Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Set Used
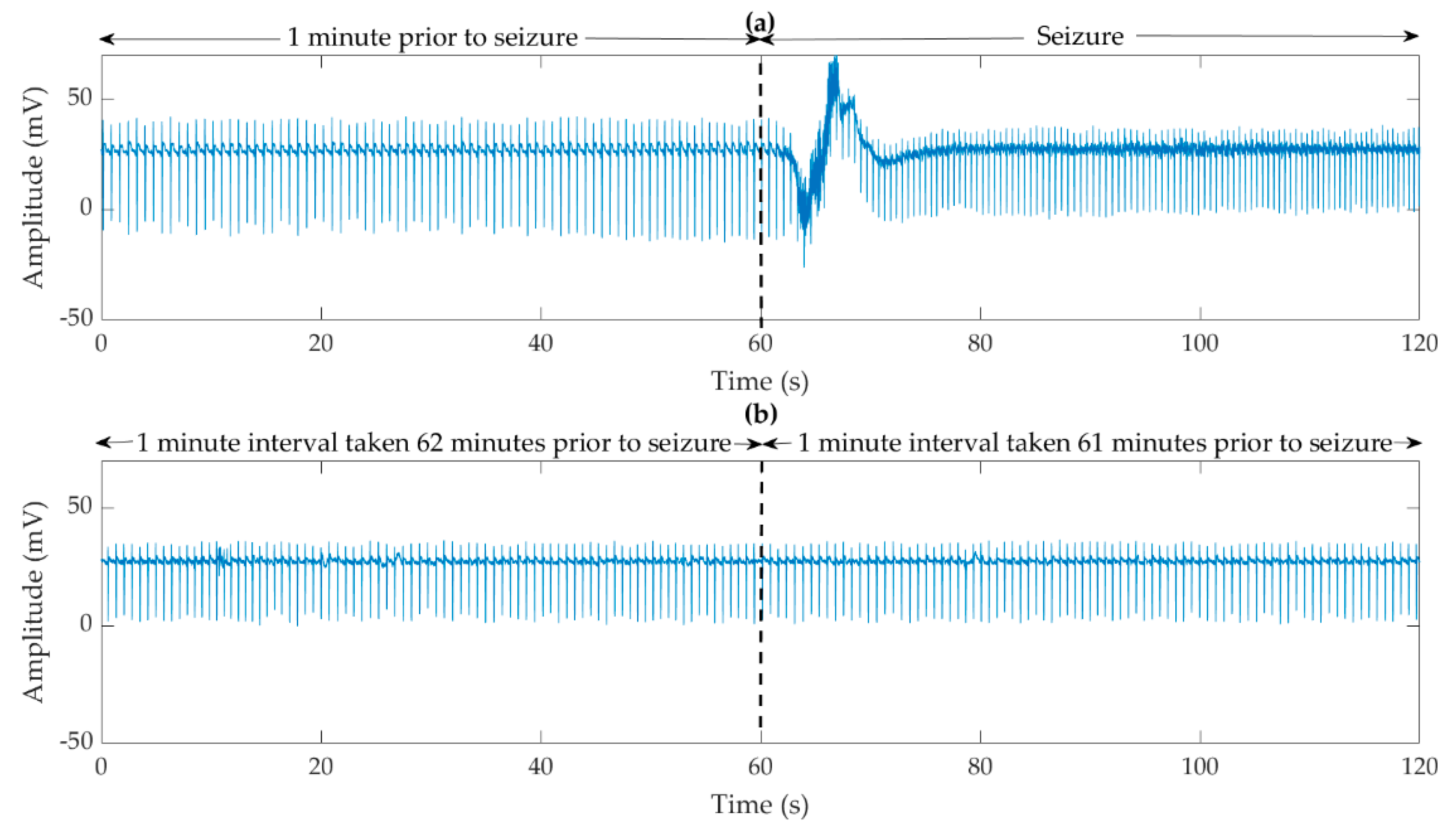
2.2. Preparation of the ECG Signals
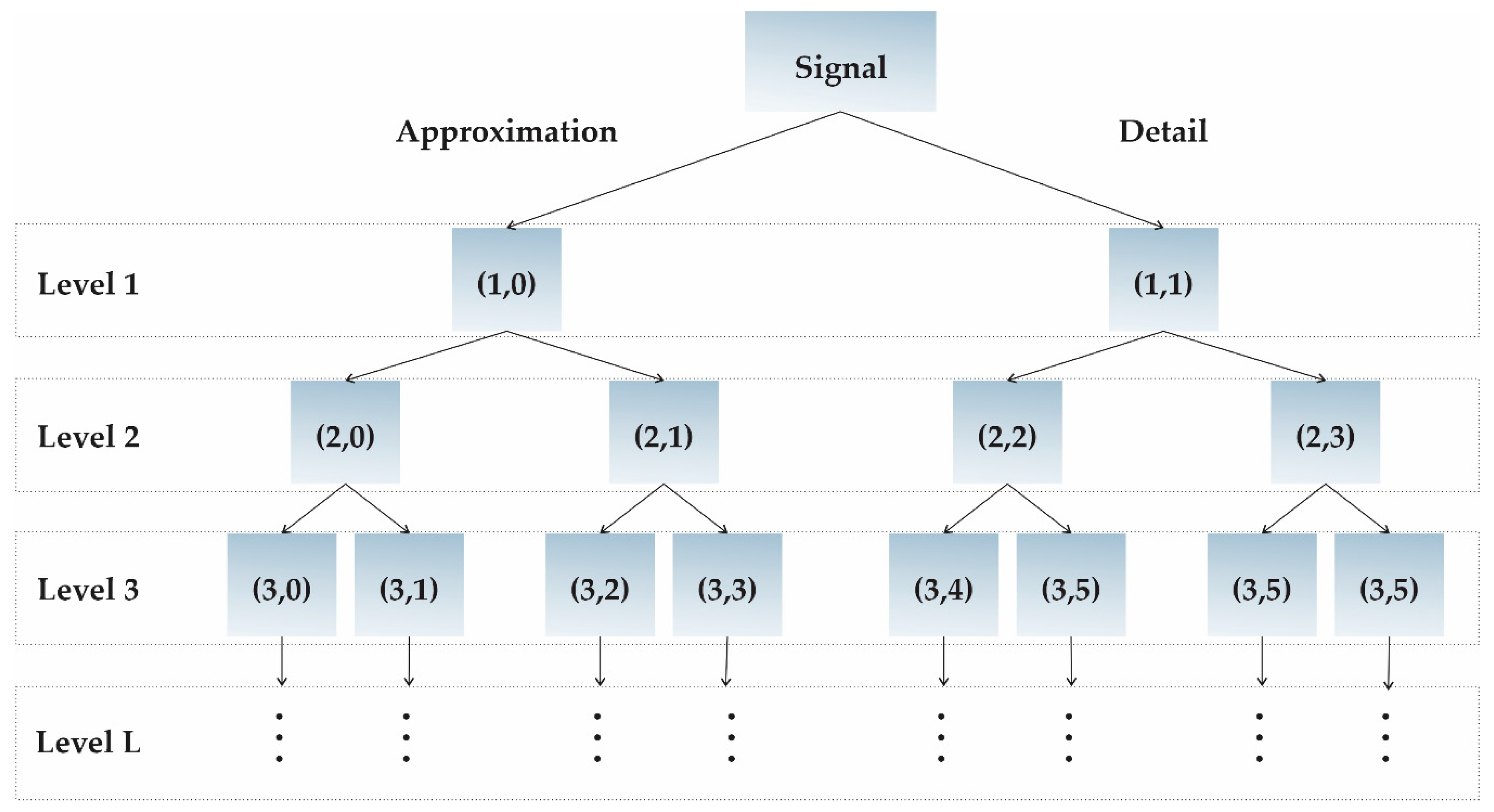
2.3. Wavelet Packet Transform
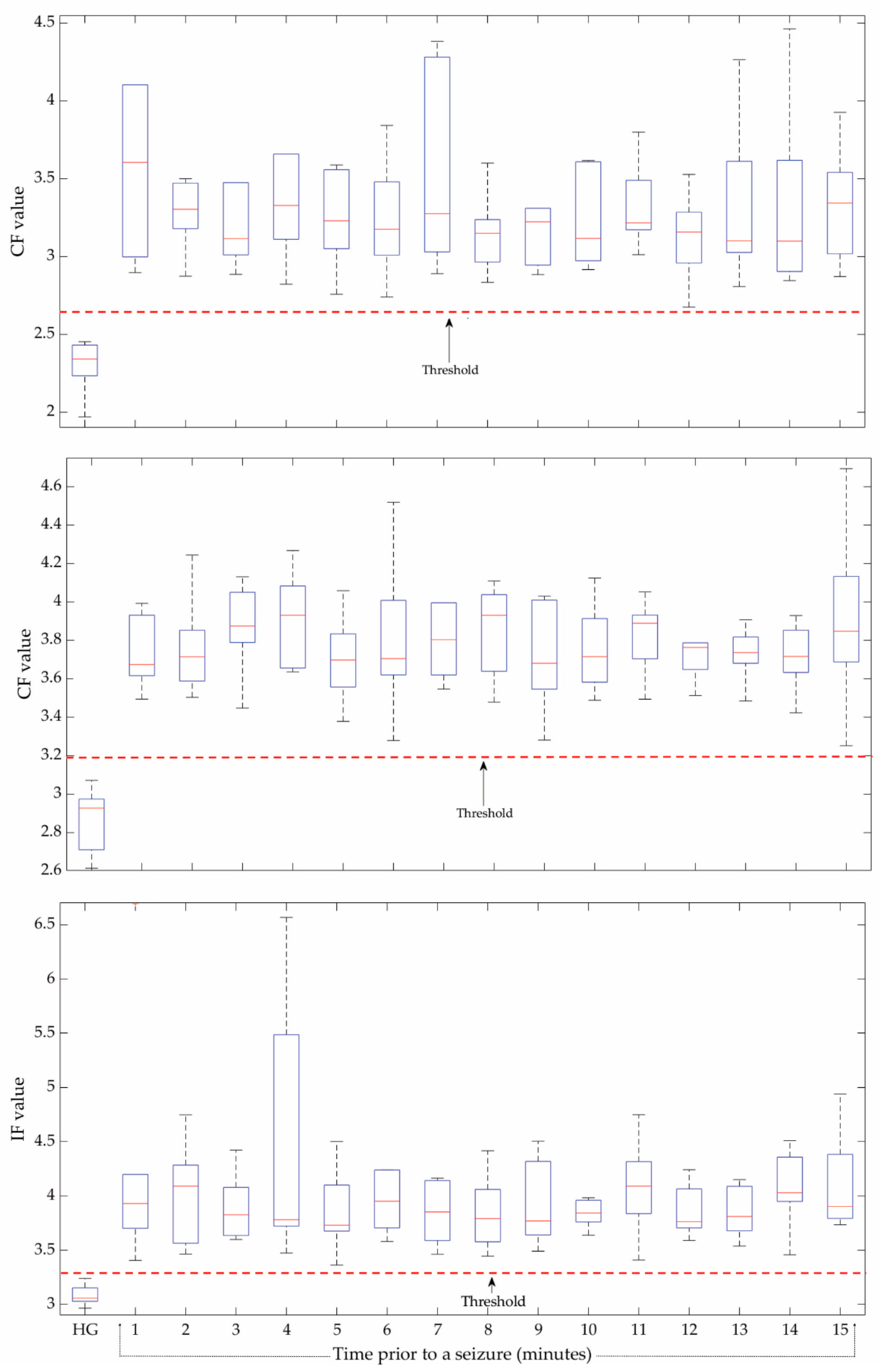
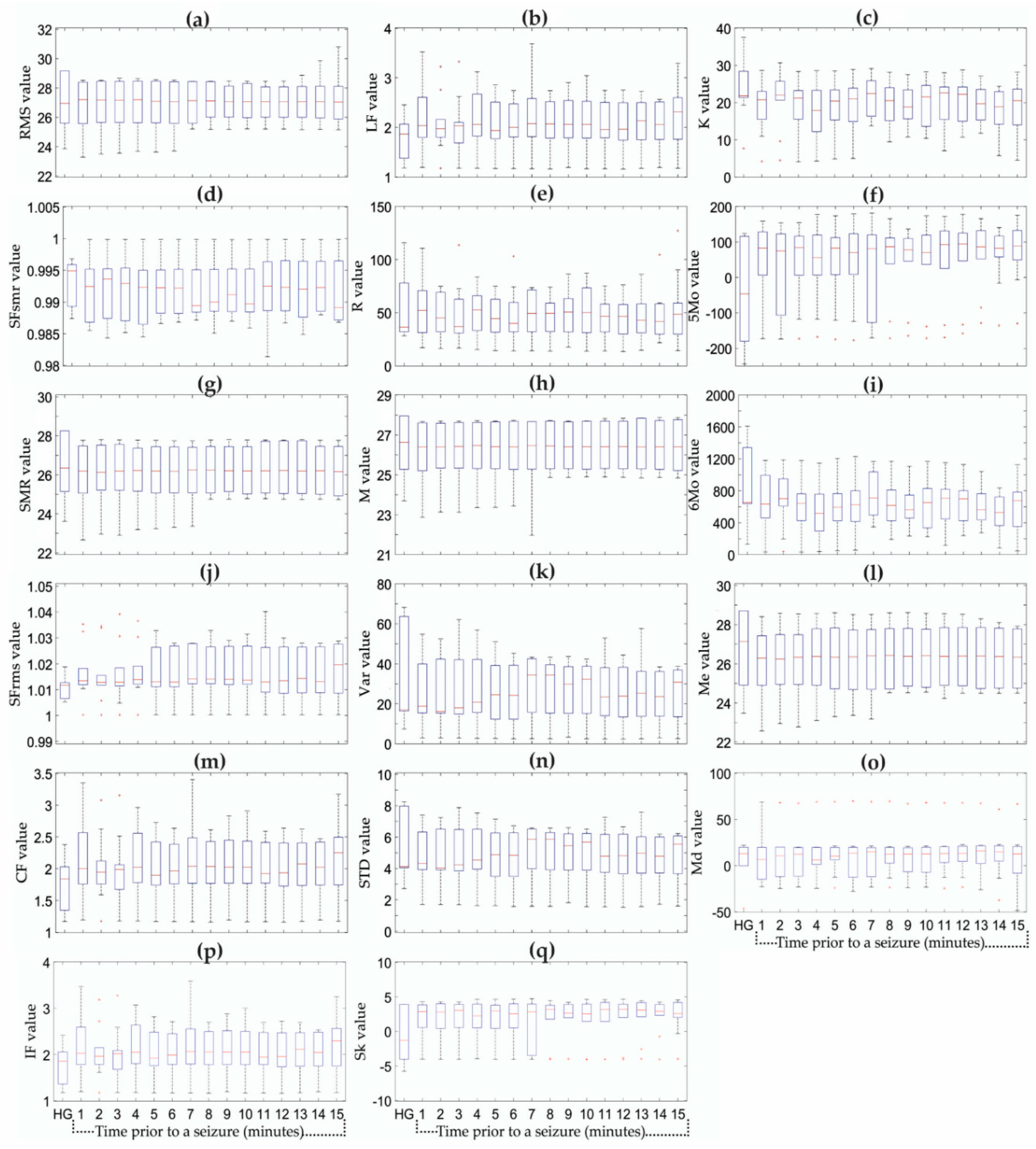
2.4. Statistical Time Features
2.5. Kruskal–Wallis Method
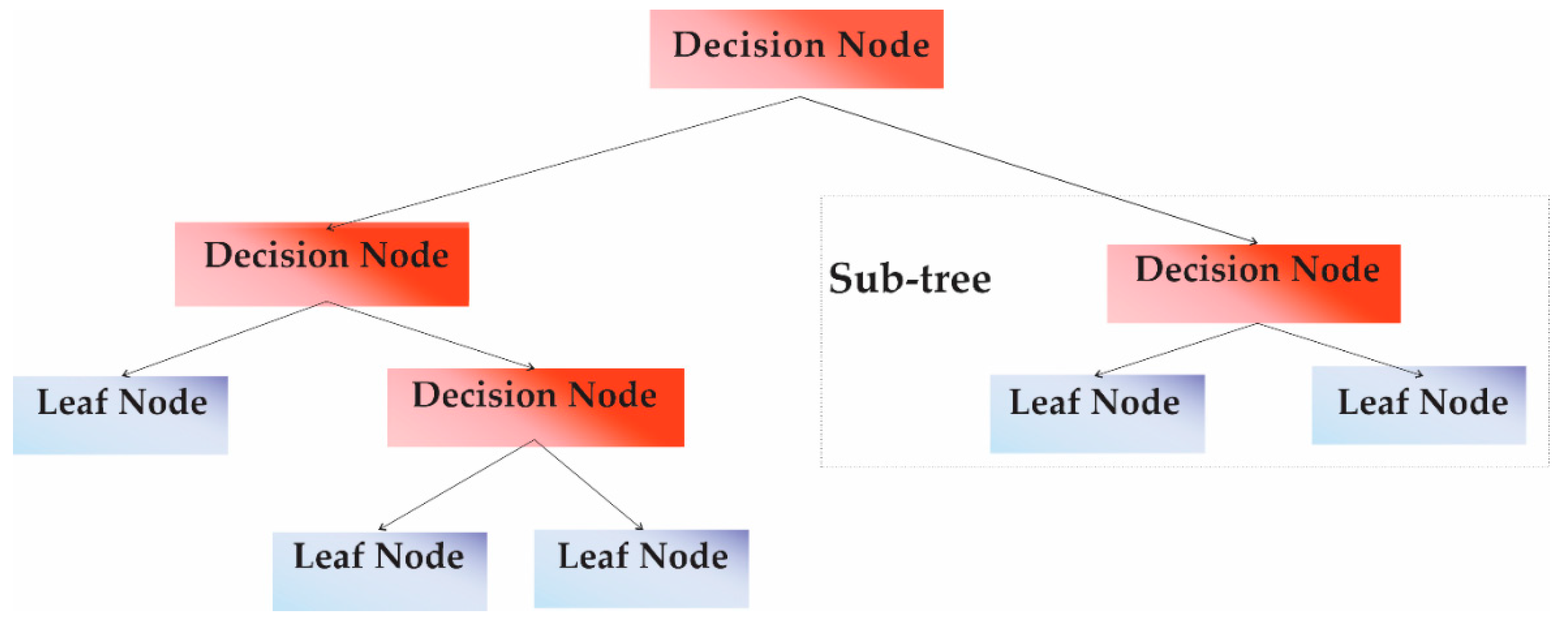
2.6. Decision Tree Classifier
- Rule splitting node selection;
- Set of the terminal nodes;
- Assignment of the corresponding class label to the terminal nodes.
- Design a logical test for each feature that will be used as an input to the DTC;
- For each logical test, use a subset of the training data to verify that the outcome to the corresponding terminal node assigns the expected label;
- Repeat this procedure for all the terminal nodes.
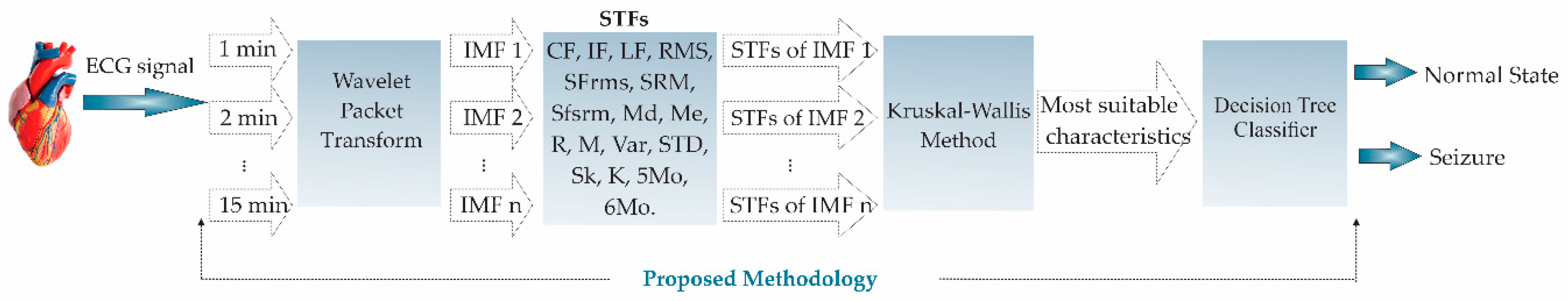
3. Methodology
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alarcón, G. What is epilepsy. In Introduction to Epilepsy; Cambridge University Press: Cambridge, UK, 2012; Volume 1, pp. 6–7. [Google Scholar]
- Siuly, S.; Li, Y. Discriminating the brain activities for brain–computer interface applications through the optimal allocation-based approach. Neural Comput. Appl. 2015, 26, 799–811. [Google Scholar] [CrossRef]
- Truccolo, W.; Donoghue, J.A.; Hochberg, L.R.; Eskandar, E.N.; Madsen, J.R.; Anderson, W.S.; Brown, E.N.; Halgren, E.; Cash, S.S. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011, 14, 635–643. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Epilepsy: A Public Health Imperative. 2019. Available online: https://www.who.int/mental_health/neurology/epilepsy/report_2019/en/ (accessed on 15 August 2020).
- England, M.J.; Liverman, C.T.; Schultz, A.M.; Strawbridge, L.M. Quality of Life and Community Resources. In Epilepsy Across the Spectrum: Promoting Health and Understanding; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Vaurio, L.; Karantzoulis, S.; Barr, W.B. The impact of epilepsy on quality of life. In Changes in the Brain; Springer: New York, NY, USA, 2017; pp. 167–187. [Google Scholar]
- Taghvayi, N.E.; Aazhang, B. Application of Embedded Dynamic Mode Decomposition on Epileptic Data for Seizure Prediction. In Proceedings of the Signals, Systems and Computers, Pacific Grove, CA, USA, 3–6 November 2019; pp. 1705–1708. [Google Scholar]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput. Biol. Med. 2018, 100, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sharif, B.; Jafari, A.H. Prediction of epileptic seizures from EEG using analysis of ictal rules on Poincaré plane. Comput. Methods Progr. Biomed. 2017, 145, 11–22. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Chen, L. Epileptic Seizure Prediction Using Diffusion Distance and Bayesian Linear Discriminate Analysis on Intracranial EEG. Int. J. Neural Syst. 2018, 28, 1750043. [Google Scholar] [CrossRef]
- Usman, S.M.; Latif, S.; Beg, A. Principle components analysis for seizures prediction using wavelet transform. Int. J. Adv. Appl. Sci. 2019, 6, 50–55. [Google Scholar]
- Usman, S.M.; Khalid, S.; Aslam, M.H. Epileptic seizures prediction using deep learning techniques. IEEE Access 2020, 8, 39998–40007. [Google Scholar] [CrossRef]
- Ibrahim, F.; El-Gindy, S.A.E.; El-Dolil, S.M.; El-Fishawy, A.S.; El-Rabaie, E.S.M.; Dessouky, M.I.; Abd El-Samie, F.E. A statistical framework for EEG channel selection and seizure prediction on mobile. Int. J. Speech Technol. 2019, 22, 191–203. [Google Scholar] [CrossRef]
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Ippolito, S.; Kavehei, O. Convolutional neural networks for seizure prediction using intracranial and scalp electroencephalogram. Neural Netw. 2018, 105, 104–111. [Google Scholar] [CrossRef]
- Cui, S.; Duan, L.; Qiao, Y.; Xiao, Y. Learning EEG synchronization patterns for epileptic seizure prediction using bag-of-wave features. J. Ambient Intell. Humaniz. Comput. 2018, in press. [Google Scholar] [CrossRef]
- Khan, H.; Marcuse, L.; Fields, M.; Swann, K.; Yener, B. Focal onset seizure prediction using convolutional networks. IEEE Trans. Biomed. Eng. 2017, 65, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Kolsal, E.; Serdaroğlu, A.; Çilsal, E.; Kula, S.; Soysal, A.Ş.; Kurt, A.N.Ç.; Arhan, E. Can heart rate variability in children with epilepsy be used to predict seizures? Seizure 2014, 23, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012, 53, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, R.J.; Thijs, R.D.; Laffan, A.; Langan, Y.; Sander, J.W. Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia 2012, 53, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Lagae, L. Cardiac changes in epilepsy. Seizure 2010, 19, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Panichev, O.; Karplyuk, Y.; Smirnov, Y.; Zaunseder, S.; Kharytonov, V. Heart beat-to-beat intervals classification for epileptic seizure prediction. In Proceedings of the Signal Process: Symposium (SPSympo 2017), Jachranka, Poland, 12–14 September 2017; pp. 2–5. [Google Scholar]
- Pavei, J.; Heinzen, R.G.; Novakova, B.; Walz, R.; Serra, A.J.; Reuber, M.; Marques, J.L. Early seizure detection based on cardiac autonomic regulation dynamics. Front. Physiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Varon, C.; Caicedo, A.; Jansen, K.; Lagae, L.; Van Huffel, S. Detection of epileptic seizures from single lead ECG by means of phase rectified signal averaging. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2014), Chicago, IL, USA, 26–30 August 2014; pp. 3789–3790. [Google Scholar]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef]
- Giannakakis, G.; Tsiknakis, M.; Vorgia, P. Focal epileptic seizures anticipation based on patterns of heart rate variability parameters. Comput. Methods Progr. Biomed. 2019, 178, 123–133. [Google Scholar] [CrossRef]
- MIT/BIH-PIHROPE. Available online: https://physionet.org/content/szdb/1.0.0/ (accessed on 1 February 2020).
- Cao, F.; Budhota, A.; Chen, H.; Rajput, K.S. Feature matching-based ECG generative network for arrhythmia event augmentation. In Proceedings of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 296–299. [Google Scholar]
- Vargas-Lopez, O.; Amezquita-Sanchez, J.P.; De-Santiago-Perez, J.J.; Rivera-Guillen, J.R.; Valtierra-Rodriguez, M.; Toledano-Ayala, M.; Perez-Ramirez, C.A. A new methodology based on EMD and nonlinear measurements for sudden cardiac death detection. Sensors 2020, 20, 9. [Google Scholar] [CrossRef]
- Amezquita-Sanchez, J.P.; Valtierra-Rodriguez, M.; Adeli, H.; Perez-Ramirez, C.A. A Novel Wavelet Transform-Homogeneity Model for Sudden Cardiac Death Prediction Using ECG Signals. J. Med. Syst. 2018, 42, 1–15. [Google Scholar] [CrossRef]
- Nahak, S.; Saha, G. A fusion based classification of normal, arrhythmia and congestive heart failure in ECG. In Proceedings of the National Conference on Communications (NCC), Kharagpur, India, 21–23 February 2020; pp. 1–6. [Google Scholar]
- Fujita, H.; Acharya, U.R.; Sudarshan, V.K.; Ghista, D.N.; Sree, S.V.; Eugene, L.W.J.; Koh, J.E. Sudden cardiac death (SCD) prediction based on nonlinear heart rate variability features and SCD index. Appl. Soft Comput. J. 2016, 43, 510–519. [Google Scholar] [CrossRef]
- Chinara, S. Automatic classification methods for detecting drowsiness using wavelet packet transform extracted time-domain features from single-channel EEG signal. J. Neurosci. Methods 2020, 347, 108927. [Google Scholar]
- Wali, M.K. FFBPNN-based high drowsiness classification using EMG and WPT. Biomed. Eng. Appl. Basis Commun. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Walczak, B.; Massart, D.L. Noise suppression and spinal compression using the wavelet packet transform. Chemom. Intell. Lab. Syst. 1997, 36, 81–94. [Google Scholar] [CrossRef]
- Shestakov, O. Wavelet Thresholding risk estimate for the model with random samples and correlated noise. Mathematics 2020, 8, 377. [Google Scholar] [CrossRef]
- Rafiee, J.; Rafiee, M.A.; Prause, N.; Schoen, M.P. Wavelet basis functions in biomedical signal processing. Expert Syst. Appl. 2011, 38, 6190–6201. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, Q.; Ye, Q. Classification of gait rhythm signals between patients with neuro-degenerative diseases and normal subjects: Experiments with statistical features and different classification models. Biomed. Signal Process. Control 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Ghorbani Afkhami, R.; Azarnia, G.; Tinati, M.A. Cardiac arrhythmia classification using statistical and mixture modeling features of ECG signals. Pattern Recognit. Lett. 2016, 70, 45–51. [Google Scholar] [CrossRef]
- Hassan, A.R.; Bhuiyan, M.I. Automatic sleep scoring using statistical features in the EMD domain and ensemble methods. Biocybern. Biomed. Eng. 2016, 36, 248–255. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Zhao, Z.; Wang, J. Bearing fault diagnosis based on statistical locally linear embedding. Sensors 2015, 15, 16225–16247. [Google Scholar] [CrossRef]
- Caesarendra, W.; Tjahjowidodo, T. A Review of feature extraction methods in vibration-based condition monitoring and its application for degradation trend estimation of low-speed slew bearing. Machines 2017, 5, 21. [Google Scholar] [CrossRef]
- Yanez-Borjas, J.J.; Valtierra-Rodriguez, M.; Camarena-Martinez, D.; Amezquita-Sanchez, J.P. Statistical time features for global corrosion assessment in a truss bridge from vibration signals. Meas. J. Int. Meas. Confed. 2020, 160, 107858. [Google Scholar] [CrossRef]
- Abdullah, S. Wavelet Bump Extraction (WBE) for Editing Variable Amplitude Fatigue Loadings. Ph.D. Thesis, University of Sheffield United Kingdom, Sheffield, UK, 2005. [Google Scholar]
- Devore, J.L.; Berk, K.N. Overview and descriptive statistics. In Modern Mathematical Statistics with Applications; Springer: New York, NY, USA, 2013; pp. 1–49. [Google Scholar]
- Palaniswamy, U.R.; Palaniswamy, K.M. Teaching and Research in Plant and Crop Science; Food Products and The Haworth Refrence Press: New York, NY, USA, 2005. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Morales, J.F.; Willems, R.; Van Huffel, S.; Varon, C. Evaluation of the ECG Derived Respiration in the Presence of Irregular Heart Beats. In Proceedings of the 11th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Pisa, Italy, 15 July 2020; pp. 20–21. [Google Scholar]
- Bajaj, V.; Taran, S.; Khare, S.K.; Sengur, A. Feature extraction method for classification of alertness and drowsiness states EEG signals. Appl. Acoust. 2020, 163, 107224. [Google Scholar] [CrossRef]
- Hecke, T.V. Power study of anova versus Kruskal-Wallis test. J. Stat. Manag. Syst. 2012, 15, 241–247. [Google Scholar] [CrossRef]
- Nisbet, R.; Miner, G.; Yale, K. What is classification. In Handbook of Statistical Analysis and Data Mining Applications, 2nd ed.; Nisbet, R., Miner, G., Yale, K., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 169–186. [Google Scholar]
- Ardhapure, O.; Patil, G.; Udani, D.; Jetha, K. Comparative study of classification algorithm for text based categorization. Int. J. Res. Eng. Technol. 2016, 5, 217–220. [Google Scholar]
- Shobha, G.; Rangaswamy, S. Machine Learning. Handbook of Statistics, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 38, pp. 197–228. [Google Scholar]
- Jukic, S.; Saracevic, M.; Subasi, A.; Kevric, J. Comparison of ensemble machine learning methods for automated classification of focal and non-focal epileptic EEG signals. Mathematics 2020, 8, 1481. [Google Scholar] [CrossRef]
- Joloudari, J.H.; Hassannataj Joloudari, E.; Saadatfar, H.; GhasemiGol, M.; Razavi, S.M.; Mosavi, A.; Nadai, L. Coronary artery disease diagnosis; ranking the significant features using a random trees model. Int. J. Environ. Res. Public Health 2020, 17, 731. [Google Scholar] [CrossRef]
- Wei, J.K.E.; Jahmunah, V.; Pham, T.H.; Oh, S.L.; Ciaccio, E.J.; Acharya, U.R.; Ramli, N. Automated detection of Alzheimer’s disease using bi-directional empirical model decomposition. Pattern Recognit. Lett. 2020, 135, 106–113. [Google Scholar] [CrossRef]
- Borah, P.; Ahmed, H.A.; Bhattacharyya, D.K. A statistical feature selection technique. Netw. Modl. Anal. Health Inform. Bioinform. 2014, 3, 55. [Google Scholar] [CrossRef]
- Rasoul Safavian, S.; Landgrebe, D. A Survey of Decision Tree Classifier Methodology. IEEE Trans. Syst. Man Cybern. Syst. 1990, 21, 660–674. [Google Scholar] [CrossRef]
- Polat, K.; Günes, S. Classification of epileptiform EEG using a hybrid system based on decision tree classifier and fast Fourier transform. Appl. Math. Compt. 2007, 187, 1017–1026. [Google Scholar] [CrossRef]
- Li, T.; Zhou, M. ECG classification using wavelet packet entropy and random forests. Entropy 2016, 18, 285. [Google Scholar] [CrossRef]
- Hamzenejad, A.; Jafarzadeh Ghoushchi, S.; Baradaran, V.; Mardani, A. A robust algorithm for classification and diagnosis of brain disease using local linear approximation and generalized autoregressive conditional heteroscedasticity model. Mathematics 2020, 8, 1268. [Google Scholar] [CrossRef]
- Willigenburg, N.W.; Daffertshofer, A.; Kingma, I.; Van Dieën, J.H. Removing ECG contamination from EMG recordings: A comparison of ICA-based and other filtering procedures. J. Electromyogr. Kinesiol. 2012, 22, 485–493. [Google Scholar] [CrossRef]
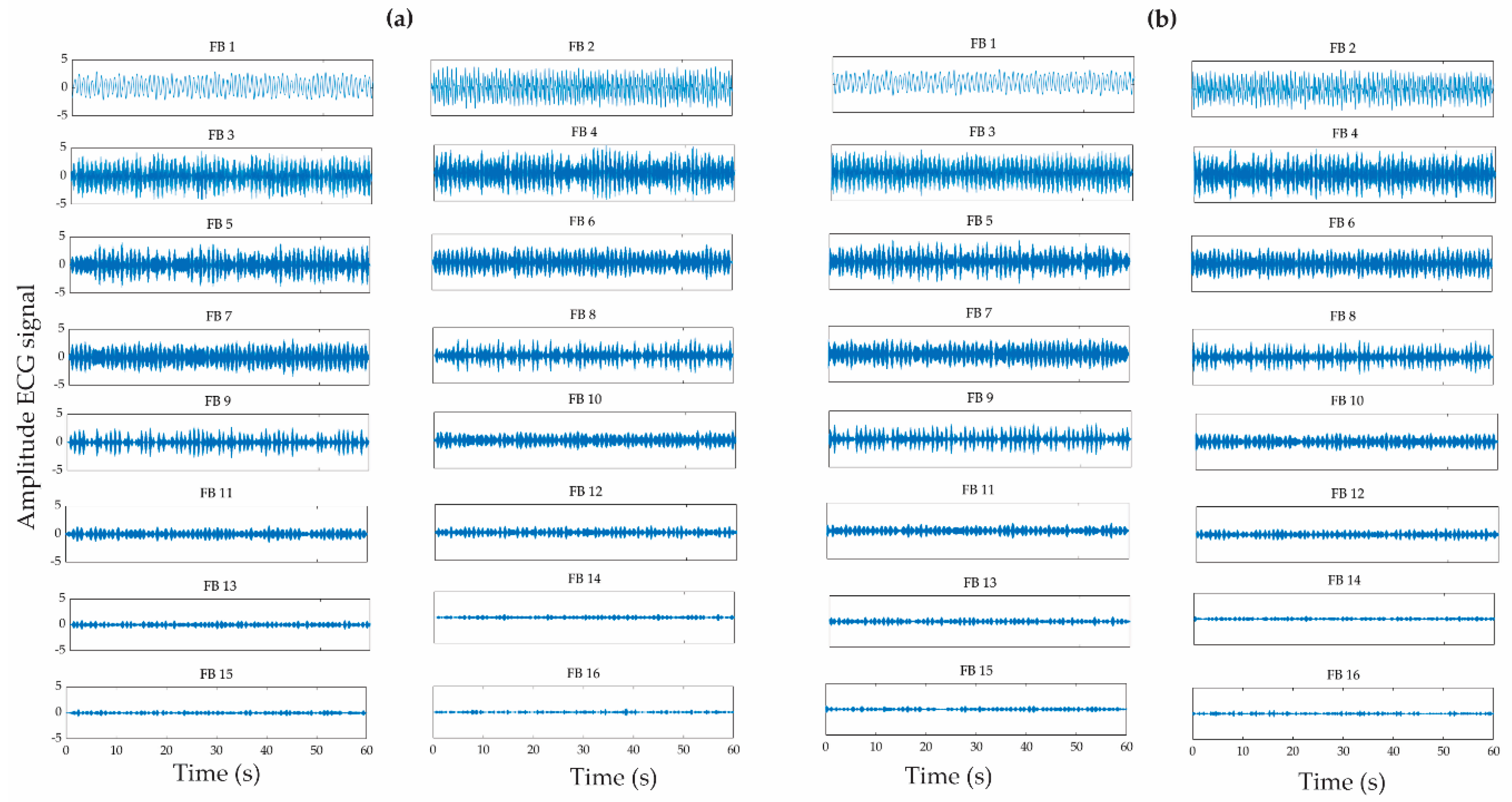
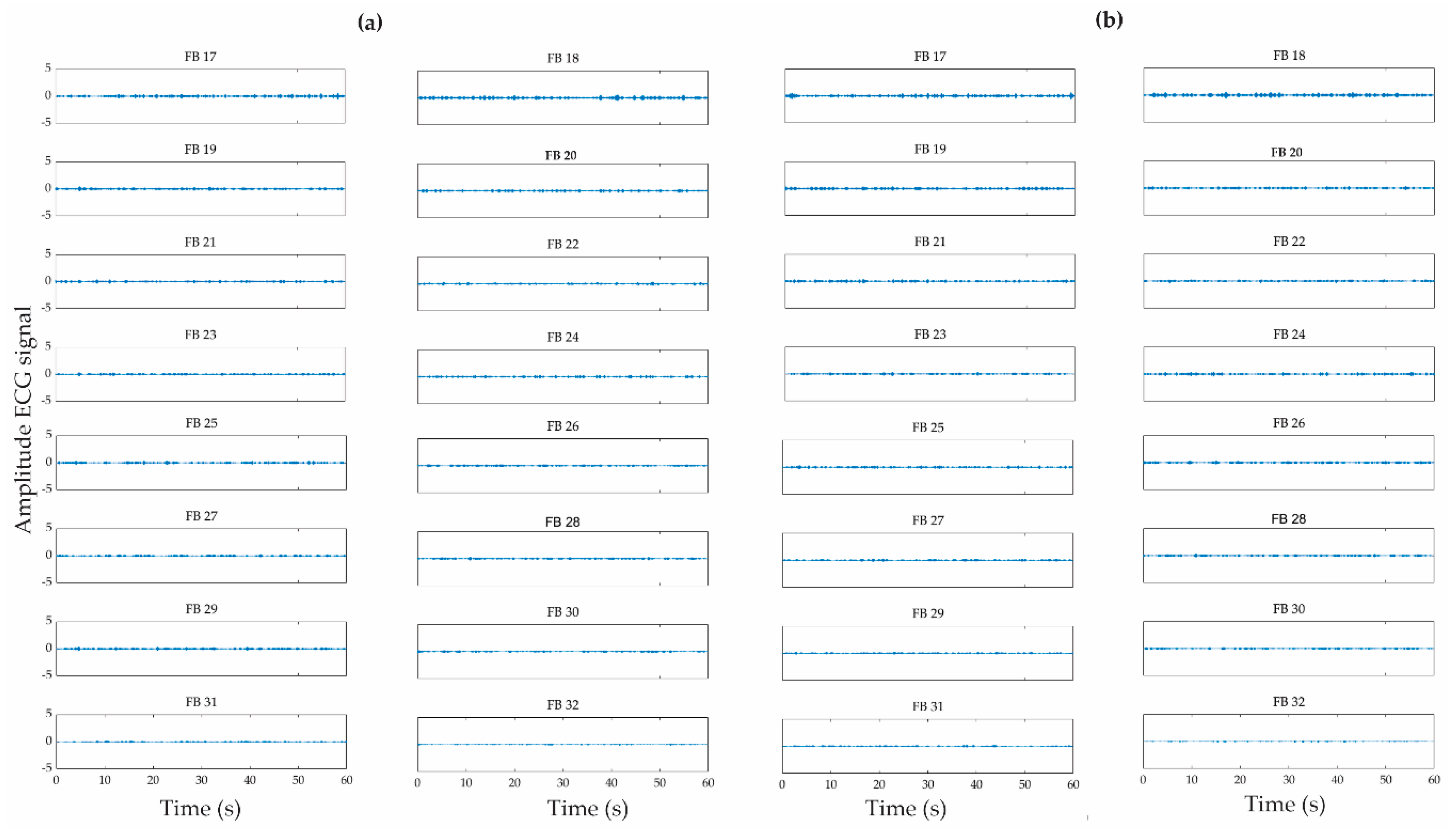
| Patient | Seizure Onset Time (Hours:Minutes:Seconds) |
|---|---|
| 1 | 00:14:36 |
| 2 | 01:02:43 |
| 02:55:51 | |
| 3 | 01:24:34 |
| 4 | 02:34:27 |
| 5 | 00:20:10 |
| 6 | 00:24:07 |
| 00:51:25 | |
| 7 | 02:04:45 |
| 1 min Interval | p-Value | ||
|---|---|---|---|
| CF (FB-9) | CF(FB-13) | IF (FB-12) | |
| 1 | 2.08 × 10−4 | 3.75 × 10−4 | 2.08 × 10−4 |
| 2 | 2.80 × 10−4 | 2.08 × 10−4 | 2.08 × 10−4 |
| 3 | 2.08 × 10−4 | 6.60 × 10−4 | 1.54 × 10−4 |
| 4 | 1.54 × 10−4 | 3.75 × 10−4 | 2.08 × 10−4 |
| 5 | 3.75 × 10−4 | 3.75 × 10−4 | 2.08 × 10−4 |
| 6 | 2.08 × 10−4 | 6.60 × 10−4 | 1.58 × 10−4 |
| 7 | 2.08 × 10−4 | 4.99 × 10−4 | 2.08 × 10−4 |
| 8 | 2.08 × 10−4 | 6.60 × 10−4 | 2.08 × 10−4 |
| 9 | 3.75 × 10−4 | 4.99 × 10−4 | 1.54 × 10−4 |
| 10 | 2.08 × 10−4 | 6.60 × 10−4 | 1.58 × 10−4 |
| 11 | 2.80 × 10−4 | 2.80 × 10−4 | 2.08 × 10−4 |
| 12 | 2.80 × 10−4 | 6.60 × 10−4 | 1.54 × 10−4 |
| 13 | 2.80 × 10−4 | 8.68 × 10−4 | 1.58 × 10−4 |
| 14 | 2.80 × 10−4 | 6.60 × 10−4 | 2.08 × 10−4 |
| 15 | 2.80 × 10−4 | 4.99 × 10−4 | 1.54 × 10−4 |
| Minute | Accuracy (%) | Minute | Accuracy (%) | Minute | Accuracy (%) |
|---|---|---|---|---|---|
| 1 | 100 | 6 | 100 | 11 | 100 |
| 2 | 100 | 7 | 100 | 12 | 100 |
| 3 | 100 | 8 | 100 | 13 | 100 |
| 4 | 100 | 9 | 100 | 14 | 100 |
| 5 | 100 | 10 | 100 | 15 | 100 |
| Work | Signal | Methodology | Prediction Time (Accuracy %) |
|---|---|---|---|
| Popov et al. [21] | HRV |
| 20 min (76.2%) |
| Pavei et al. [22] | HRV |
| 5 min (95.6%) |
| Varon et al. [23] | RRI |
| 30 s (86.1%) |
| Billeci et al. [24] | RRI |
| 10 min (74.6%) |
| Giannakakis et al. [25] | HRV |
| 21.8 s (77.1%) |
| This work | ECG |
| 15 min (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Sanchez, A.V.; Perez-Ramirez, C.A.; Valtierra-Rodriguez, M.; Dominguez-Gonzalez, A.; Amezquita-Sanchez, J.P. Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals. Mathematics 2020, 8, 2125. https://doi.org/10.3390/math8122125
Perez-Sanchez AV, Perez-Ramirez CA, Valtierra-Rodriguez M, Dominguez-Gonzalez A, Amezquita-Sanchez JP. Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals. Mathematics. 2020; 8(12):2125. https://doi.org/10.3390/math8122125
Chicago/Turabian StylePerez-Sanchez, Andrea V., Carlos A. Perez-Ramirez, Martin Valtierra-Rodriguez, Aurelio Dominguez-Gonzalez, and Juan P. Amezquita-Sanchez. 2020. "Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals" Mathematics 8, no. 12: 2125. https://doi.org/10.3390/math8122125
APA StylePerez-Sanchez, A. V., Perez-Ramirez, C. A., Valtierra-Rodriguez, M., Dominguez-Gonzalez, A., & Amezquita-Sanchez, J. P. (2020). Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals. Mathematics, 8(12), 2125. https://doi.org/10.3390/math8122125







