Backward Bifurcation and Optimal Control Analysis of a Trypanosoma brucei rhodesiense Model
Abstract
1. Introduction
2. Methods and Results
2.1. Model Formulation
2.2. Positivity and Boundedness of Solutions
2.3. The Basic Reproduction Number
2.4. Existence and Uniqueness of the Endemic Equilibria
- (i)
- A unique endemic equilibrium if and cases 2 and 4 are satisfied;
- (ii)
- More than one endemic equilibrium if and part of case 3 holds;
- (iii)
- No endemic equilibrium if , and cases 1 and part of case 3 are satisfied.
2.5. Optimal Control
2.5.1. Existence and Uniqueness Results
2.5.2. Characterization of an Optimal Control Pair
3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NTD | Neglected Tropical Disease |
| HAT | Human African Trypanosomiasis |
| ICT | Information and Communication Technology |
| ODE | Ordinary Differential Equation |
References
- World Health Organization. Human African trypanosomiasis (sleeping sickness): Epidemiological update. Wkly. Epidemiol. Rec. 2018, 81, 71–80. [Google Scholar]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar]
- Lutumba, P.; Makieya, E.; Shaw, A.; Meheus, F.; Boelaert, M. Human African Trypanosomiasis in a Rural Community, Democratic Republic of Congo. Emerging Infectious Diseases. Available online: www.cdc.gov/eid (accessed on 8 May 2012).
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. Soc. Lond. Ser. Math. Phys. Eng. Sci. 1927, 115, 700–721. [Google Scholar] [CrossRef]
- Mushayabasa, S.; Posny, D.; Wang, J. Modeling the intrinsic dynamics of foot–and–mouth disease. Math. Biosci. Eng. 2016, 13, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Okosun, K.O.; Ouifki, R.; Marcus, N. Optimal control analysis of a malaria disease transmission model that includes treatment and vaccination with waning immunity. BioSystems 2011, 106, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, X.; Tuncer, N.; Martcheva, M.; Lashari, A. Optimal control of a malaria model with asymptomatic class and superinfection. Math. Biosci. 2017, 288, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kalinda, C.; Mushayabasa, S.; Chimbari, J.M.; Mukaratirwa, S. Optimal control applied to a temperature dependent schistosomiasis model. Biosystems 2017, 175, 47–56. [Google Scholar] [CrossRef]
- Lolika, O.P.; Mushayabasa, S. On the role of short-term animal movements on the persistence of brucellosis. Mathematics 2018, 6, 154. [Google Scholar] [CrossRef]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2018, 70, 1272–1296. [Google Scholar] [CrossRef]
- Mushayabasa, S.; Bhunu, C.P. Modelling the impact of early therapy for latent tuberculosis patients and its optimal control analysis. J. Biol. Phys. 2013, 39, 723–747. [Google Scholar] [CrossRef][Green Version]
- Hargrove, J.W.; Ouifki, R.; Kajunguri, D.; Vale, G.A.; Torr, S.J. Modeling the control of trypanosomiasis using trypanocides or insecticide-treated livestock. PLoS Negl. Trop. Dis. 2012, 6, e1615. [Google Scholar] [CrossRef] [PubMed]
- Kajunguri, D.; Hargrove, J.W.; Ouifki, R.; Mugisha, J.Y.T.; Coleman, P.G.; Welburn, S.C. Modelling the use of insecticide-treated cattle to control tsetse and Trypanosoma brucei rhodiense in a multi-host population. Bull. Math. Biol. 2014, 76, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Shrestha, S.; Tomlinson, K.W.; Vuong, H. Predicting the effect of climate change on African trypanosomiasis: Integrating epidemiology with parasite and vector biology. J. R. Soc. Interface 2012, 9, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.L.; Bouyer, J. Mathematical modeling, spatial complexity, and critical decisions in tsetse control. J. Econ. Entomol. 2012, 105, 1477–1486. [Google Scholar] [CrossRef]
- Stone, C.M.; Chitnis, N. Implications of Heterogeneous Biting Exposure and Animal Hosts on Trypanosomiasis brucei gambiense Transmission and Control. Plos Comput. Biol. 2015, 11, e1004514. [Google Scholar] [CrossRef]
- Artzrouni, M.; Gouteux, J.-P. Estimating tsetse population parameters: Application of a mathematical model with density-dependence. Med. Vet. Entomol. 2003, 17, 272–279. [Google Scholar] [CrossRef]
- Artzrouni, M.; Gouteux, J.-P. A model of Gambian sleeping sickness with open vector populations. Math. Med. Biol. 2001, 18, 99–117. [Google Scholar] [CrossRef]
- Artzrouni, M.; Gouteux, J.-P. Population dynamics of sleeping sickness: A microsimulation. Simul. Gaming 2001, 32, 215–227. [Google Scholar] [CrossRef]
- Artzrouni, M.; Gouteux, J.-P. A compartmental model of sleeping sickness in Central Africa. J. Biol. Syst. 1996, 4, 459–477. [Google Scholar] [CrossRef]
- Rogers, D.J. A general model for the African trypanosomiases. Parasitology 1998, 97, 193–212. [Google Scholar] [CrossRef]
- Rock, K.S.; Ndeffo-Mbah, M.L.; Castaño, S. Assessing strategies against Gambiense sleeping sickness through mathematical modeling. Clin. Infect. Dis. 2018, 66, S286–S292. [Google Scholar] [CrossRef] [PubMed]
- Ndondo, A.M.; Munganga, J.M.W.; Mwambakana, J.N.; Saad-Roy, M.C.; Van den Driessche, P.; Walo, O.R. Analysis of a model of gambiense sleeping sickness in human and cattle. J. Biol. Dyn. 2016, 10, 347–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilbert, J.A.; Medlock, J.; Townsend, J.P.; Aksoy, S.; Mbah, M.N.; Galvani, A.P. Determinants of Human African Trypanosomiasis Elimination via Paratransgenesis. PLoS Negl. Trop. Dis. 2016, 10, e0004465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rock, K.S.; Torr, S.J.; Lumbala, C.; Keeling, M.J. Predicting the impact of intervention strategies for sleeping sickness in two high-endemicity health zones of the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2017, 11, e0005162. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.S.; Torr, S.J.; Lumbala, C.; Keeling, M.J. Quantitative evaluation of the strategy to eliminate human African trypanosomiasis in the Democratic Republic of Congo. Parasit. Vectors 2015, 8, 532. [Google Scholar] [CrossRef]
- Rock, K.S.; Stone, C.M.; Hastings, I.M.; Keeling, M.J.; Torr, S.J.; Chitnis, N. Mathematical models of human African trypanosomiasis epidemiology. Adv. Parasitol. 2015, 87, 53–133. [Google Scholar]
- Randolph, W.; Viswanath, K. Lessons learned from public health mass media campaigns: marketing health in a crowded media world. Annu. Rev Public Health 2004, 25, 419–437. [Google Scholar] [CrossRef]
- Apollonio, D.E.; Malone, R.E. Turning negative into positive: Public health mass media campaigns and negative advertising. Health Educ. Res. 2009, 24, 483–495. [Google Scholar] [CrossRef]
- Noar, S.M. A 10-year retrospective of research in health mass media campaigns: where do we go from here? J. Health Commun. 2006, 11, 21–42. [Google Scholar] [CrossRef]
- Leak, S.G.A. Tsetse vector population dynamics: ILRAD’s Requirements. In Modelling Vector-Borne and Other Parasitic Diseases; Hansen, J.W., Perry, B.D., Eds.; International Livestock Research Institute (ILRI): Nairobi, Kenya, 1994; p. 36. Available online: https://books.google.co.zw/books?isbn=9290552972 (accessed on 8 May 2012).
- van den Driessche, P.; Watmough, J. Reproduction number and subthreshold endemic equilibria for compartment models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Gumel, A.B. Causes of backward bifurcation in some epidemiological models. J. Math. Anal. Appl. 2012, 395, 355–365. [Google Scholar] [CrossRef]
- Silva, C.J.; Maurer, H.; Torres, D.F.M. Optimal control of a tuberculosis model with state and control delays. Math. Biosci. Eng. 2017, 14, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Lukes, D.L. Differential Equations: Classical to Controlled, Mathematics in Science and Engineering; Academic Press: New York, NY, USA, 1982; Volume 162. [Google Scholar]
- Pontryagin, L.S.; Boltyanskii, V.T.; Gamkrelidze, R.V.; Mishcheuko, E.F. The Mathematical Theory of Optimal Processes; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models; Chapman and Hall/CRC: London, UK, 2007. [Google Scholar]
- Chávez, J.P.; Götz, T.; Siegmund, S.; Wijaya, K.P. Wijaya An SIR-Dengue transmission model with seasonal effects and impulsive control. Math. Biosci. 2017, 1, 29–39. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Description | Value | Units | |
|---|---|---|---|---|
| Transmission rate of HAT disease from infected human to susceptible vector | day | [14] | ||
| Transmission rate of HAT disease from infected animal to susceptible vector | day | [14] | ||
| Transmission rate of HAT disease from infected vector to susceptible human | day | [14] | ||
| Transmission rate of HAT disease from infected vector to susceptible animal | day | [14] | ||
| Progression rate of human population from recovered to susceptible class | day | [23] | ||
| Progression rate of animal population from recovered to susceptible class | day | [23] | ||
| Rate at which humans become aware of the disease | 0.2 | day | ||
| Natural mortality rate of human population | day | [23] | ||
| Natural mortality rate of animal population | day | [23] | ||
| Natural mortality rate of vector population | day | [23] | ||
| Recovery rate of infected human | day | [23] | ||
| Recovery rate of infected animal | day | [23] |
| Case | A | B | C | Reproduction Number | No. of Sign Changes | No. of Possible Positive Real Roots |
|---|---|---|---|---|---|---|
| 1 | + | + | + | 0 | 0 | |
| 2 | + | + | - | 1 | 1 | |
| 3 | + | - | + | 2 | 0,2 | |
| 4 | + | - | - | 1 | 1 |
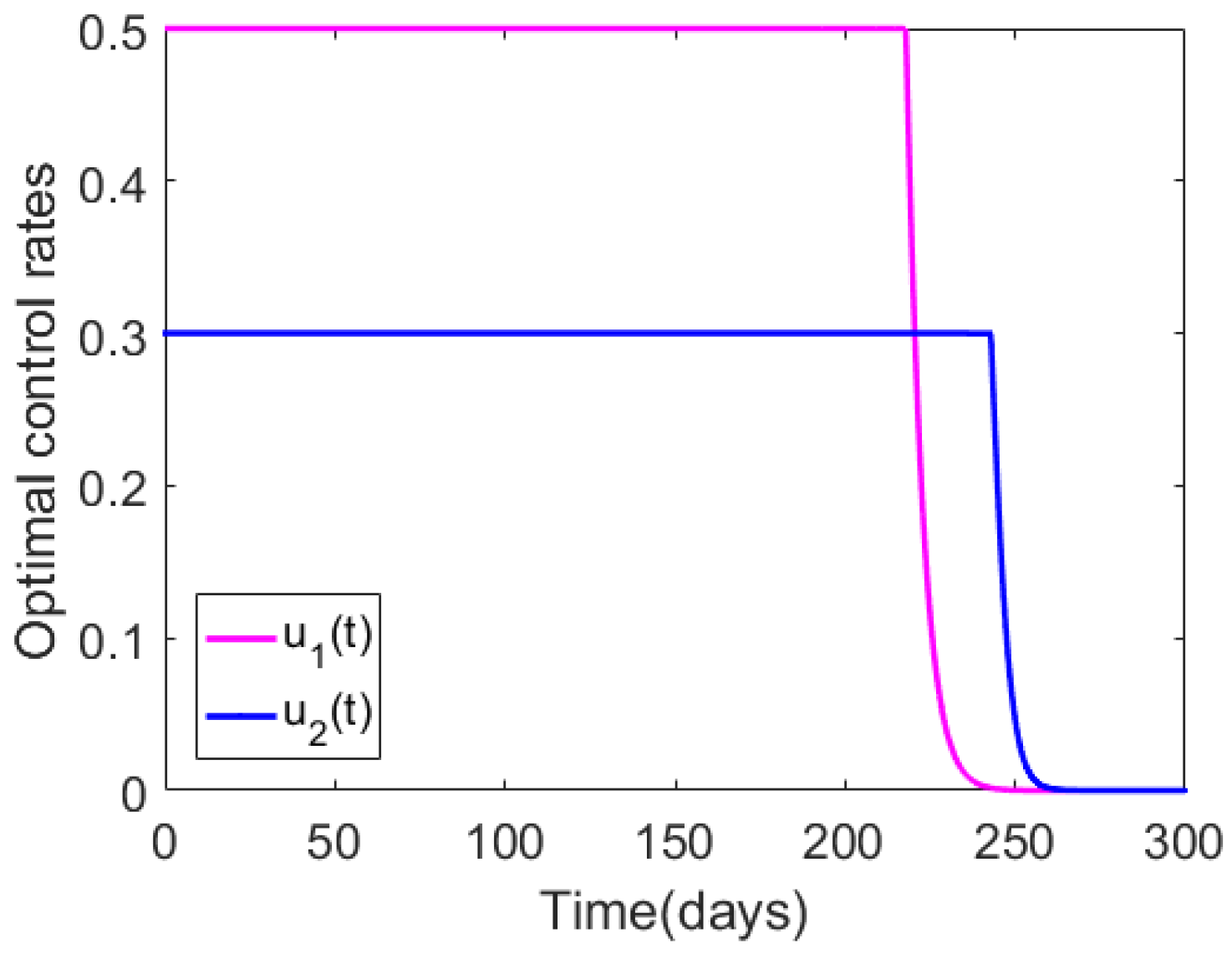
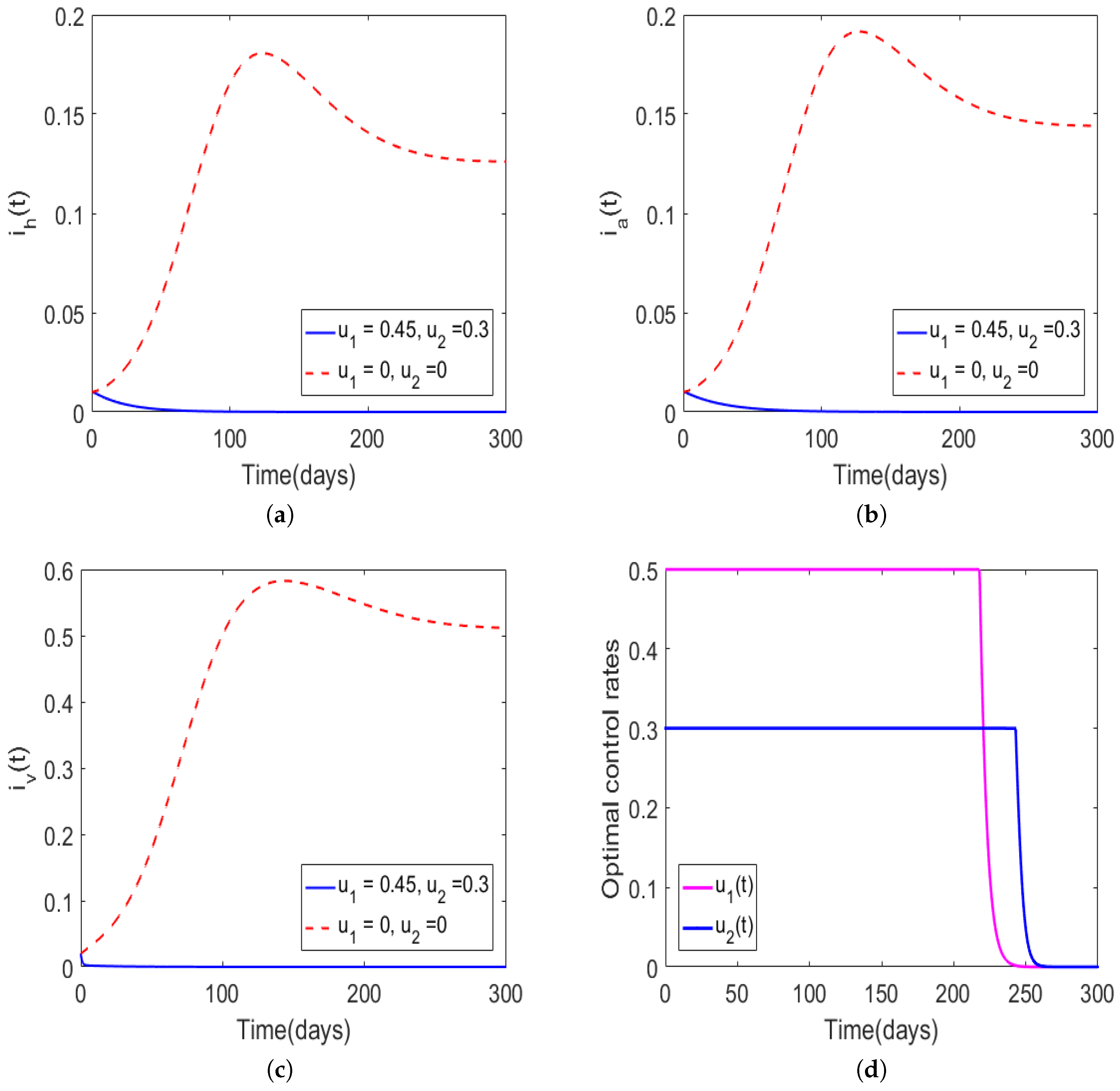
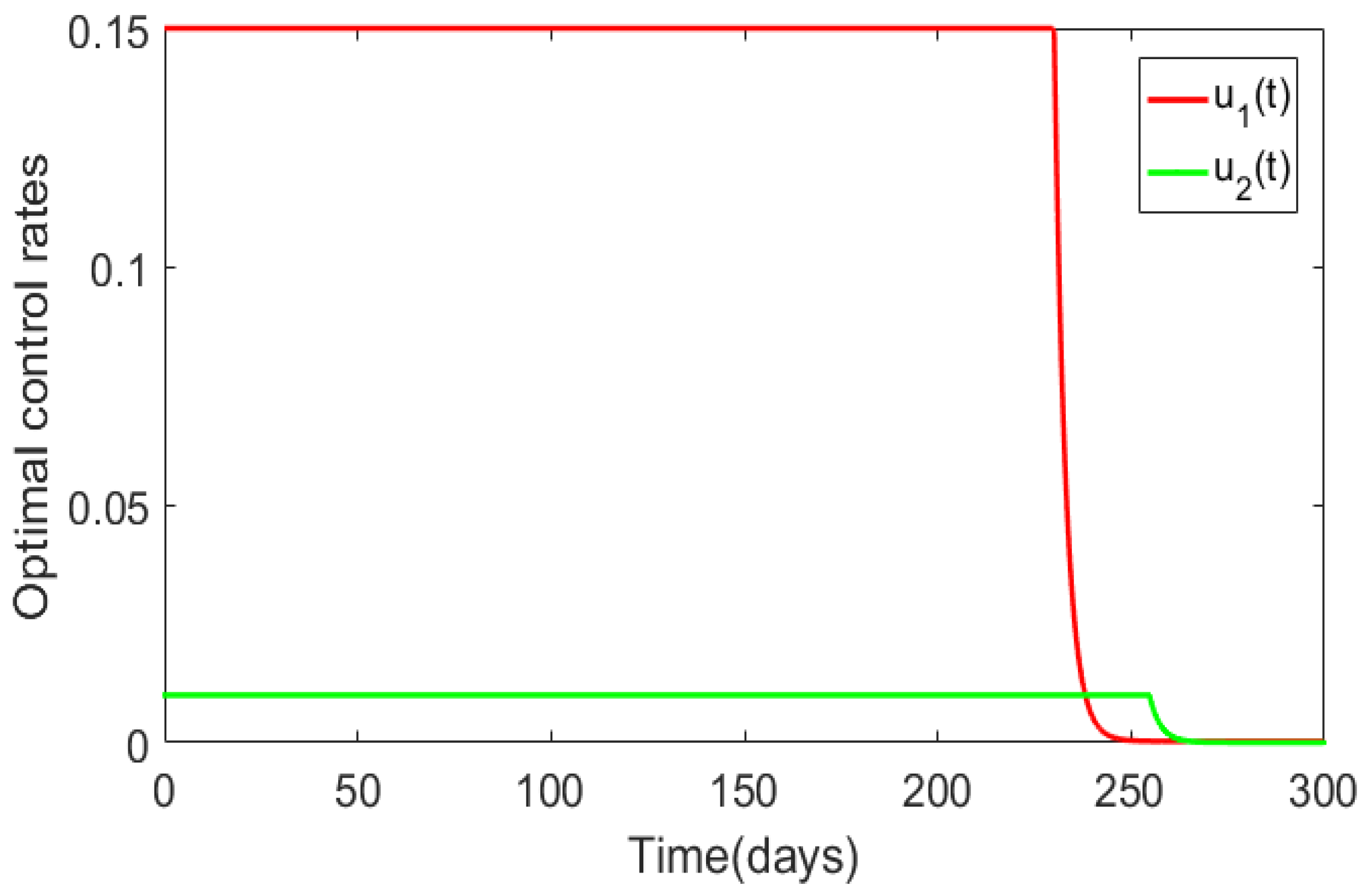
| Case | Host | Total Number of New Infections Observed with Optimal Control | Infections Averted Due to Implementation of Optimal Control |
|---|---|---|---|
| Figure 2 | Human population | ||
| Animal population | |||
| Figure 4 | Human population | ||
| Animal population | |||
| Figure 5 | Human population | ||
| Animal population |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helikumi, M.; Kgosimore, M.; Kuznetsov, D.; Mushayabasa, S. Backward Bifurcation and Optimal Control Analysis of a Trypanosoma brucei rhodesiense Model. Mathematics 2019, 7, 971. https://doi.org/10.3390/math7100971
Helikumi M, Kgosimore M, Kuznetsov D, Mushayabasa S. Backward Bifurcation and Optimal Control Analysis of a Trypanosoma brucei rhodesiense Model. Mathematics. 2019; 7(10):971. https://doi.org/10.3390/math7100971
Chicago/Turabian StyleHelikumi, Mlyashimbi, Moatlhodi Kgosimore, Dmitry Kuznetsov, and Steady Mushayabasa. 2019. "Backward Bifurcation and Optimal Control Analysis of a Trypanosoma brucei rhodesiense Model" Mathematics 7, no. 10: 971. https://doi.org/10.3390/math7100971
APA StyleHelikumi, M., Kgosimore, M., Kuznetsov, D., & Mushayabasa, S. (2019). Backward Bifurcation and Optimal Control Analysis of a Trypanosoma brucei rhodesiense Model. Mathematics, 7(10), 971. https://doi.org/10.3390/math7100971





