Fractional Modeling and Dynamic Analysis of COVID-19 Transmission with Computational Simulations
Abstract
1. Introduction
2. Preliminaries
3. Fractional COVID-19 Model with an Immunization Structure
3.1. Definition of State Variables and Model Parameters
3.1.1. Susceptible Class
3.1.2. Recovered Class
3.1.3. Vaccinated Class
3.1.4. Mild Infection Class
3.1.5. Severe Infection Class
3.1.6. Death Class
3.2. A Fractional Approach Utilizing the Caputo Operator
4. Qualitative Analysis of a Fractional COVID-19 Model
- There is a constant verifying:
- Consider a nonnegative, nondecreasing continuous mapping that complies with the condition:alongside a suitable function such that
- Let be constants chosen in accordance with the inequality:
5. Fixed Points and Basic Reproduction Number
5.1. Disease-Free Equilibrium (DFE)
5.2. Endemic Equilibrium (EE)
5.3. Basic Reproduction Number
6. Stability Insights
6.1. Stability Analysis Near the Disease-Free Equilibrium
6.2. Stability of Ulam–Hyers
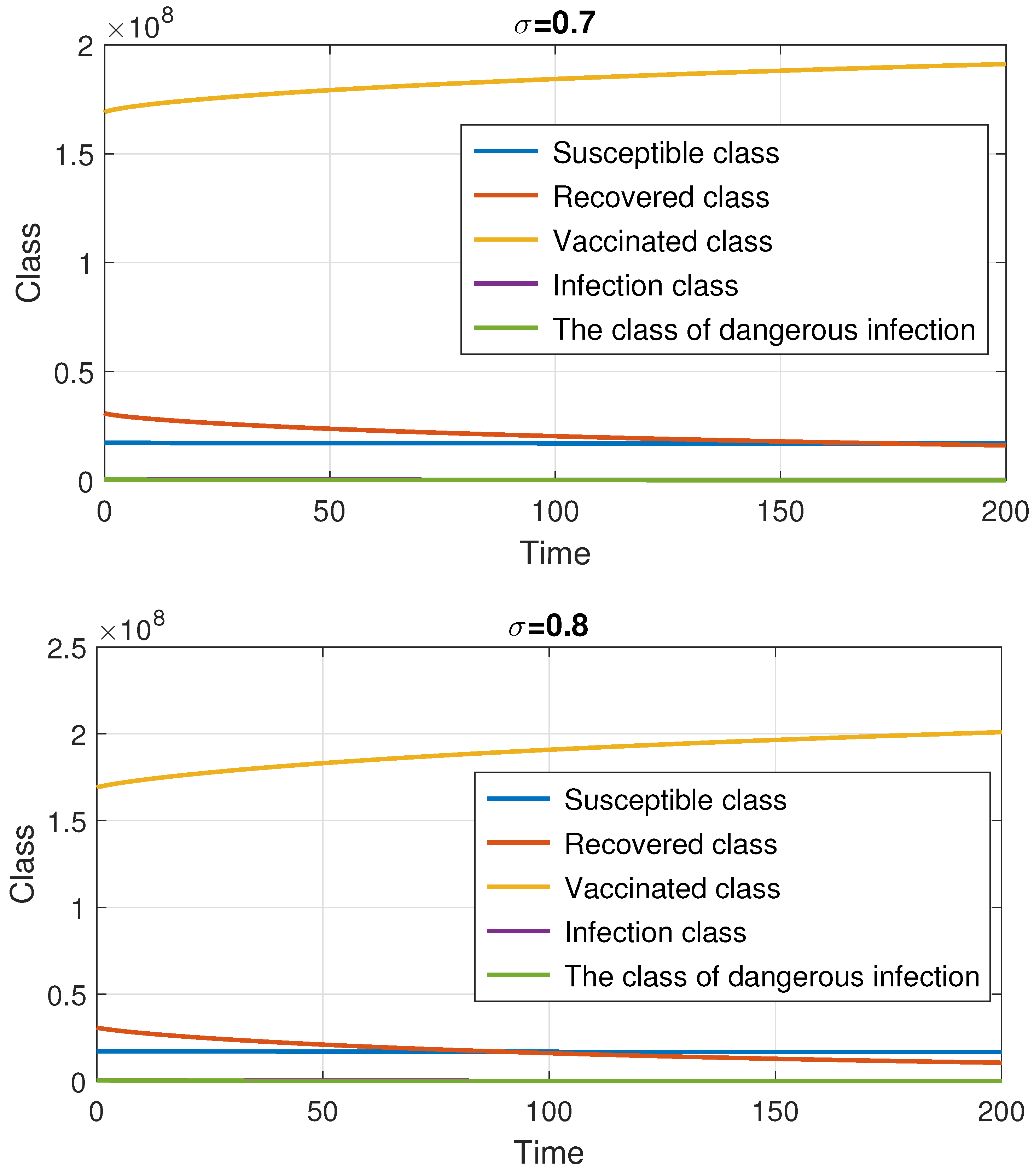
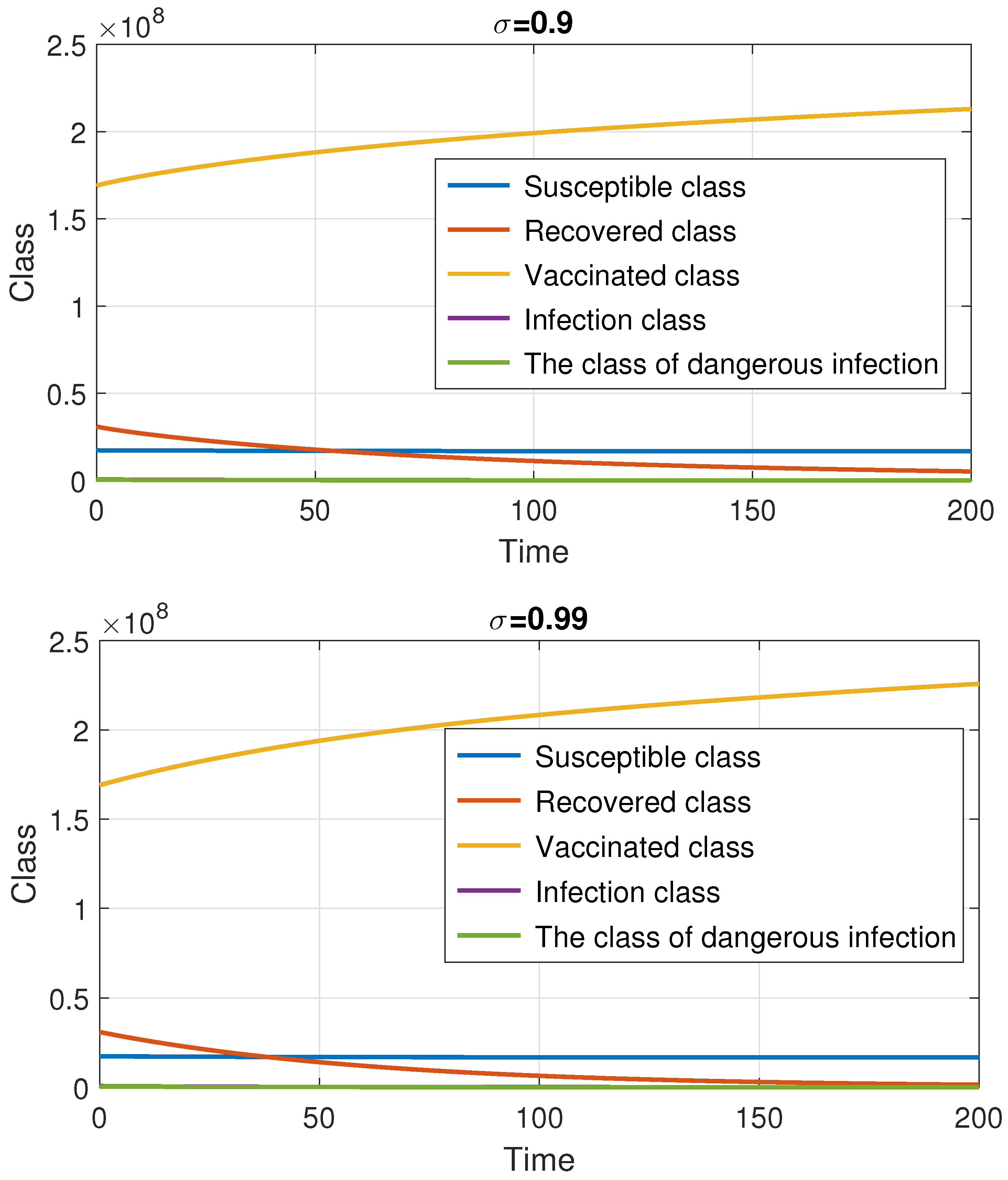
7. Numerical Method and Simulations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thanin, S.; Anwar, Z.; Saowaluck, C.; Zohreh, E.; Mouhcine, T.; Salih, D. Analysis of a discrete mathematical COVID-19 model. Results Phys. 2021, 28, 104668. [Google Scholar] [CrossRef]
- Kermack, W.; McKendrick, A. Contributions to the mathematical theory of epidemics. Proc. R. Soc. A 1927, 115, 700–721. [Google Scholar]
- Ruan, S.; Wang, W. Dynamical behavior of an epidemic mode with a nonlinear incidence rate. J. Differ. Equ. 2003, 188, 135–163. [Google Scholar] [CrossRef]
- Korobeinikov, A. Global properties of infectious disease models with nonlinear incidence. Bull. Math. Biol. 2007, 69, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- De la Sen, M.; Alonso-Quesada, S. Vaccination strategies based on feedback control techniques for a general SEIR-epidemic model. Appl. Math. Comput. 2011, 218, 3888–3904. [Google Scholar] [CrossRef]
- Korobeinikov, A. Global properties of basic virus dynamics models. Bull. Math. Biol. 2004, 66, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.O.; Zubelli, J.P. Global stability for a class of virus models with cytotoxic T lymphocyte immune response and antigenic variation. Bull. Math. Biol. 2011, 73, 609–625. [Google Scholar] [CrossRef]
- Huo, H.F.; Dang, Y.S.-J.; Li, N. Stability of a two-strain tuberculosis model with general contact rate. Abstr. Appl. Anal. 2010, 2010, 293747. [Google Scholar] [CrossRef]
- Hethcote, H.W. Three basic epidemiological models. In Applied Mathematical Ecology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 119–144. [Google Scholar]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Baba, I.A.; Nasidi, B.A. Fractional order epidemic model for the dynamics of novel COVID-19. Alex. Eng. J. 2021, 60, 537–548. [Google Scholar] [CrossRef]
- Bhatter, S.; Bhatia, B.; Kumawat, S.; Purohit, S.D. Modeling and Simulation of COVID-19 Disease Dynamics via Caputo Fabrizio Fractional Derivative. Comput. Methods Differ. Equ. 2024, 13, 494–504. [Google Scholar]
- Kittaneh, F. Numerical radius inequalities for Hilbert space operators. Stud. Math. 2005, 168, 73–80. [Google Scholar] [CrossRef]
- Sababheh, M.; Moradi, H.R. More accurate numerical radius inequalities (I). Linear Multilinear Algebra 2021, 69, 1964–1973. [Google Scholar] [CrossRef]
- Barakat, M.A.; Saadeh, R.; Hyder, A.-A.; Qazza, A.; Aly, A.M. A Novel Fractional Model Combined with Numerical Simulation to Examine the Impact of Lockdown on COVID-19 Spread. Fractal Fract. 2024, 8, 702. [Google Scholar] [CrossRef]
- Gozalpour, N.; Badfar, E.; Nikoofard, A. Transmission dynamics of novel coronavirus SARS-CoV-2 among healthcare workers, a case study in Iran. Nonlinear Dyn. 2021, 105, 3749–3761. [Google Scholar] [CrossRef] [PubMed]
- De la Sen, M.; Alonso-Quesada, S.; Ibeas, A. On a discrete SEIR epidemic model with exposed infectivity, feedback vaccination and partial delayed re-susceptibility. Mathematics 2021, 9, 520. [Google Scholar] [CrossRef]
- Kumar, P.; Erturk, V.S.; Murillo-Arcila, M. A new fractional mathematical modelling of COVID-19 with the availability of vaccine. Results Phys. 2021, 24, 104213. [Google Scholar] [CrossRef]
- De la Sen, M.; Ibeas, A. On an SE(Is)(Ih)AR epidemic model with combined vaccination and antiviral controls for COVID-19 pandemic. Adv. Differ. Equ. 2021, 2021, 92. [Google Scholar] [CrossRef]
- De la Sen, M.; Ibeas, A.; Nistal, R. About partial reachability issues in an SEIR epidemic model and related infectious disease tracking in finite time under vaccination and treatment controls. Discret. Dyn. Nat. Soc. 2021, 2021, 1026–1226. [Google Scholar] [CrossRef]
- Barakat, M.A.; Hyder, A.A.; Rizk, D. New fractional results for Langevin equations through extensive fractional operators. AIMS Math. 2023, 8, 6119–6135. [Google Scholar] [CrossRef]
- Rashid, S.; Jarad, F.; Alsharidi, A.K. Numerical investigation of fractional-order cholera epidemic model with transmission dynamics via fractal–fractional operator technique. Chaos Solitons Fractals 2022, 162, 112477. [Google Scholar] [CrossRef]
- Rashid, S.; Kubra, K.T.; Sultana, S.; Agarwal, P.; Osman, M.S. An approximate analytical view of physical and biological models in the setting of Caputo operator via Elzaki transform decomposition method. J. Comput. Appl. Math. 2022, 413, 114378. [Google Scholar] [CrossRef]
- Selvam, A.; Sabarinathan, S.; Noeiaghdam, S.; Govindan, V. Fractional Fourier Transform and Ulam Stability of Fractional Differential Equation with Fractional Caputo-Type Derivative. J. Funct. Spaces 2022, 2022, 3777566. [Google Scholar] [CrossRef]
- Agarwal, P.; Baleanu, D.; Chen, Y.; Momani, S.; Machado, J.T. Fractional calculus. In Proceedings of the International Workshop on Advanced Theory and Applications of Fractional Calculus (ICFDA), Amman, Jordan, 16–18 July 2019. [Google Scholar]
- Omame, A.; Okuonghae, D.; Nwajeri, U.K.; Onyenegecha, C.P. A fractional-order multi-vaccination model for COVID-19 with non-singular kernel. Alex. Eng. J. 2022, 61, 6089–6104. [Google Scholar] [CrossRef]
- Al-Shbeil, I.; Djenina, N.; Jaradat, A.; Al-Husban, A.; Ouannas, A.; Grassi, G. A New COVID-19 Pandemic Model including the Compartment of Vaccinated Individuals: Global Stability of the Disease-Free Fixed Point. Mathematics 2023, 11, 576. [Google Scholar] [CrossRef]
- Damag, F.H. On Comparing Analytical and Numerical Solutions of Time Caputo Fractional Kawahara Equations via Some Techniques. Mathematics 2025, 13, 2995. [Google Scholar] [CrossRef]
- Abbas, M.; Ciobanescu, C.; Asghar, M.W.; Omame, A. Solution Approximation of Fractional Boundary Value Problems and Convergence Analysis Using AA-Iterative Scheme. AIMS Math. 2024, 9, 13129–13158. [Google Scholar] [CrossRef]
- Kilbas, A.; Srivastava, H.; Trujillo, J. Theory and Applications of Fractional Differential Equations; North-Holland Mathematics Studies—Elsevier: Amsterdam, The Netherlands, 2006; Volume 204. [Google Scholar]
- Dunford, N.; Schwartz, J. Linear Operators. Part I: General Theory; Interscience Publishers (John Wiley & Sons): New York, NY, USA, 1958; Interscience Tracts in Pure and Applied Mathematics, Volume 7. [Google Scholar]
- Zeidler, E. Nonlinear Functional Analysis and Its Applications: Fixed-Point Theorems; Springer: New York, NY, USA, 1986. [Google Scholar]
- Diethelm, K.; Ford, N.J.; Freed, A.D. Detailed error analysis for a fractional Adams method. Numer. Algorithms 2004, 36, 31–52. [Google Scholar] [CrossRef]
- Diethelm, K.; Ford, N.J.; Freed, A.D. A predictor-corrector approach for the numerical solution of fractional differential equations. Nonlinear Dyn. 2002, 29, 3–22. [Google Scholar] [CrossRef]
| Symbol | Meaning |
|---|---|
| Rate at which new individuals enter | |
| Proportion of infected individuals with mild symptoms | |
| Infection rate from and to | |
| Reinfection rate from and to | |
| Infection rate for vaccinated individuals in | |
| Vaccination rate for and | |
| Rate at which individuals recover (from and to ) | |
| Natural mortality rate | |
| Epidemic-related death rate (applies to ) | |
| Average number of contacts per individual per unit time | |
| Probability of infection (subscript i indicates the class) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarady, M.M.; Barakat, M.A.; Darwish, M.M. Fractional Modeling and Dynamic Analysis of COVID-19 Transmission with Computational Simulations. Mathematics 2025, 13, 3619. https://doi.org/10.3390/math13223619
Alarady MM, Barakat MA, Darwish MM. Fractional Modeling and Dynamic Analysis of COVID-19 Transmission with Computational Simulations. Mathematics. 2025; 13(22):3619. https://doi.org/10.3390/math13223619
Chicago/Turabian StyleAlarady, Mohamed. M., Mohamed A. Barakat, and Mohamed M. Darwish. 2025. "Fractional Modeling and Dynamic Analysis of COVID-19 Transmission with Computational Simulations" Mathematics 13, no. 22: 3619. https://doi.org/10.3390/math13223619
APA StyleAlarady, M. M., Barakat, M. A., & Darwish, M. M. (2025). Fractional Modeling and Dynamic Analysis of COVID-19 Transmission with Computational Simulations. Mathematics, 13(22), 3619. https://doi.org/10.3390/math13223619







