 | |
| EfficientNetB1 | 32 |
| Model | Precision | Recall | F1-score | Accuracy |
|---|---|---|---|---|
| VGG19 | 0.860 ± 0.015 | 0.859 ± 0.014 | 0.856 ± 0.017 | 0.859 ± 0.014 |
| ResNet101 | 0.859 ± 0.014 | 0.845 ± 0.020 | 0.845 ± 0.018 | 0.845 ± 0.020 |
| InceptionV3 | 0.849 ± 0.017 | 0.851 ± 0.014 | 0.849 ± 0.016 | 0.851 ± 0.014 |
| Xception | 0.830 ± 0.011 | 0.824 ± 0.013 | 0.829 ± 0.010 | 0.824 ± 0.013 |
| EfficientNetB1 | 0.864 ± 0.023 | 0.857 ± 0.020 | 0.858 ± 0.023 | 0.857 ± 0.020 |
| Model | Precision | Recall | F1-score | Accuracy |
|---|---|---|---|---|
| VGG19 | 0.833 ± 0.032 | 0.823 ± 0.045 | 0.825 ± 0.039 | 0.823 ± 0.045 |
| ResNet101 | 0.835 ± 0.019 | 0.824 ± 0.023 | 0.821 ± 0.025 | 0.824 ± 0.023 |
| InceptionV3 | 0.823 ± 0.013 | 0.814 ± 0.018 | 0.816 ± 0.014 | 0.814 ± 0.018 |
| Xception | 0.812 ± 0.018 | 0.794 ± 0.033 | 0.798 ± 0.032 | 0.794 ± 0.033 |
| EfficientNetB1 | 0.841 ± 0.016 | 0.834 ± 0.013 | 0.835 ± 0.012 | 0.834 ± 0.013 |
References
- Messer, D.L.; Adler, B.H.; Brink, F.W.; Xiang, H.; Agnew, A.M. Radiographic timelines for pediatric healing fractures: A systematic review. Pediatr. Radiol. 2020, 50, 1041–1048. [Google Scholar] [CrossRef]
- Cappella, A.; de Boer, H.H.; Cammilli, P.; De Angelis, D.; Messina, C.; Sconfienza, L.M.; Sardanelli, F.; Sforza, C.; Cattaneo, C. Histologic and radiological analysis on bone fractures: Estimation of posttraumatic survival time in skeletal trauma. Forensic Sci. Int. 2019, 302, 109909. [Google Scholar] [CrossRef]
- Prosser, I.; Maguire, S.; Harrison, S.K.; Mann, M.; Sibert, J.R.; Kemp, A.M. How old is this fracture? Radiologic dating of fractures in children: A systematic review. Am. J. Roentgenol. 2005, 184, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, K.E.; Broderick, N.; Somers, J.; Hawkes, R. Dating fractures in infants. Clin. Radiol. 2011, 66, 1049–1054. [Google Scholar] [CrossRef]
- Prosser, I.; Lawson, Z.; Evans, A.; Harrison, S.; Morris, S.; Maguire, S.; Kemp, A.M. A timetable for the radiologic features of fracture healing in young children. Am. J. Roentgenol. 2012, 198, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine learning for medical imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Cho, Y.J.; Lee, S.; Lee, J.; Lee, S.; Choi, Y.H.; Cheon, J.E.; Ha, J.Y. Using a dual-input convolutional neural network for automated detection of pediatric supracondylar fracture on conventional radiography. Investig. Radiol. 2020, 55, 101–110. [Google Scholar] [CrossRef]
- Gan, K.; Xu, D.; Lin, Y.; Shen, Y.; Zhang, T.; Hu, K.; Zhou, K.; Bi, M.; Pan, L.; Wu, W.; et al. Artificial intelligence detection of distal radius fractures: A comparison between the convolutional neural network and professional assessments. Acta Orthop. 2019, 90, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczak, J.; Fahlberg, N.; Maki, A.; Razavian, A.S.; Jilert, A.; Stark, A.; Sköldenberg, O.; Gordon, M. Artificial intelligence for analyzing orthopedic trauma radiographs: Deep learning algorithms—Are they on par with humans for diagnosing fractures? Acta Orthop. 2017, 88, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Guo, H.; Ma, K.; Wu, J.; Weng, Z.; Zheng, Y. X2CT-GAN: Reconstructing CT from biplanar X-rays with generative adversarial networks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Long Beach, CA, USA, 15–20 June 2019; pp. 10619–10628. [Google Scholar]
- Hržić, F.; Žužić, I.; Tschauner, S.; Štajduhar, I. Cast suppression in radiographs by generative adversarial networks. J. Am. Med Informatics Assoc. 2021, 28, 2687–2694. [Google Scholar] [CrossRef]
- Haglin, J.M.; Jimenez, G.; Eltorai, A.E. Artificial neural networks in medicine. Health Technol. 2019, 9, 150–154. [Google Scholar] [CrossRef]
- Sorantin, E.; Grasser, M.G.; Hemmelmayr, A.; Tschauner, S.; Hrzic, F.; Weiss, V.; Lacekova, J.; Holzinger, A. The augmented radiologist: Artificial intelligence in the practice of radiology. Pediatr. Radiol. 2021, 688. [Google Scholar] [CrossRef]
- Holzinger, A.; Biemann, C.; Pattichis, C.S.; Kell, D.B. What do we need to build explainable AI systems for the medical domain? arXiv 2017, arXiv:1712.09923. [Google Scholar]
- Hedström, E.M.; Svensson, O.; Bergström, U.; Michno, P. Epidemiology of fractures in children and adolescents: Increased incidence over the past decade: A population-based study from northern Sweden. Acta Orthop. 2010, 81, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Pietka, E.; Gertych, A.; Pospiech, S.; Cao, F.; Huang, H.; Gilsanz, V. Computer-assisted bone age assessment: Image preprocessing and epiphyseal/metaphyseal ROI extraction. IEEE Trans. Med. Imaging 2001, 20, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Ebner, T.; Stern, D.; Donner, R.; Bischof, H.; Urschler, M. Towards automatic bone age estimation from MRI: Localization of 3D anatomical landmarks. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Boston, MA, USA, 14–18 September 2014; pp. 421–428. [Google Scholar]
- Thodberg, H.H.; Kreiborg, S.; Juul, A.; Pedersen, K.D. The BoneXpert method for automated determination of skeletal maturity. IEEE Trans. Med Imaging 2008, 28, 52–66. [Google Scholar] [CrossRef]
- Halabi, S.S.; Prevedello, L.M.; Kalpathy-Cramer, J.; Mamonov, A.B.; Bilbily, A.; Cicero, M.; Pan, I.; Pereira, L.A.; Sousa, R.T.; Abdala, N.; et al. The RSNA pediatric bone age machine learning challenge. Radiology 2019, 290, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; George, J.; Junge, A.; Hodler, J. Age determination by magnetic resonance imaging of the wrist in adolescent male football players. Br. J. Sport. Med. 2007, 41, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Shi, L.; Zhan, M.J.; Fan, F.; Peng, Z.; Zhang, K.; Deng, Z.H. Age estimation based on magnetic resonance imaging of the ankle joint in a modern Chinese Han population. Int. J. Leg. Med. 2020, 134, 1843–1852. [Google Scholar] [CrossRef]
- Lu, T.; Qiu, L.R.; Ren, B.; Shi, L.; Fan, F.; Deng, Z.H. Forensic age estimation based on magnetic resonance imaging of the proximal humeral epiphysis in Chinese living individuals. Int. J. Leg. Med. 2021, 135, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Palazzo, S.; Giordano, D.; Aldinucci, M.; Leonardi, R. Deep learning for automated skeletal bone age assessment in X-ray images. Med. Image Anal. 2017, 36, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.M.; Hassan, N.; Ahmed, A. Bone age assessment methods: A critical review. Pak. J. Med Sci. 2014, 30, 211. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B. Learning from imbalanced data: Open challenges and future directions. Prog. Artif. Intell. 2016, 5, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Lee, J.H.; Kim, Y.J.; Kim, K.G. Bone age estimation using deep learning and hand X-ray images. Biomed. Eng. Lett. 2020, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Widek, T.; Genet, P.; Ehammer, T.; Schwark, T.; Urschler, M.; Scheurer, E. Bone age estimation with the Greulich-Pyle atlas using 3T MR images of hand and wrist. Forensic Sci. Int. 2021, 319, 110654. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In International Conference on Machine Learning; PMLR: Cambridge, MA, USA, 2019; pp. 6105–6114. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- Chollet, F. Xception: Deep learning with depthwise separable convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 1251–1258. [Google Scholar]
- Gawlikowski, J.; Tassi, C.R.N.; Ali, M.; Lee, J.; Humt, M.; Feng, J.; Kruspe, A.; Triebel, R.; Jung, P.; Roscher, R.; et al. A survey of uncertainty in deep neural networks. arXiv 2021, arXiv:2107.03342. [Google Scholar]
- Loquercio, A.; Segu, M.; Scaramuzza, D. A general framework for uncertainty estimation in deep learning. IEEE Robot. Autom. Lett. 2020, 5, 3153–3160. [Google Scholar] [CrossRef] [Green Version]
- Jospin, L.V.; Buntine, W.; Boussaid, F.; Laga, H.; Bennamoun, M. Hands-on Bayesian Neural Networks–A Tutorial for Deep Learning Users. arXiv 2020, arXiv:2007.06823. [Google Scholar]
- Gal, Y.; Ghahramani, Z. Dropout as a bayesian approximation: Representing model uncertainty in deep learning. In International Conference on Machine Learning; PMLR: Cambridge, MA, USA, 2016; pp. 1050–1059. [Google Scholar]
- Dudley, R.M. Sample functions of the Gaussian process. Sel. Work. Dudley 2010, 187–224. [Google Scholar] [CrossRef]
- Linacre, J.M. Overlapping normal distributions. Rasch Meas. Trans. 1996, 10, 487–488. [Google Scholar]
- Fushiki, T. Estimation of prediction error by using K-fold cross-validation. Stat. Comput. 2011, 21, 137–146. [Google Scholar] [CrossRef]
- Aurelio, Y.S.; de Almeida, G.M.; de Castro, C.L.; Braga, A.P. Learning from imbalanced data sets with weighted cross-entropy function. Neural Process. Lett. 2019, 50, 1937–1949. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative adversarial networks: An overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Adedokun, O.A.; Burgess, W.D. Analysis of paired dichotomous data: A gentle introduction to the McNemar test in SPSS. J. Multidiscip. Eval. 2012, 8, 125–131. [Google Scholar]
- Huh, M.; Agrawal, P.; Efros, A.A. What makes ImageNet good for transfer learning? arXiv 2016, arXiv:1608.08614. [Google Scholar]

| Fold | Precision | Recall | F1-score | Accuracy |
|---|---|---|---|---|
| Fold-1 | 0.869 | 0.859 | 0.861 | 0.859 |
| Fold-2 | 0.880 | 0.880 | 0.879 | 0.880 |
| Fold-3 | 0.866 | 0.849 | 0.855 | 0.849 |
| Fold-4 | 0.882 | 0.875 | 0.878 | 0.875 |
| Fold-5 | 0.820 | 0.825 | 0.815 | 0.825 |
| Mean ± stdev | 0.864 ± 0.023 | 0.857 ± 0.020 | 0.858 ± 0.023 | 0.857 ± 0.020 |
| Fold | Precision | Recall | F1-score | Accuracy |
|---|---|---|---|---|
| Fold-1 | 0.846 | 0.825 | 0.833 | 0.825 |
| Fold-2 | 0.844 | 0.833 | 0.836 | 0.833 |
| Fold-3 | 0.852 | 0.853 | 0.851 | 0.853 |
| Fold-4 | 0.854 | 0.843 | 0.840 | 0.843 |
| Fold-5 | 0.811 | 0.818 | 0.813 | 0.818 |
| Mean ± stdev | 0.841 ± 0.016 | 0.834 ± 0.013 | 0.835 ± 0.012 | 0.834 ± 0.013 |
| Fold | Precision | Recall | F1-score | Accuracy |
|---|---|---|---|---|
| Fold-1 | 0.894 | 0.888 | 0.890 | 0.888 |
| Fold-2 | 0.887 | 0.887 | 0.886 | 0.887 |
| Fold-3 | 0.880 | 0.878 | 0.878 | 0.878 |
| Fold-4 | 0.892 | 0.892 | 0.892 | 0.892 |
| Fold-5 | 0.841 | 0.845 | 0.843 | 0.845 |
| Mean ± stdev | 0.879± 0.019 | 0.878± 0.017 | 0.878± 0.018 | 0.878 ± 0.017 |
| Fold | AP result | Lateral results | System results |
|---|---|---|---|
| (Mean ± Stdev) | (Mean ± Stdev) | (Mean ± Stdev) | |
| Fold-1 | 0.041 ± 0.041 | 0.051 ± 0.052 | 0.055 ± 0.057 |
| Fold-2 | 0.020 ± 0.035 | 0.043 ± 0.047 | 0.066 ± 0.044 |
| Fold-3 | 0.053 ± 0.036 | 0.035 ± 0.041 | 0.066 ± 0.053 |
| Fold-4 | 0.036 ± 0.036 | 0.046 ± 0.067 | 0.046 ± 0.059 |
| Fold-5 | 0.021 ± 0.030 | 0.037 ± 0.050 | 0.086 ± 0.035 |
| Mean ± stdev | 0.034 ± 0.014 | 0.043 ± 0.007 | 0.063 ± 0.015 |
| Fold | AP result | Lateral results | System results |
|---|---|---|---|
| (Mean ± Stdev) | (Mean ± Stdev) | (Mean ± Stdev) | |
| Fold-1 | 0.072 ± 0.022 | 0.108 ± 0.047 | 0.138 ± 0.039 |
| Fold-2 | 0.081 ± 0.044 | 0.102 ± 0.028 | 0.136 ± 0.031 |
| Fold-3 | 0.080 ± 0.026 | 0.085 ± 0.038 | 0.138 ± 0.035 |
| Fold-4 | 0.073 ± 0.021 | 0.132 ± 0.052 | 0.141 ± 0.042 |
| Fold-5 | 0.073 ± 0.023 | 0.111 ± 0.038 | 0.145 ± 0.029 |
| Mean ± stdev | 0.076 ± 0.004 | 0.108 ± 0.017 | 0.140 ± 0.003 |
| Fold | Precision | Recall | F1-Score | Accuracy |
|---|---|---|---|---|
| Fold-1 | 0.913 | 0.907 | 0.909 | 0.907 |
| Fold-2 | 0.907 | 0.908 | 0.907 | 0.908 |
| Fold-3 | 0.909 | 0.908 | 0.908 | 0.908 |
| Fold-4 | 0.919 | 0.919 | 0.918 | 0.919 |
| Fold-5 | 0.886 | 0.888 | 0.887 | 0.888 |
| Mean ± stdev | 0.907 ± 0.012 | 0.906 ± 0.011 | 0.906 ± 0.012 | 0.906 ± 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hržić, F.; Janisch, M.; Štajduhar, I.; Lerga, J.; Sorantin, E.; Tschauner, S. Modeling Uncertainty in Fracture Age Estimation from Pediatric Wrist Radiographs. Mathematics 2021, 9, 3227. https://doi.org/10.3390/math9243227
Hržić F, Janisch M, Štajduhar I, Lerga J, Sorantin E, Tschauner S. Modeling Uncertainty in Fracture Age Estimation from Pediatric Wrist Radiographs. Mathematics. 2021; 9(24):3227. https://doi.org/10.3390/math9243227
Chicago/Turabian StyleHržić, Franko, Michael Janisch, Ivan Štajduhar, Jonatan Lerga, Erich Sorantin, and Sebastian Tschauner. 2021. "Modeling Uncertainty in Fracture Age Estimation from Pediatric Wrist Radiographs" Mathematics 9, no. 24: 3227. https://doi.org/10.3390/math9243227
APA StyleHržić, F., Janisch, M., Štajduhar, I., Lerga, J., Sorantin, E., & Tschauner, S. (2021). Modeling Uncertainty in Fracture Age Estimation from Pediatric Wrist Radiographs. Mathematics, 9(24), 3227. https://doi.org/10.3390/math9243227







