Toward Non-Invasive Estimation of Blood Glucose Concentration: A Comparative Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
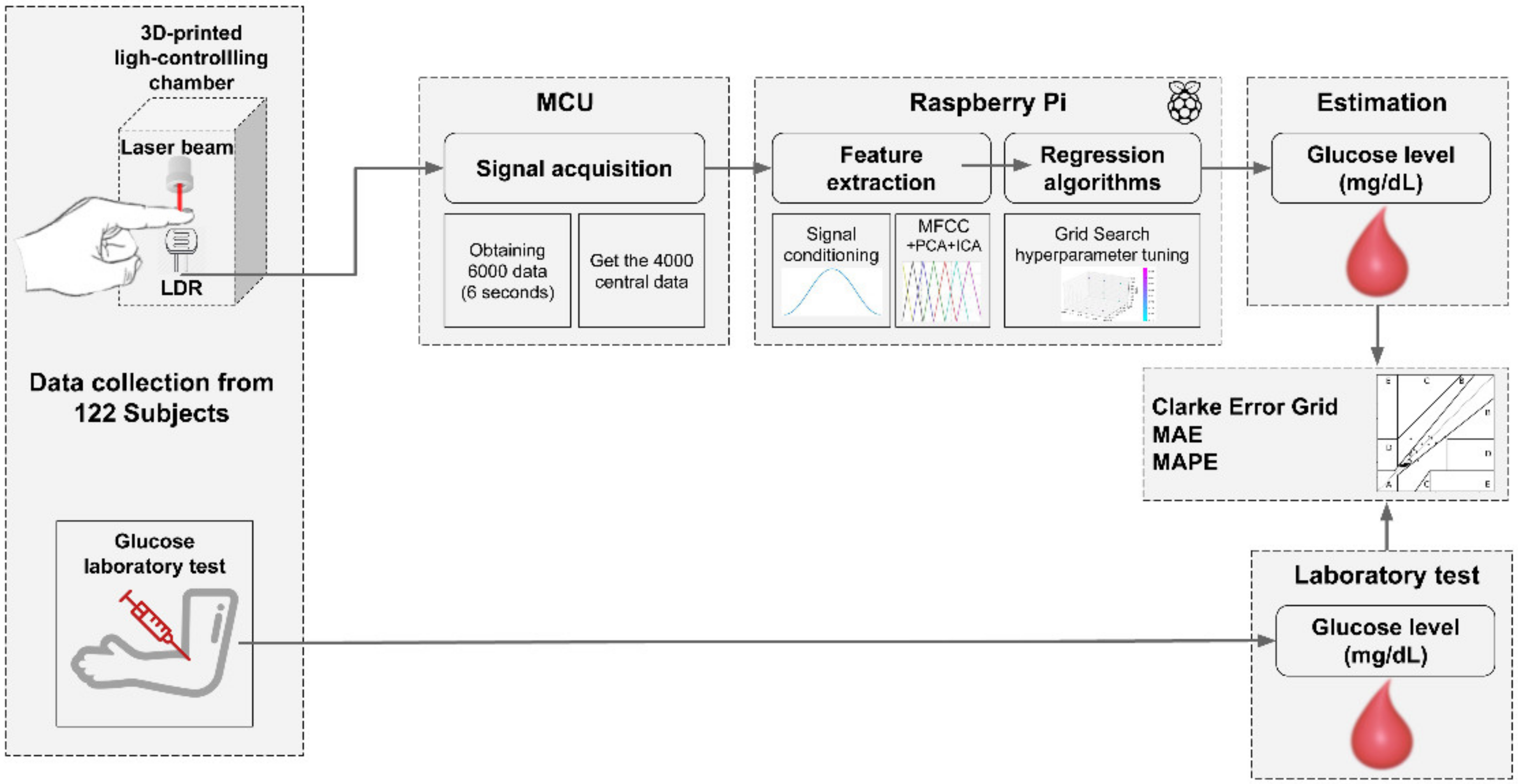
2.2. Data Collection
2.3. Feature Extraction
2.4. Regression Models
2.4.1. AdaBoost
2.4.2. Bayesian Ridge
2.4.3. Decision Tree
2.4.4. Elastic Net
2.4.5. k-Nearest Neighbors Regressor (k-NNr)
2.4.6. Linear Regression
2.4.7. Multilayer Perceptron Regressor (MLPr)
2.4.8. Random Forest
2.4.9. Ridge Regression
2.4.10. Support Vector Regression (SVR)
2.5. Algorithm Performance
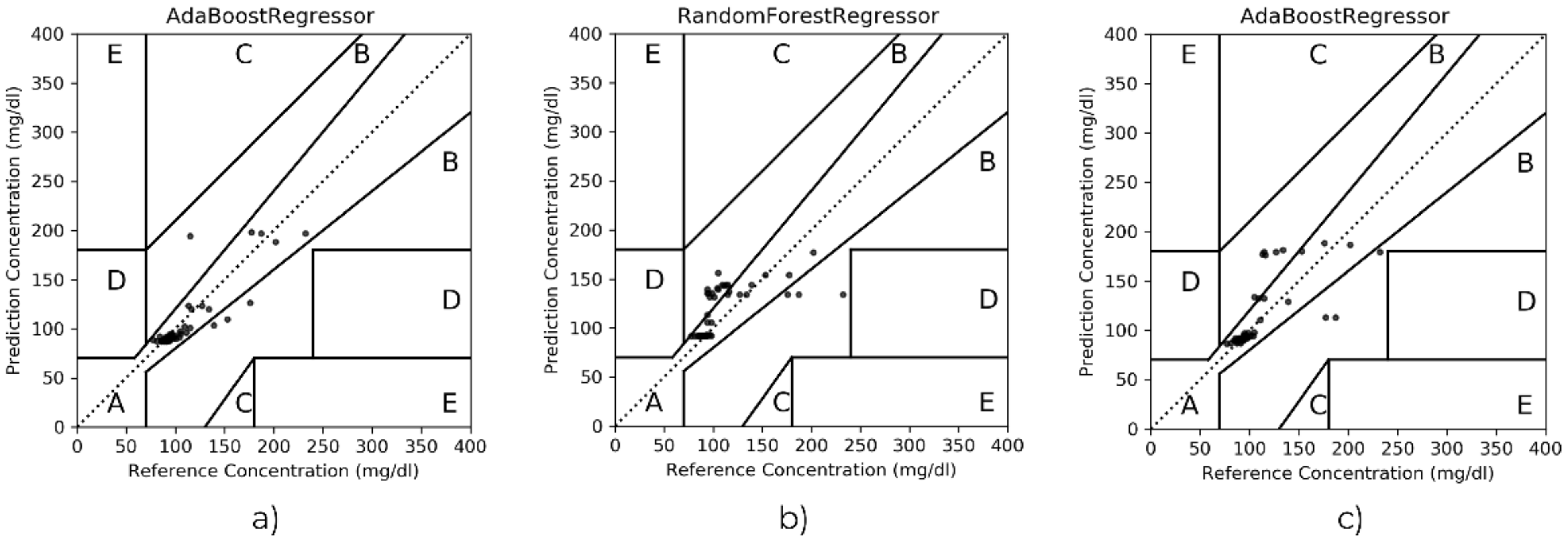
- Evaluation metrics. The mean absolute error (MAE), mean absolute percentage error (MAPE) and the Clarke error grid were considered. MAE, computed as in Equation (1), constitutes the average difference between the estimated values vs. the lab test; in addition, MAPE represents the same difference but expressed in percentage (see Equation (2)). The Clarke error grid is a graph divided into five zones, for which the success of the results depends on where the reference glucose values versus the algorithm outcomes are plotted. That is, zones A and B stand for accurate or acceptable estimation, zone C is commonly associated with unnecessary treatments, but zones D and E are representative of potentially dangerous mistreatment caused by confusing hyperglycemia and hypoglycemia.
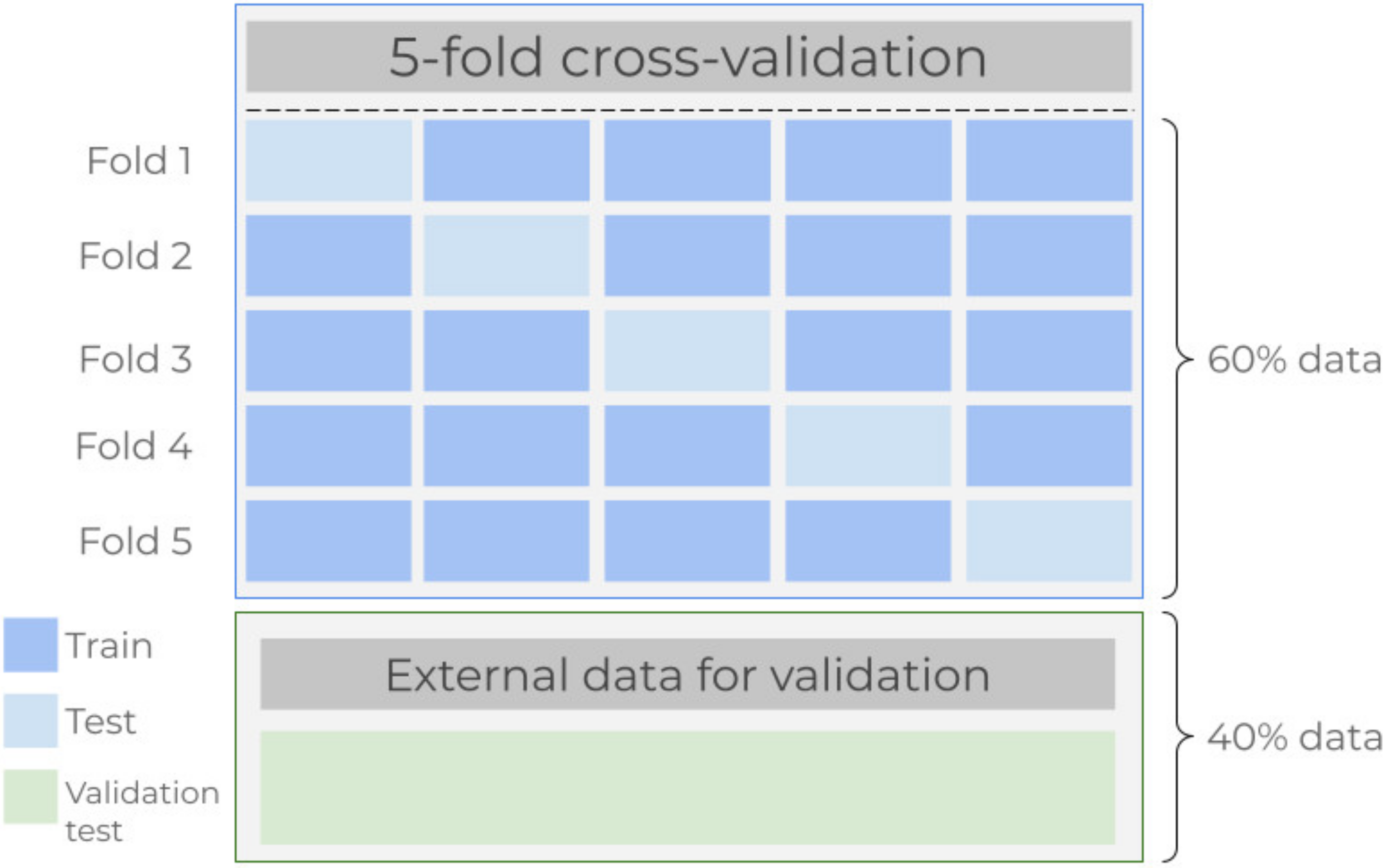
- Cross-validation. Data from all participants were divided into two mutually exclusive partitions. Here, the k-fold cross-validation model was applied onto 60% of the data, and the remaining 40% were intended for testing the model once the algorithms were fitted. In this study, the five-fold cross-validation scheme was preferred. First, data are divided into five subsets, commonly referred to as folds, and repeatedly perform a procedure for which at the i-th step the corresponding i-th fold is taken as a test subset while the remaining four folds are used to train the regressor [42]. This paragraph is graphically outlined in Figure 2.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.; Weil, C.; Unwin, N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res. Clin. Pract. 2011, 94, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Atlas, D. Five questions on the IDF Diabetes Atlas. Diabetes Res. Clin. Pract. 2013, 102, 147–148. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes Factsheets; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ramasahayam, S.; Koppuravuri, S.H.; Arora, L.; Chowdhury, S.R. Noninvasive blood glucose sensing using near infra-red spectroscopy and artificial neural networks based on inverse delayed function model of neuron. J. Med. Syst. 2015, 39, 166. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Kong, J.; Yun, K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: A meta-analysis of observational studies. J. Diabetes Res. 2020, 2020, 2315607. [Google Scholar] [CrossRef] [Green Version]
- Lawrenson, J.G.; Bourmpaki, E.; Bunce, C.; Stratton, I.M.; Gardner, P.; Anderson, J.; EROS Study Group. Trends in diabetic retinopathy screening attendance and associations with vision impairment attributable to diabetes in a large nationwide cohort. Diabet. Med. 2021, 38, e14425. [Google Scholar] [CrossRef]
- Goyal, A.; Gupta, Y.; Singla, R.; Kalra, S.; Tandon, N. American Diabetes Association Standards of Medical Care—2020 for Gestational Diabetes Mellitus: A Critical Appraisal. Diabetes Ther. 2020, 11, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Vibha, S.P.; Kulkarni, M.M.; Ballala, A.B.K.; Kamath, A.; Maiya, G.A. Community based study to assess the prevalence of diabetic foot syndrome and associated risk factors among people with diabetes mellitus. BMC Endocr. Disord. 2018, 18, 43. [Google Scholar] [CrossRef]
- De Freitas, G.R.; Ferraz, G.A.M.; Gehlen, M.; Skare, T.L. Dry eyes in patients with diabetes mellitus. Prim. Care Diabetes 2021, 15, 184–186. [Google Scholar] [CrossRef]
- John, T.M.; Jacob, C.N.; Kontoyiannis, D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. J. Fungi 2021, 7, 298. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Gribovschi, M. The methodology of glucose monitoring in type 2 diabetes mellitus. Clujul Med. 2013, 86, 93. [Google Scholar] [PubMed]
- Clark, V.L.; Kruse, J.A. Clinical methods: The history, physical, and laboratory examinations. Jama 1990, 264, 2808–2809. [Google Scholar]
- Lin, T.; Gal, A.; Mayzel, Y.; Horman, K.; Bahartan, K. Non-invasive glucose monitoring: A review of challenges and recent advances. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Talib, A.J.; Alkahtani, M.; Jiang, L.; Alghannam, F.; Brick, R.; Gomes, C.L.; Scully, M.O.; Sokolov, A.V.; Hemmer, P.R. Lanthanide ions doped in vanadium oxide for sensitive optical glucose detection. Opt. Mater. Express 2018, 8, 3277–3287. [Google Scholar] [CrossRef] [Green Version]
- Blum, Z.; Pankratov, D.; Shleev, S. Powering electronic contact lenses: Current achievements, challenges, and perspectives. Expert Rev. Ophthalmol. 2014, 9, 269–273. [Google Scholar] [CrossRef] [Green Version]
- So, C.-F.; Choi, K.-S.; Wong, T.K.S.; Chung, J.W.Y. Recent advances in noninvasive glucose monitoring. Med. Devices 2012, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In vivo blood glucose quantification using Raman spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef] [Green Version]
- Tura, A.; Sbrignadello, S.; Cianciavicchia, D.; Pacini, G.; Ravazzani, P. A low frequency electromagnetic sensor for indirect measurement of glucose concentration: In vitro experiments in different conductive solutions. Sensors 2010, 10, 5346–5358. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Lo, Y.-L.; Liao, C.-C.; Phan, Q.-H. Noninvasive measurement of glucose concentration on human fingertip by optical coherence tomography. J. Biomed. Opt. 2018, 23, 47001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, A.; Smith, B.; Ainslie, P.; Smith, K. Near-Infrared Spectroscopy, Applied Aspects of Ultrasonography in Humans; IntechOpen: London, UK, 2012; ISBN 978-953-51-0522-0. [Google Scholar] [CrossRef] [Green Version]
- Yatim, N.N.M.; Zain, Z.M.; Jaafar, M.Z.; Yusof, Z.M.; Laili, A.R.; Laili, M.H.; Hisham, M.H. Noninvasive glucose level determination using diffuse reflectance near infrared spectroscopy and chemometrics analysis based on in vitro sample and human skin. In Proceedings of the IEEE Conference on Systems, Process and Control (ICSPC), Kuala Lumpur, Malaysia, 12–14 December 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 30–35. [Google Scholar]
- Malin, S.F.; Ruchti, T.L.; Blank, T.B.; Thennadil, S.N.; Monfre, S.L. Noninvasive prediction of glucose by near-infrared diffuse reflectance spectroscopy. Clin. Chem. 1999, 45, 1651–1658. [Google Scholar] [CrossRef] [Green Version]
- Jeon, K.J.; Hwang, I.D.; Hahn, S.J.; Yoon, G. Comparison between transmittance and reflectance measurements in glucose determination using near infrared spectroscopy. J. Biomed. Opt. 2006, 11, 14022. [Google Scholar] [CrossRef] [Green Version]
- Amerov, A.K.; Chen, J.; Small, G.W.; Arnold, M.A. Scattering and absorption effects in the determination of glucose in whole blood by near-infrared spectroscopy. Anal. Chem. 2005, 77, 4587–4594. [Google Scholar] [CrossRef]
- Elhaj, F.A.; Salim, N.; Harris, A.R.; Swee, T.T.; Ahmed, T. Arrhythmia recognition and classification using combined linear and nonlinear features of ECG signals. Comput. Methods Programs Biomed. 2016, 127, 52–63. [Google Scholar] [CrossRef]
- Martis, R.J.; Acharya, U.R.; Lim, C.M.; Suri, J.S. Characterization of ECG beats from cardiac arrhythmia using discrete cosine transform in PCA framework. Knowledge-Based Syst. 2013, 45, 76–82. [Google Scholar] [CrossRef]
- Lakshmi, M.R.; Prasad, T.V.; Prakash, D.V.C. Survey on EEG signal processing methods. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2014, 4, 84–91. [Google Scholar]
- Subasi, A.; Gursoy, M.I. EEG signal classification using PCA, ICA, LDA and support vector machines. Expert Syst. Appl. 2010, 37, 8659–8666. [Google Scholar] [CrossRef]
- Salamea, C.; Narvaez, E.; Montalvo, M. Database Proposal for Correlation of Glucose and Photoplethysmography Signals. In Proceedings of the International Conference on Advances in Emerging Trends and Technologies, Quito, Ecuador, 27–29 October 2019; Springer: Berlin, Germany, 2019; pp. 44–53. [Google Scholar]
- Francisco-García, V.; Guzmán-Guzmán, I.P.; Salgado-Rivera, R.; Alonso-Silverio, G.A.; Alarcón-Paredes, A. Non-invasive Glucose Level Estimation: A Comparison of Regression Models Using the MFCC as Feature Extractor. In Proceedings of the Mexican Conference on Pattern Recognition, Querétaro, Mexico, 26–29 June 2019; Springer: Berlin, Germany, 2019; pp. 206–215. [Google Scholar]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer—Lambert law revisited. Phys. Med. Biol. 2006, 51, N91. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed. Signal Process. Control. 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Davis, S.; Mermelstein, P. Comparison of parametric representations for monosyllabic word recognition in continuously spoken sentences. IEEE Trans. Acoust. 1980, 28, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 2065. [Google Scholar] [CrossRef]
- Hyvärinen, A. Independent component analysis: Recent advances. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 1984. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, S.; Gawali, B.; Yannawar, P.; Mehrotra, S. Feature extraction using fusion MFCC for continuous marathi speech recognition. In Proceedings of the India Conference (INDICON), 2011 Annual IEEE, Hyderabad, India, 16–18 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–5. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin, Germany, 2013; Volume 6. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning; Springer: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31073-2. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Routledge: Boca Raton, FL, USA, 2017; ISBN 1315139472. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Mack, Y.-P. Local properties of k-NN regression estimates. SIAM J. Algebr. Discret. Methods 1981, 2, 311–323. [Google Scholar] [CrossRef]
- Murtagh, F. Multilayer perceptrons for classification and regression. Neurocomputing 1991, 2, 183–197. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Platt, J. Probabilistic outputs for SVMs and comparisons to regularized likelihood methods. Adv. Large Margin Classif. 1999, 10, 61–74. [Google Scholar]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Alarcon-Paredes, A.; Rebolledo-Nandi, Z.; Guzmán-Guzmán, I.P.; Yanez-Marquez, C.; Alonso, G.A. A non-invasive glucose level estimation in a multi-sensing health care monitoring system. Technol. Health Care 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Segman, Y. Device and Method for Noninvasive Glucose Assessment. J. Diabetes Sci. Technol. 2018, 12, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Dataset | Age | Gender (%) F–M | T2D 1 (%) Diagnosed | Glucose Concentration |
|---|---|---|---|---|
| All participants | 36.7 ± 14.2 (21,76) | 52.5–47.5 | 20.50 | 108.8 ± 38.1 (75,259) |
| Training subset | 36.4 ± 13.93 (21,76) | 58.3–41.7 | 19.44 | 107.1 ± 39.7 (75,259) |
| Validation subset | 37.1 ± 14.16 (21,70) | 44.0–56.0 | 22.00 | 111.3 ± 35.5 (77,232) |
| Regression Model | Selected Parameters |
|---|---|
| AdaBoost | learning_rate:1.0; loss:square; n_estimators:50 |
| Bayesian Ridge | alpha_1:1e-6; alpha_2:0.001; fit_intercept:True; lambda_1:0.001; lambda_2:0.001; n_iter:200; tol:0.01 |
| Decision Tree | criterion:mse; max_features:sqrt; min_samples_leaf:3; min_samples_split:2 |
| Elastic Net | alpha:1.0; l1_ratio:0.9; max_iter:100 |
| k-NNr 1 | leaf_size:2; n_neighbors:13 |
| Linear Regression | fit_intercept:False; normalize:True |
| MLPr 2 | activation:tanh; alpha:1.0; max_iter:500; solver:lbfgs |
| Random Forest | max_depth:2; n_estimators:30 |
| Ridge Regression | alpha:0.1; max_iter:50; solver:sag; tol:0.8 |
| SVR 3 | C:100.0; gamma:0.01; kernel:rbf |
| Regression Model | MAE | MAPE | Clark Error Grid Region (%) (A–B–C–D–E) |
|---|---|---|---|
| AdaBoost | 11.62 | 10.78 | 92–8–0–0–0 |
| Bayesian Ridge | 22.56 | 18.71 | 57–43–0–0–0 |
| Decision Tree | 23.65 | 21.70 | 53–47–0–0–0 |
| Elastic Net | 19.40 | 16.29 | 76–24–0–0–0 |
| k-NNr 1 | 21.97 | 19.88 | 61–39–0–0–0 |
| Linear Regression | 35.21 | 99.05 | 41–57–2–0–0 |
| MLPr 2 | 33.12 | 20.55 | 57–39–4–0–0 |
| Random Forest | 11.66 | 10.21 | 90–10–0–0–0 |
| Ridge Regression | 26.23 | 21.89 | 57–43–0–0–0 |
| SVR 3 | 17.87 | 17.20 | 73–27–0–0–0 |
| Regression Model | MAE | MAPE | Clark Error Grid Region (%) (A–B–C–D–E) |
|---|---|---|---|
| AdaBoost | 14.62 | 10.52 | 80–20–0–0–0 |
| Bayesian Ridge | 19.04 | 14.64 | 59–41–0–0–0 |
| Decision Tree | 24.24 | 21.99 | 61–37–2–0–0 |
| Elastic Net | 18.88 | 14.53 | 59–41–0–0–0 |
| k-NNr 1 | 14.95 | 10.49 | 76–24–0–0–0 |
| Linear Regression | 18.87 | 14.52 | 59–41–0–0–0 |
| MLPr 2 | 24.04 | 18.70 | 53–47–0–0–0 |
| Random Forest | 13.98 | 10.42 | 82–18–0–0–0 |
| Ridge Regression | 19.11 | 14.68 | 57–43–0–0–0 |
| SVR 3 | 19.76 | 21.24 | 80–20–0–0–0 |
| Regression Model | MAE | MAPE | Clark Error Grid Region (%) (A–B–C–D–E) |
|---|---|---|---|
| AdaBoost | 16.75 | 15.21 | 82–18–0–0–0 |
| Bayesian Ridge | 17.73 | 14.38 | 78–22–0–0–0 |
| Decision Tree | 25.80 | 26.13 | 69–29–2–0–0 |
| Elastic Net | 18.62 | 16.10 | 78–22–0–0–0 |
| k-NNr 1 | 16.73 | 12.87 | 69–31–0–0–0 |
| Linear Regression | 17.71 | 14.08 | 69–31–0–0–0 |
| MLPr 2 | 31.19 | 21.36 | 55–45–0–0–0 |
| Random Forest | 18.26 | 13.85 | 65–35–0–0–0 |
| Ridge Regression | 18.53 | 16.00 | 78–22–0–0–0 |
| SVR 3 | 19.06 | 20.19 | 82–18–0–0–0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Silverio, G.A.; Francisco-García, V.; Guzmán-Guzmán, I.P.; Ventura-Molina, E.; Alarcón-Paredes, A. Toward Non-Invasive Estimation of Blood Glucose Concentration: A Comparative Performance. Mathematics 2021, 9, 2529. https://doi.org/10.3390/math9202529
Alonso-Silverio GA, Francisco-García V, Guzmán-Guzmán IP, Ventura-Molina E, Alarcón-Paredes A. Toward Non-Invasive Estimation of Blood Glucose Concentration: A Comparative Performance. Mathematics. 2021; 9(20):2529. https://doi.org/10.3390/math9202529
Chicago/Turabian StyleAlonso-Silverio, Gustavo A., Víctor Francisco-García, Iris P. Guzmán-Guzmán, Elías Ventura-Molina, and Antonio Alarcón-Paredes. 2021. "Toward Non-Invasive Estimation of Blood Glucose Concentration: A Comparative Performance" Mathematics 9, no. 20: 2529. https://doi.org/10.3390/math9202529
APA StyleAlonso-Silverio, G. A., Francisco-García, V., Guzmán-Guzmán, I. P., Ventura-Molina, E., & Alarcón-Paredes, A. (2021). Toward Non-Invasive Estimation of Blood Glucose Concentration: A Comparative Performance. Mathematics, 9(20), 2529. https://doi.org/10.3390/math9202529






