Study of Transition Zones in the Carbon Monoxide Catalytic Oxidation on Platinum Using the Network Simulation Method
Abstract
1. Introduction
2. Governing Equations
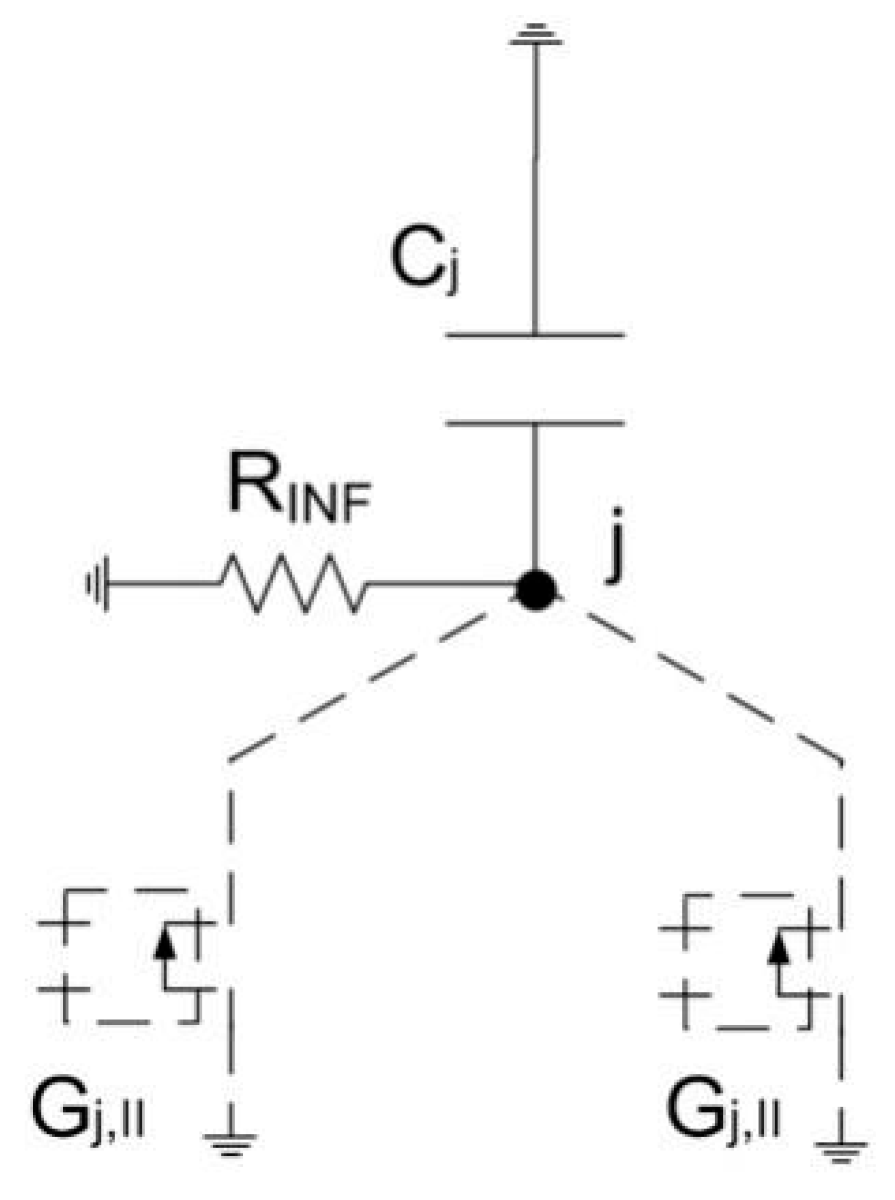
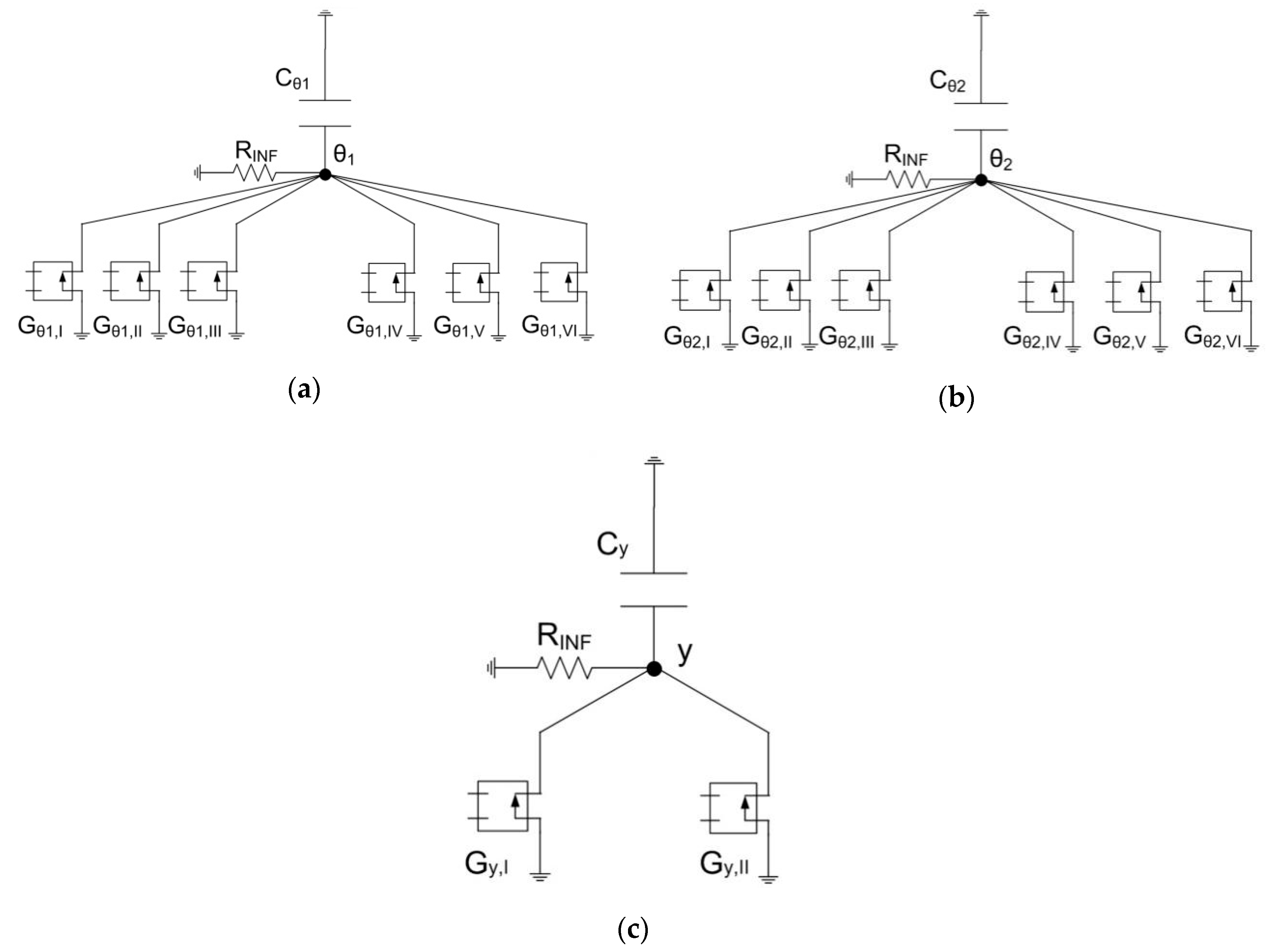
3. The Network Model
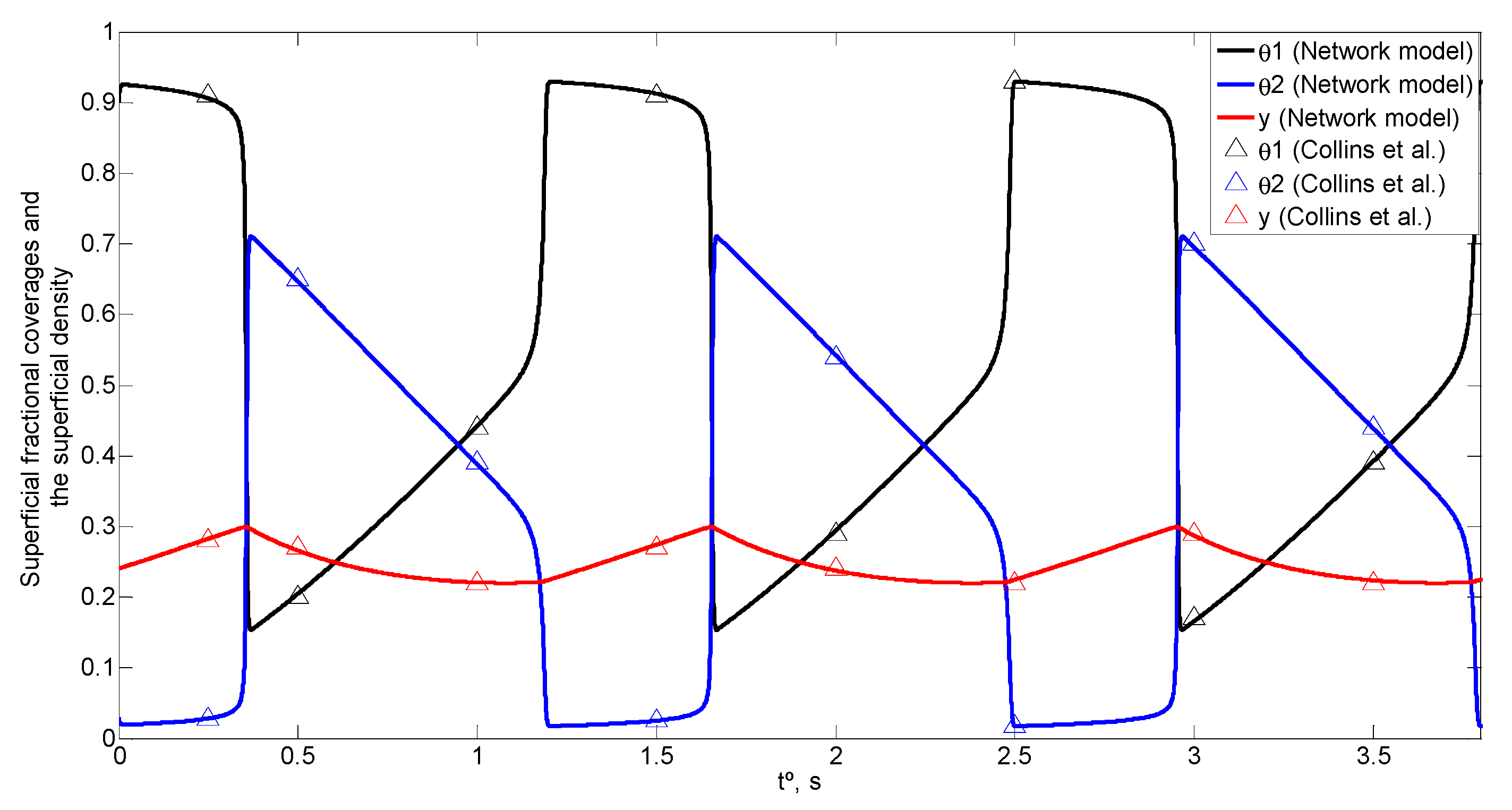
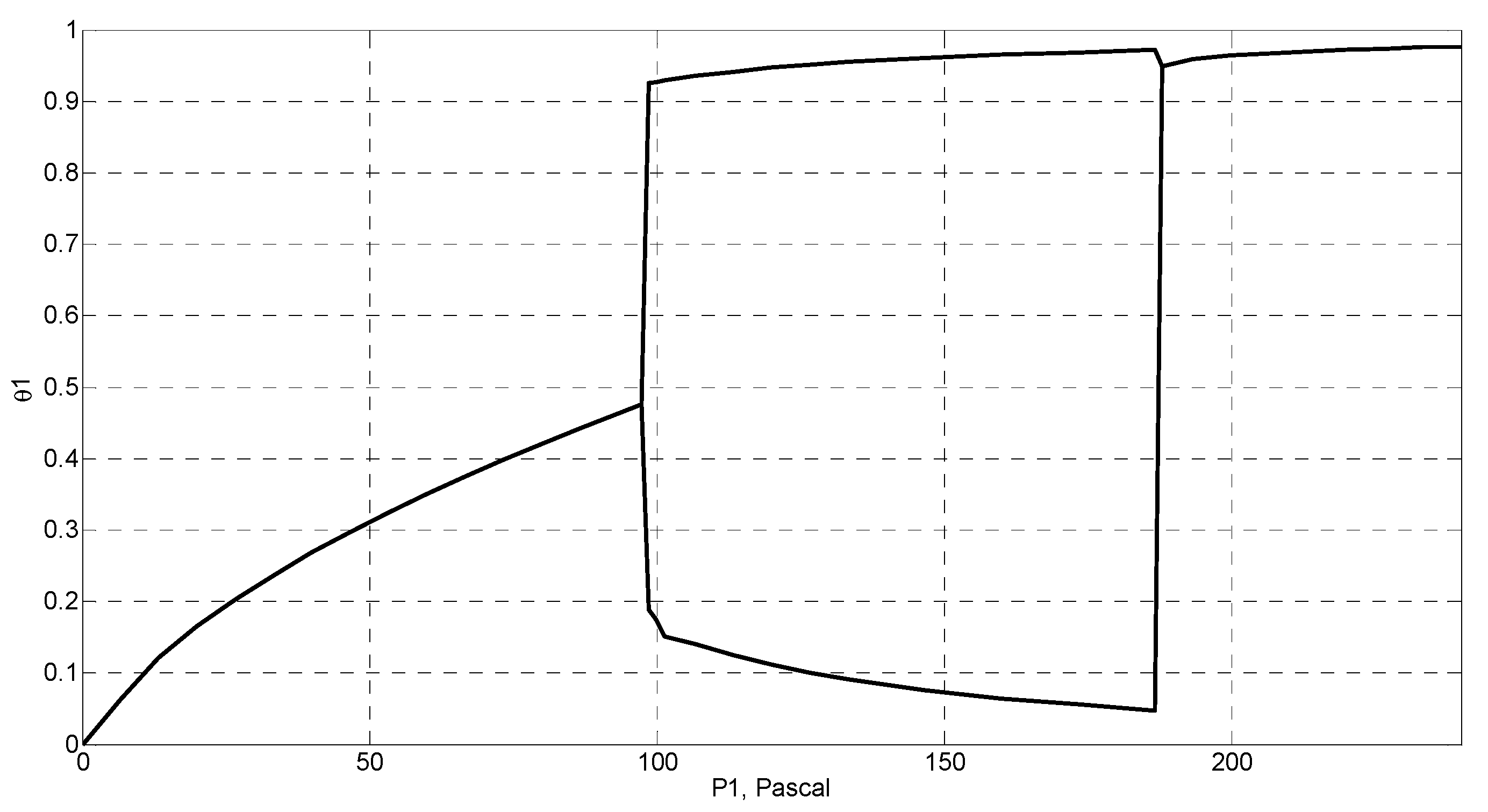
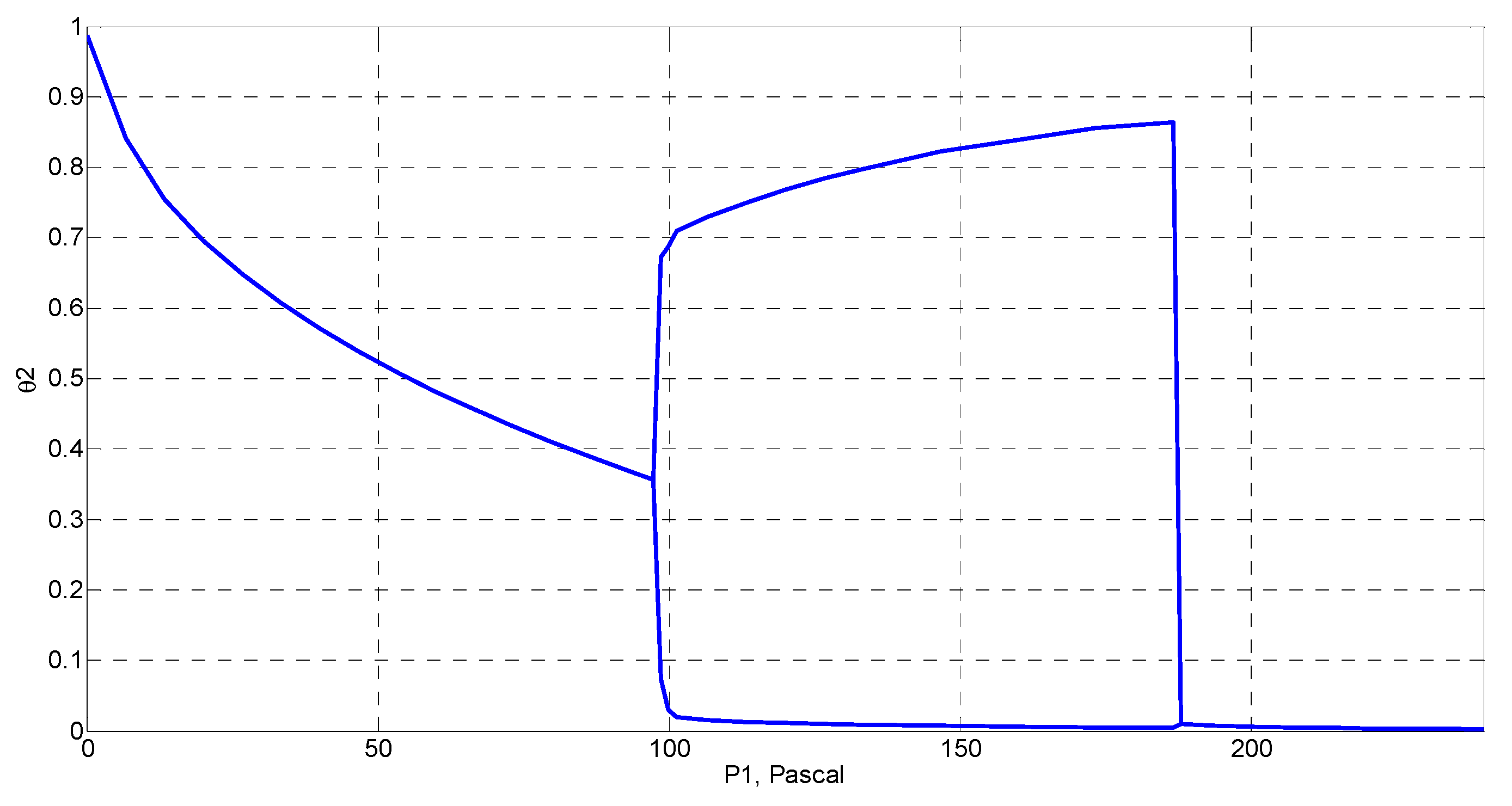
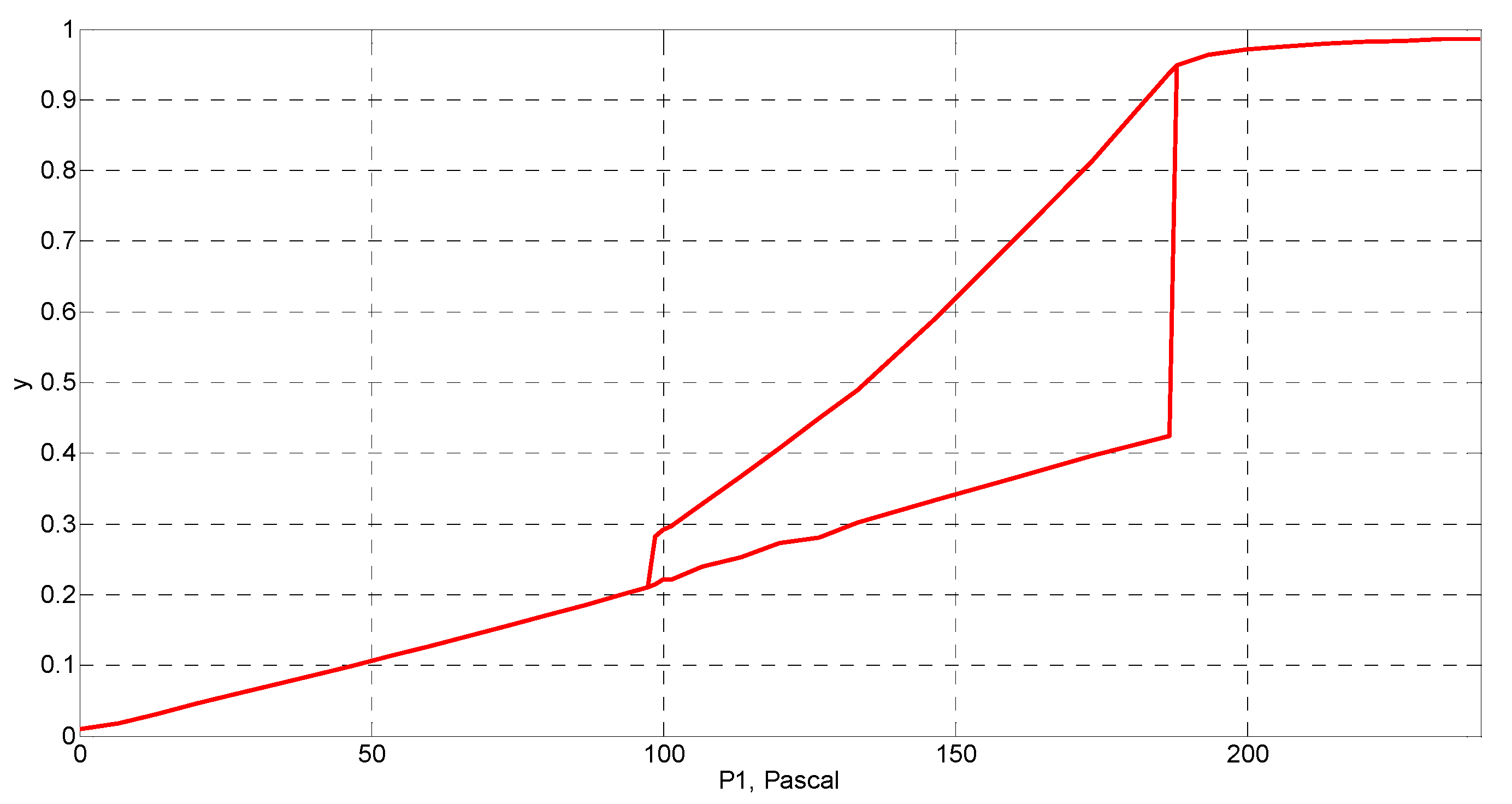
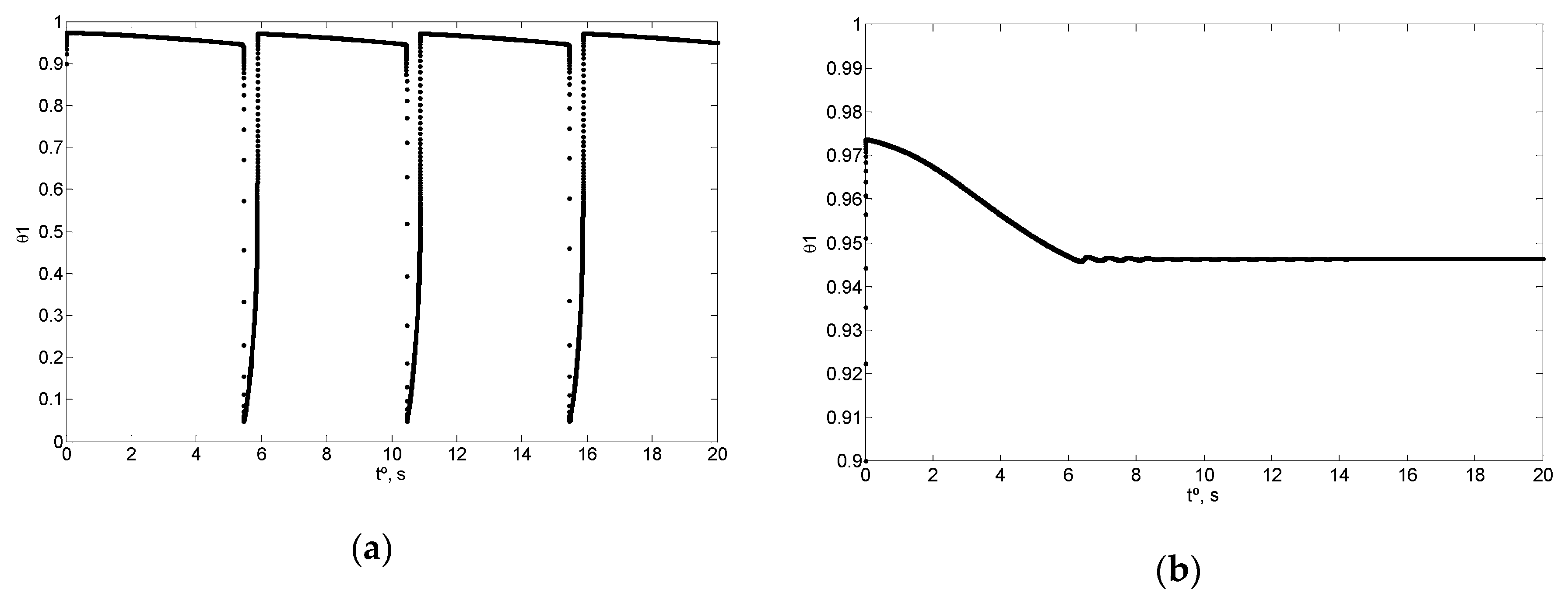
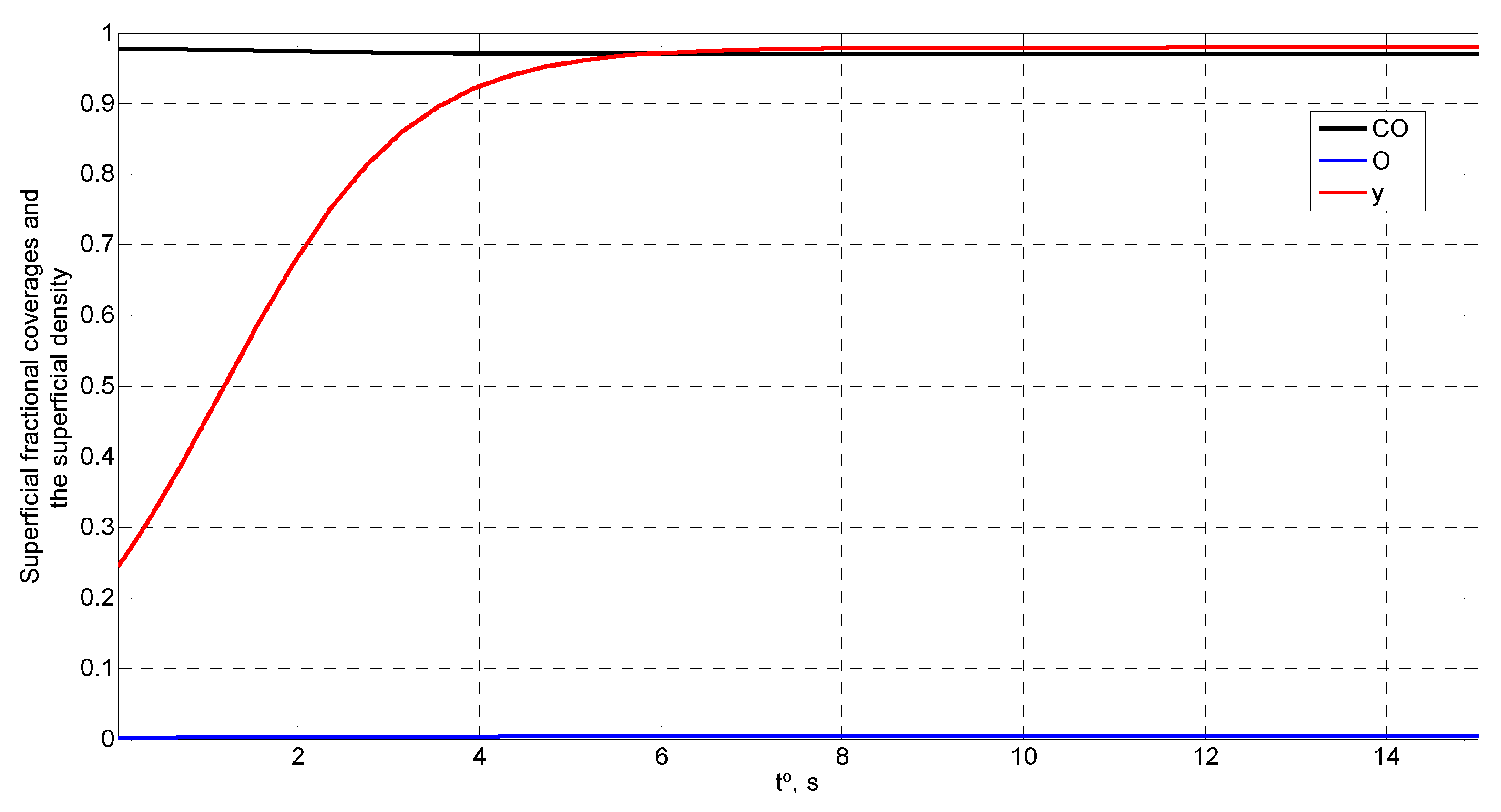
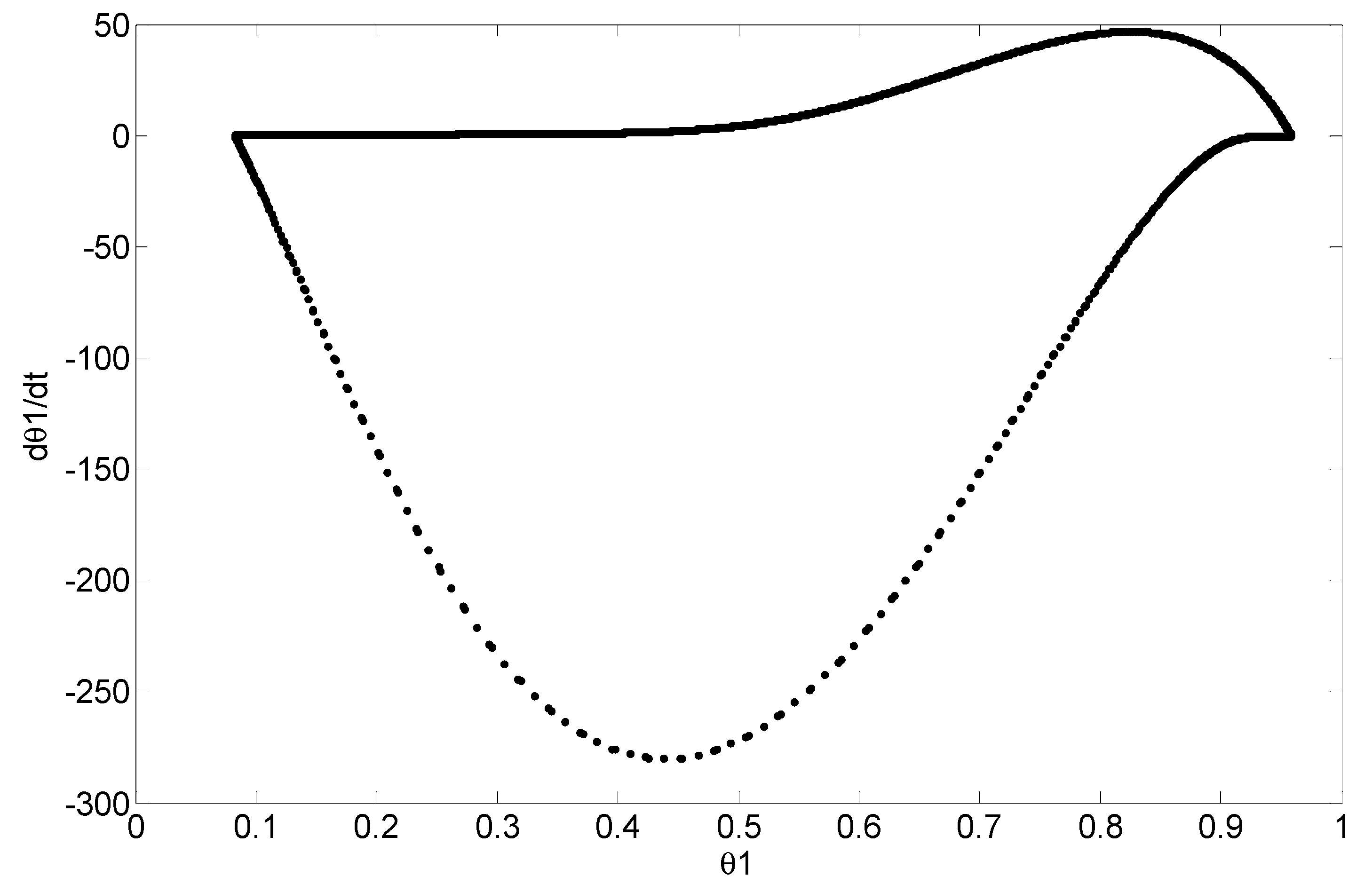
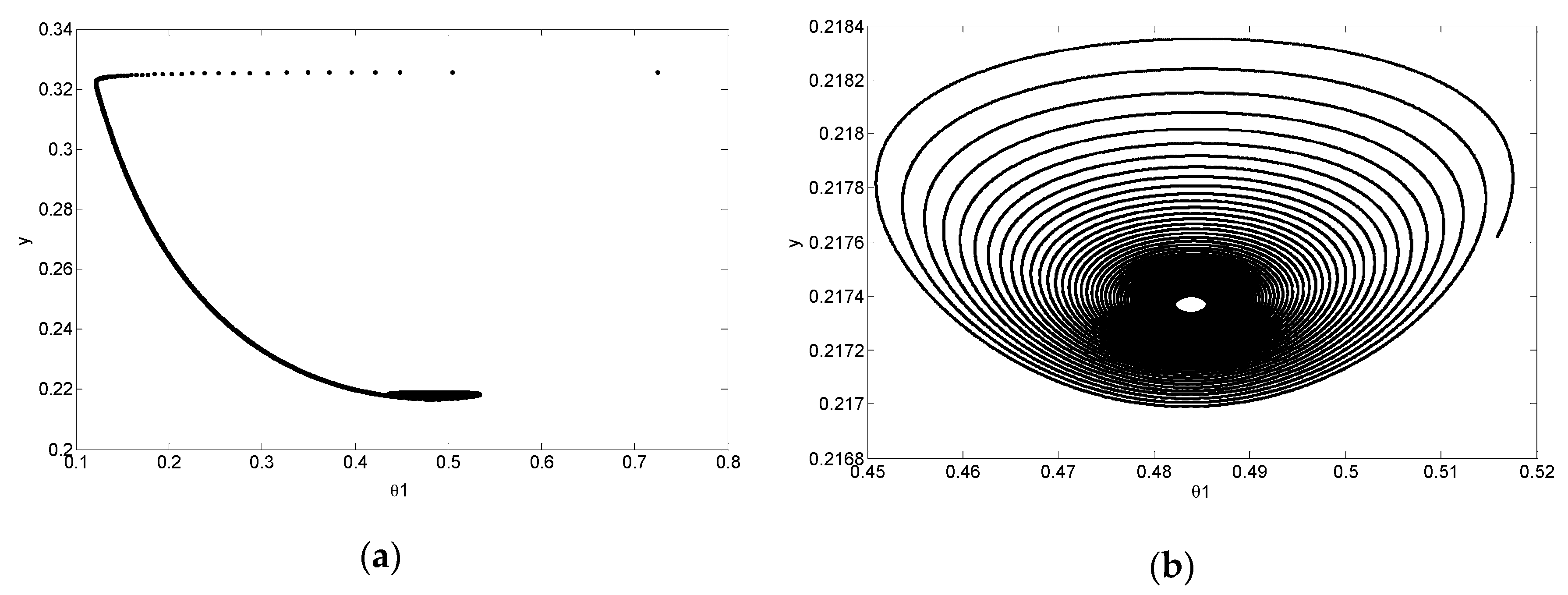
4. Simulation and Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinfelt, J.H. Role of surface science in catalysis. Surf. Sci. 2002, 500, 923–946. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Surface Science: Foundations of Catalysis and Nanoscience; Wiley: Hoboken, NJ, USA, 2012; ISBN 9781119990369. [Google Scholar]
- Hendriksen, B.L.M.; Bobaru, S.C.; Frenken, J.W.M. Bistability and oscillations in CO oxidation studied with scanning tunnelling microscopy inside a reactor. Catal. Today 2005, 105, 234–243. [Google Scholar] [CrossRef]
- Pattas, K.N.; Stamatelos, A.M.; Pistikopoulos, P.K.; Koltsakis, G.C.; Konstandinidis, P.A.; Volpi, E.; Leveroni, E. Transient modeling of 3-way catalytic converters. In Proceedings of the SAE Technical Papers, Detroit, MI, USA, 1 March 1994. [Google Scholar]
- Oh, S.H.; Cavendish, J.C. Transients of Monolithic Catalytic Converters: Response to Step Changes in Feedstream Temperature as Related to Controlling Automobile Emissions. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 29–37. [Google Scholar] [CrossRef]
- Chen, D.K.S.; Bissett, E.J.; Oh, S.H.; Van Ostrom, D.L. A three-dimensional model for the analysis of transient thermal and conversion characteristics of monolithic catalytic converters. In Proceedings of the SAE Technical Papers, Detroit, MI, USA, 1 February 1988; pp. 177–189. [Google Scholar]
- Katashiba, H.; Nishida, M.; Washino, S.; Takahashi, A.; Hashimoto, T.; Miyake, M. Fuel injection control systems that improve three way catalyst conversion efficiency. In Proceedings of the SAE Technical Papers, Detroit, MI, USA, 1 January 1991; pp. 196–204. [Google Scholar]
- Gottberg, I.; Rydquist, J.E.; Backlund, O.; Wallman, S.; Maus, W.; Brück, R.; Swars, H. New potential exhaust gas aftertreatment technologies for “clean Car” legislation. In Proceedings of the SAE Technical Papers, Detroit, MI, USA, 1 January 1991; pp. 343–360. [Google Scholar]
- Montreuil, C.N.; Williams, S.C.; Adamczyk, A.A. Modeling current generation catalytic converters: Laboratory experiments and kinetic parameter optimization-steady state kinetics. In Proceedings of the SAE Technical Papers, Detroit, MI, USA, 1 February 1992. [Google Scholar]
- Messerer, A.; Niessner, R.; Pöschl, U. Comprehensive kinetic characterization of the oxidation and gasification of model and real diesel soot by nitrogen oxides and oxygen under engine exhaust conditions: Measurement, Langmuir-Hinshelwood, and Arrhenius parameters. Carbon N. Y. 2006, 44, 307–324. [Google Scholar] [CrossRef]
- Vasanth Kumar, K.; Porkodi, K.; Selvaganapathi, A. Constrain in solving Langmuir-Hinshelwood kinetic expression for the photocatalytic degradation of Auramine O aqueous solutions by ZnO catalyst. Dye. Pigment. 2007, 75, 246–249. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Xu, W.; Kong, J.S.; Chen, P. Single-molecule kinetic theory of heterogeneous and enzyme catalysis. J. Phys. Chem. C 2009, 119, 2393–2404. [Google Scholar] [CrossRef]
- Khezrianjoo, S.; Revanasiddappa, H.D. Langmuir-Hinshelwood Kinetic Expression for the Photocatalytic Degradation of Metanil Yellow Aqueous Solutions by ZnO Catalyst. Chem. Sci. J. 2012. [Google Scholar] [CrossRef]
- Burrows, V.A.; Sundaresan, S.; Chabal, Y.J.; Christman, S.B. Studies on self-sustained reaction-rate oscillations: I. Real-time surface infrared measurements during oscillatory oxidation of carbon monoxide on platinum. Surf. Sci. 1985, 160, 122–138. [Google Scholar] [CrossRef]
- Burrows, V.A.; Sundaresan, S.; Chabal, Y.J.; Christman, S.B. Studies on self-sustained reaction-rate oscillations II. The role of carbon and oxides in the oscillatory oxidation of carbon monoxide on platinum. Surf. Sci. Lett. 1987, 180, 110–135. [Google Scholar] [CrossRef]
- Collins, N.A.; Sundaresan, S.; Chabal, Y.J. Studies on self-sustained reaction-rate oscillations. III. The carbon model. Surf. Sci. 1987, 180, 136–152. [Google Scholar] [CrossRef]
- Keren, I.; Sheintuch, M. Modeling and analysis of spatiotemporal oscillatory patterns during CO oxidation in the catalytic converter. Chem. Eng. Sci. 2000, 55, 1461–1475. [Google Scholar] [CrossRef]
- Turner, J.E.; Sales, B.C.; Maple, M.B. Oscillatory oxidation of Co over a Pt catalyst. Surf. Sci. 1981, 103, 54–74. [Google Scholar] [CrossRef]
- Sales, B.C.; Turner, J.E.; Maple, M.B. Oscillatory oxidation of CO over Pt, Pd and Ir catalysts: Theory. Surf. Sci. 1982, 114, 381–394. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. Catalytic oxidation of CO on platinum. J. Therm. Anal. Calorim. 2013, 111, 145–154. [Google Scholar] [CrossRef]
- Neugebohren, J.; Borodin, D.; Hahn, H.W.; Altschäffel, J.; Kandratsenka, A.; Auerbach, D.J.; Campbell, C.T.; Schwarzer, D.; Harding, D.J.; Wodtke, A.M.; et al. Velocity-resolved kinetics of site-specific carbon monoxide oxidation on platinum surfaces. Nature 2018, 558, 280–283. [Google Scholar] [CrossRef]
- Harding, D.J.; Neugebohren, J.; Auerbach, D.J.; Kitsopoulos, T.N.; Wodtke, A.M. Using Ion Imaging to Measure Velocity Distributions in Surface Scattering Experiments. J. Phys. Chem. A 2015, 119, 12255–12262. [Google Scholar] [CrossRef]
- Rodger, J.A. Advances in multisensor information fusion: A Markov–Kalman viscosity fuzzy statistical predictor for analysis of oxygen flow, diffusion, speed, temperature, and time metrics in CPAP. Expert Syst. 2018, 35, e12270. [Google Scholar] [CrossRef]
- González-Fernández, C.F.; Alhama, F. Heat Transfer and the Network Simulation Method; Horno, J., Ed.; Transworld Research Network: Trivandrum, India, 2001. [Google Scholar]
- Sánchez-Pérez, J.F.; Marín, F.; Morales, J.L.; Cánovas, M.; Alhama, F. Modeling and simulation of different and representative engineering problems using network simulation method. PLoS ONE 2018, 13, e0193828. [Google Scholar] [CrossRef]
- Nagel, L.W.; Pederson, D.O. SPICE (Simulation Program with Integrated Circuit Emphasis); EECS Department: Berkeley, CA, USA, 1973. [Google Scholar]
- PSPICE 6.0; MicroSim Corporation Fairbanks: Irvine, CA, USA, 1994; Available online: http://robustdesignconcepts.com/files/pspice/index.htm (accessed on 15 January 2020).
- Sourceforge. NgSpice. Available online: http://ngspice.sourceforge.net/index.html (accessed on 15 January 2020).
- Horno, J.; González-Fernández, C.F.; Hayas, A. The network method for solutions of oscillating reaction-diffusion systems. J. Comput. Phys. 1995, 118, 310–319. [Google Scholar] [CrossRef]
- González Fernández, C.F.; Alhama, F.; López Sánchez, J.F.; Horno, J. Application of the network method to heat conduction processes with polynomial and potential-exponentially varying thermal properties. Numer. Heat Transf. Part. A Appl. 1998, 33, 549–559. [Google Scholar] [CrossRef]
- Zueco, J.; Alhama, F. Inverse estimation of temperature dependent emissivity of solid metals. J. Quant. Spectrosc. Radiat. Transf. 2006, 101, 73–86. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Alhama, F.; Moreno, J.A. An efficient and reliable model based on network method to simulate CO2 corrosion with protective iron carbonate films. Comput. Chem. Eng. 2012, 39, 57–64. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.F.; Moreno, J.A.; Alhama, F. Numerical Simulation of High-Temperature Oxidation of Lubricants Using the Network Method. Chem. Eng. Commun. 2015, 202, 982–991. [Google Scholar] [CrossRef]
- Sánchez-Pérez, J.F.; Alhama, F.; Moreno, J.A.; Cánovas, M. Study of main parameters affecting pitting corrosion in a basic medium using the network method. Results Phys. 2019, 12, 1015–1025. [Google Scholar] [CrossRef]
- Nagel, L.W. SPICE2: A Computer Program To Simulate Semiconductor Circuits. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1975. [Google Scholar]
- Vogt, H.; Marcel, H.; Paolo, N. Ngspice User’s Manual Version 32; Sourceforge: San Diego, CA, USA, 2020; Available online: http://ngspice.sourceforge.net/index.html (accessed on 20 July 2020).
- Gear, C.W. The Automatic Integration of Ordinary Differential Equations. Commun. ACM 1971, 14, 176–179. [Google Scholar] [CrossRef]
- Beusch, H.; Fieguth, P.; Wicke, E. Thermisch und kinetisch verursachte Instabilitäten im Reaktionsverhalten einzelner Katalysatorkörner. Chem. Ing. Tech. 1972, 44, 445–451. [Google Scholar] [CrossRef]
- Lintz, H.-G.; Weisker, T. The oxidation of carbon monoxide on polycrystalline rhodium under knudsen conditions. Appl. Surf. Sci. 1985, 14, 251–258. [Google Scholar] [CrossRef]
- Lobban, L.; Luss, D. Spatial temperature oscillations during hydrogen oxidation on a nickel foil. J. Phys. Chem. 1989, 93, 6530–6533. [Google Scholar] [CrossRef]
- Tsai, P.K.; Wu, M.G.; Brian Maple, M. Oscillatory oxidation of CO over Pt at pressures from 10 to 760 torr. J. Catal. 1991, 127, 512–523. [Google Scholar] [CrossRef]
- Carlsson, P.A.; Zhdanov, V.P.; Kasemo, B. Bistable mean-field kinetics of CO oxidation on Pt with oxide formation. Appl. Surf. Sci. 2005, 239, 424–431. [Google Scholar] [CrossRef]
- Sánchez-Perez, J.F. Solución Numérica de Problemas de Oxidación Mediante el Método de Simulación por Redes. Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, 2012. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Pérez, J.F.; Nicolas, J.A.M.; Alhama, F.; Canovas, M. Study of Transition Zones in the Carbon Monoxide Catalytic Oxidation on Platinum Using the Network Simulation Method. Mathematics 2020, 8, 1413. https://doi.org/10.3390/math8091413
Sánchez-Pérez JF, Nicolas JAM, Alhama F, Canovas M. Study of Transition Zones in the Carbon Monoxide Catalytic Oxidation on Platinum Using the Network Simulation Method. Mathematics. 2020; 8(9):1413. https://doi.org/10.3390/math8091413
Chicago/Turabian StyleSánchez-Pérez, Juan Francisco, Jose Andres Moreno Nicolas, Francisco Alhama, and Manuel Canovas. 2020. "Study of Transition Zones in the Carbon Monoxide Catalytic Oxidation on Platinum Using the Network Simulation Method" Mathematics 8, no. 9: 1413. https://doi.org/10.3390/math8091413
APA StyleSánchez-Pérez, J. F., Nicolas, J. A. M., Alhama, F., & Canovas, M. (2020). Study of Transition Zones in the Carbon Monoxide Catalytic Oxidation on Platinum Using the Network Simulation Method. Mathematics, 8(9), 1413. https://doi.org/10.3390/math8091413






