Ant Colony and Whale Optimization Algorithms Aided by Neural Networks for Optimum Skin Lesion Diagnosis: A Thorough Review
Abstract
1. Introduction
- (A)
- Promotes the potential for handling big data and the analysis of complicated algebraic problems.
- (B)
- Provides a flexible investigation of nonlinear correlations.
- (C)
- Elevates rates of detection, recognition, and classification efficiency.
- The study conducts a comprehensive review of the recent literature to highlight the use of machine learning (ML) and deep learning (DL) techniques in skin cancer detection in combination with optimization algorithms for these techniques, which include the ant colony (ACO) and whale optimization (WOA) algorithms.
- The study presents a comparative analysis of ML and DL methods, including ACO, WOA, and NNs, for the classification of skin lesions.
- The study lists several limitations of these techniques, and demonstrates the importance of improving these algorithms, especially in the field of skin diseases. This will lead to accurate diagnosis, assist dermatologists in decision-making, and improve patient outcomes by facilitating early detection and treatment of skin lesions.
- The study provides recommendations and proposals to increase the level of performance of detection and classification models in this field by integrating the two algorithms, (ACO) and (WOA), with NNS.
- Section 2 explains the main research method adopted to execute the thorough overview. It clarifies the considered criteria of the secondary data collection procedure to cover essential ideas and prominent aspects of ACO and WOA in identifying different types of SLDs precisely when supported with NNs.
- Section 3 illustrates the involvement of ACO and WOA in recognizing various SLDs accurately, shedding light on variant breakthroughs and state of the arts that corresponded to more elaborations on the performance of ACO and WOA in conducting their identification tasks of SLDs.
- Section 4 indicates an overview of numerous outputs correlated with the utilization of ACO, WOA, or both in carrying out the necessary classification of SLDs from different research articles.
- Section 5 explores how healthcare providers can overcome challenges in skin lesion detection by leveraging advanced techniques such as ACO, WOA, and NNs. We will discuss the importance of accurate diagnosis and classification, particularly in cases where early detection is crucial for improved patient outcomes. Additionally, we will examine how traditional methods can be complemented or replaced by these intelligent algorithms to enhance accuracy and efficiency in skin lesion detection.
- Section 6 classifies the research’s main future work and recommendation opportunities that can offer areas for improving the robustness of this work.
- Section 7 presents concise conclusions drawn from our study. We summarize key findings regarding the application of AI, DL, ML, NNs, ACO, and WOA in skin lesion detection. Our conclusions highlight the effectiveness of integrating these techniques for improving diagnostic accuracy and patient outcomes in dermatology. We also identify potential avenues for future research to further advance the field.
- Finally, Section 8 shows the major research limitations encountered in completing this overview. Finally, all abbreviations are presented in Appendix A.
2. Materials and Methods
Validation of the Reliability, Contributions, and Efficacy of the Manuscript’s Outputs
- Expert Judgment: The manuscript may have undergone evaluation by experts in the fields of AI, DL, ML, and dermatology. These experts likely provided feedback and insights regarding the relevance, accuracy, and significance of the study’s outputs.
- Peer Review: The manuscript might have been subjected to a peer review process, where independent experts in the field critically evaluated the study’s methodology, results, and conclusions. Peer reviewers would have assessed the study’s rigor, validity, and contribution to the existing body of knowledge.
- Professional Perspectives: Input from professionals specializing in AI, DL, ML, and dermatology could have been solicited to validate the manuscript’s outputs. Their perspectives and insights would have provided additional validation and confirmation of the study’s findings.
- Comparative Analysis: The study may have compared its findings with those of other relevant research studies to ensure consistency and accuracy. This comparative analysis would help validate the reliability and contributions of the manuscript’s outputs within the broader context of existing literature.
3. Background
3.1. Brief Illustration of SLD Prevalence Universally
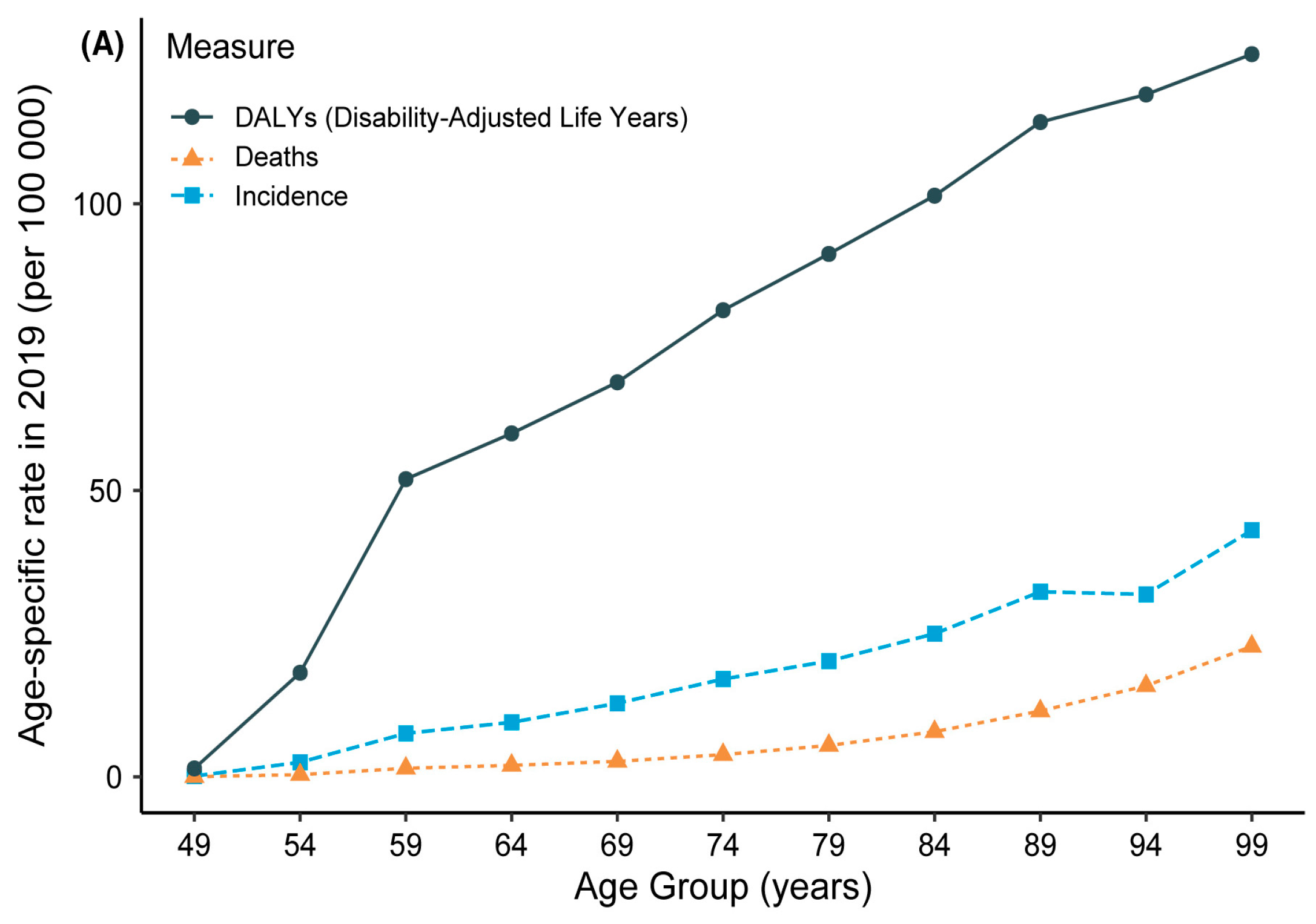
- Prevalence of Skin Lesion Disorders: In 2018, approximately 300,000 new cases of melanoma were detected globally, making it the most prevalent cancer among both men and women. Over one million new cases of Squamous Cell Carcinoma (SCC) and Basal Cell Carcinoma (BCC) were diagnosed in the same year, ranking them as the second and third most common forms of skin cancer after melanoma, respectively [7].
- Impact of Ozone Depletion and UV Exposure: The depletion of the ozone layer, which protects against harmful ultraviolet (UV) rays, has led to a considerable increase in skin cancer cases due to heightened UV exposure. Excessive exposure to harmful sunbeams, especially more than five sunburns, can double the risk of skin cancer and melanoma [7].
- Mortality Rates and Survival Rate: Skin cancer, including melanoma, is associated with significant mortality rates. Over two individuals die due to skin cancer in the USA every hour. However, the survival rate for skin cancer, especially when treated and managed during its early stages, can reach up to 90 percent [6].
- Trends in Melanoma Cases: Invasive melanoma is expected to be the fourth most recognized cancer type in both sexes in 2022, with a projected 57,180 cases in men and 42,600 occurrences in women. Melanoma cases have demonstrated an increasing trend and a higher frequency of observed cases in the USA over the past three decades, with a doubling of the rate in 2011 compared to cases recorded in 1982 [6].
3.2. Crucial Features and Practical Merits of ACO for the Medical Context
- Feature Selection: ACO can be used to select the most relevant features from skin lesion images. By considering the importance of different features (such as texture, color, and shape) through the exploration of feature space, ACO helps in identifying the most discriminative features for classification.
- Optimization of Classifier Parameters: ACO can optimize the parameters of machine learning or deep learning classifiers used for skin lesion classification. This optimization process helps in fine-tuning the classifier to achieve better performance in terms of accuracy and robustness [23].
- Enhanced Adaptability: ACO’s ability to adapt to dynamic and varying datasets is particularly advantageous in skin lesion classification, where images may exhibit diverse characteristics due to factors like lighting conditions, camera angles, and patient demographics. ACO helps classifiers adapt to these variations, leading to more consistent and reliable classification results [24].
- Improved Interpretability: ACO-based feature selection and parameter optimization techniques often result in more interpretable classifiers. This means that the reasoning behind the classification decisions can be better understood, which is crucial for medical applications like skin lesion diagnosis [25].
- Reduced Overfitting: ACO’s optimization process can help in preventing overfitting, a common issue in machine learning models where the model performs well on training data but fails to generalize to unseen data. By optimizing model parameters in a principled manner, ACO aids in building classifiers that generalize better to new skin lesion images [25].
- Scalability: ACO algorithms are scalable and can handle large datasets efficiently. This scalability is important in skin lesion classification, where datasets may contain thousands or even millions of images [26].
- Overall, by leveraging ACO techniques in skin lesion classification, researchers and practitioners can achieve more accurate, robust, and interpretable classification models, ultimately leading to improved diagnosis and patient outcomes. However, because of its workability in different classification situations of SLDs, various researchers, including, clarified ACO’s potential in classifying the type of skin problem, relying on visual optimization, preprocessing, similarity evaluation, augmentation, and filtering of investigated images.
3.3. Serviceable Practicalities and Efficient Features of WOA for the Medical Context
3.3.1. Features and Merits of WOA
- Versatility and Effectiveness: WOA has gained significant attention in the optimization community due to its effectiveness in solving diverse problems [36]. Researchers have explored extensions and hybrids to improve its performance across different domains.
- Popularity Among Researchers: Despite the no-free-lunch theorem’s assertion, WOA’s versatility in handling various optimization problems has made it a popular choice among researchers [37].
3.3.2. Feature Selection and Classification
- Feature Selection Process: Feature selection involves identifying relevant features from input data essential for accurate classification [27]. ACO and WOA algorithms optimize feature selection iteratively, aiming to maximize classification accuracy.
- Contribution to Classification: Selecting discriminative features improves classification efficiency and effectiveness. ACO and WOA algorithms optimize feature selection by considering feature importance, correlation, and interaction, leading to better classification outcomes [38].
3.3.3. Mathematical Evaluation of WOA
3.4. Engagement of ACO and WOA in SLD Identification
3.5. New Cutting-Edge Concepts and Novel Approaches Involved in Skin Lesion Detection Process
- Deep Convolutional Neural Networks (D-CNNs): D-CNNs are a type of deep learning model specifically designed for image classification tasks. They excel in detecting intricate patterns and features within medical images, particularly in dermoscopy images used for skin lesion classification. By leveraging D-CNNs, researchers can achieve higher accuracy in classifying skin lesions into different categories, such as melanoma, nevus, and seborrheic keratosis. These networks contribute to overcoming challenges by providing accurate and reliable automated screening systems for early detection of malignant skin lesions, thereby assisting clinicians in timely diagnosis and treatment.
- Ensemble Learning: Ensemble learning involves combining multiple models to improve predictive performance over any single model. In the context of skin lesion detection, researchers combine the outputs of multiple deep neural network architectures to enhance classification accuracy. By aggregating the classification results of different models, ensemble learning compensates for individual model weaknesses and enhances overall performance. This approach helps address challenges related to the limited availability of training data and variability in lesion characteristics by leveraging the collective knowledge of diverse models.
- Fusion-Based Aggregation Methods: Fusion-based aggregation methods involve combining the outputs of individual neural networks using various fusion strategies. These methods aim to integrate complementary information from different models to make more accurate predictions. By selecting the most effective fusion strategy, researchers optimize classification performance and overcome challenges associated with variability in lesion appearance and classification difficulty. Fusion-based aggregation methods enhance the robustness and reliability of skin lesion classification systems, improving diagnostic accuracy and patient outcomes.
3.5.1. Neural Networks (NNs)
3.5.2. Multi-Layered Perceptron (MLP)
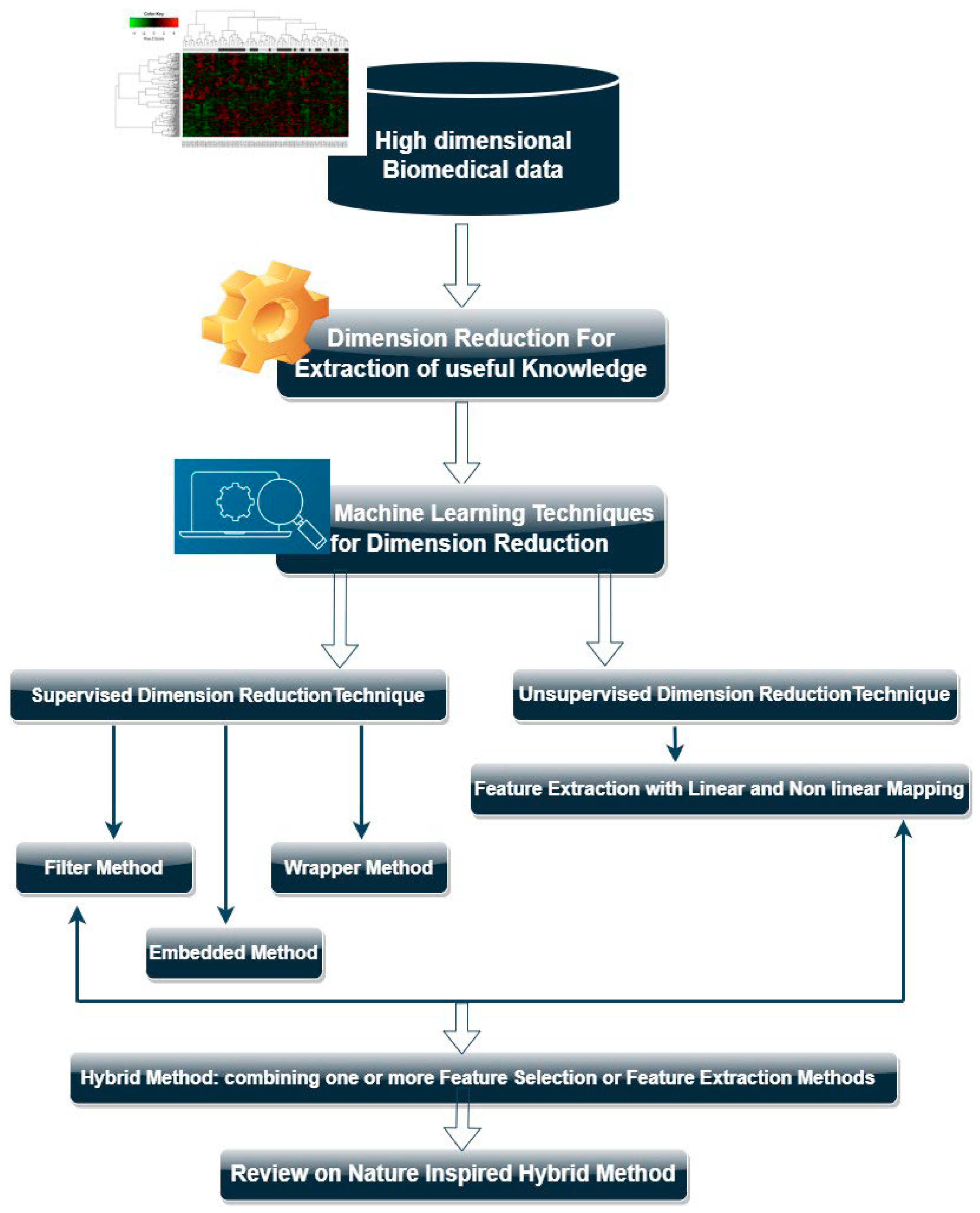
3.5.3. Data Dimensionality Reduction Techniques
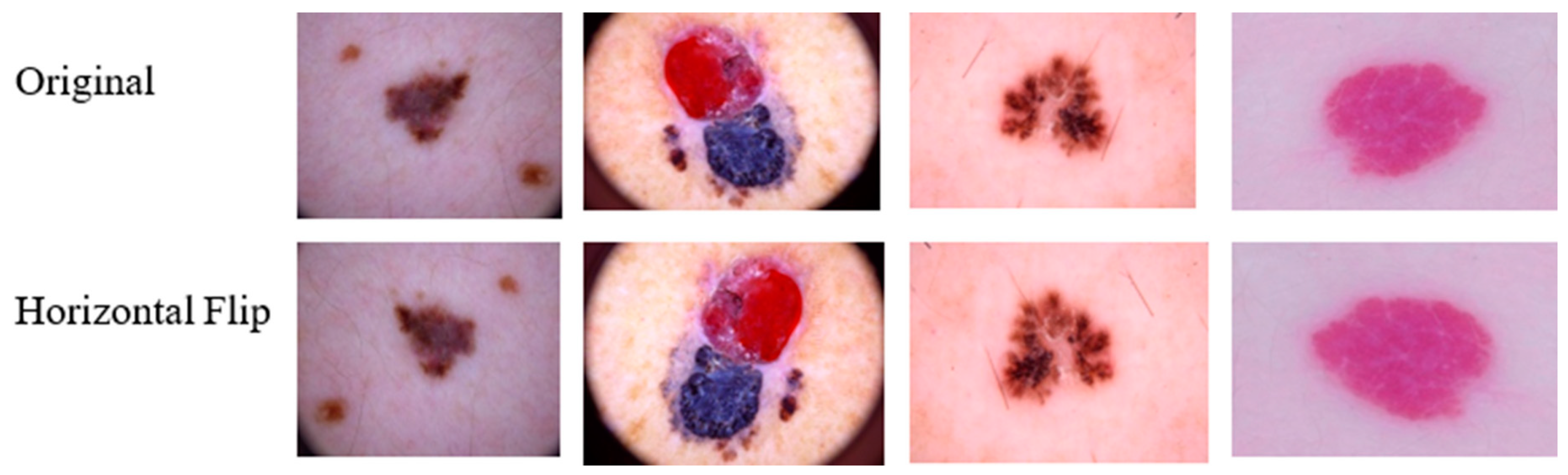
3.5.4. Data Augmentation
3.6. Commonly Examined Datasets Related to SLD
3.6.1. Prevalence of Skin Lesion Disorders
3.6.2. Impact of Ozone Depletion and UV Exposure
3.6.3. Mortality Rates and Survival Rate
3.6.4. Trends in Melanoma Cases
4. Contributary Statistical Outcomes and Medical Gains of ACO, WOA, and NNs
- Superiority of Intelligent Algorithms: The study demonstrates the superiority of intelligent algorithms such as ACO (Ant Colony Optimization) and WOA (Whale Optimization Algorithm) over traditional methods inefficiently classifying skin lesion disorders. This suggests that leveraging advanced computational techniques can lead to more accurate and reliable diagnoses [57].
- Optimal Detection of Skin Lesions: The comparative analysis reveals that AI, DL, and ML approaches offer optimal detection of skin lesions compared to conventional methods. This finding is crucial, as early and accurate detection is paramount for the timely intervention and treatment of skin disorders [2].
- Performance Metrics: This study assesses various performance metrics such as accuracy, robustness, and sensitivity across different models. Highlighting the performance of each algorithm provides insights into their effectiveness in handling diverse skin lesion datasets and informs researchers and practitioners about the most suitable approach for specific diagnostic tasks. The details performance metrics are provided in the Supplementary Materials, section 6.
- Flexible Perspective: By providing a tabulated representation of the database, the analysis offers a flexible perspective on the contributions and relevance of different models. This aids in understanding the strengths and limitations of each approach, enabling researchers to make informed decisions when selecting algorithms for skin lesion classification tasks.
- Implications for Clinical Practice: Ultimately, the findings from this comparative analysis can have direct implications for clinical practice. The adoption of advanced AI techniques in dermatology can enhance diagnostic accuracy, assist dermatologists in decision-making processes, and improve patient outcomes by facilitating early detection and treatment of skin lesions [58].
4.1. ACO with or without NNs to Perform Precise SLD Classification
- Sengupta et al. (2019) [25]: The primary limitation of the ACO model proposed by Sengupta et al. lies in its computational complexity and the potential for premature convergence to local optima. This can lead to suboptimal classification of skin lesions under varying conditions and complexities presented in visual RGB datasets.
- Anjum et al. (2020) [58]: Anjum et al.’s research, while pioneering in integrating ACO with deep learning models for skin lesion classification, faces limitations in generalizability across different datasets beyond MICCAI ISIC 2017, 2018, and 2019. The model’s performance might vary significantly, with datasets having different characteristics, potentially affecting its applicability in real-world scenarios.
- Singh et al. (2021) [59]: The novel hybrid model SLICA-CO, combining SLIC and ACO, although highly accurate, may suffer from scalability issues when dealing with very large dermatoscopic image archives. Additionally, the complexity of parameter tuning for the hybrid model can pose challenges in achieving optimal performance across diverse image sets.
- Ahmed et al. (2019) [60]: While the use of TSVM with ACO and GA for classifying dermatological issues shows high accuracy, the approach’s critical limitation is its dependency on high-quality labeled data for training. In dermatology, where subtle distinctions exist between different conditions, the lack of extensive, well-annotated datasets can hinder the model’s learning and generalization capabilities.
- Sengupta et al. (2020) [48]: The ACO-optimized Canny edge detection method, despite its robust performance, may encounter difficulties in edge detection for low-contrast skin lesion images. The limitation stems from the inherent challenge of accurately detecting edges in images where the lesion boundaries blend with the surrounding skin.
- Sarada et al. (2021) [61]: The hybrid multi-layer algorithms of K-Means and WOA, while effective in reducing detection error rates, face limitations in handling highly imbalanced datasets, where the prevalence of one class significantly outweighs others. This imbalance can skew the classification performance, affecting sensitivity and specificity.
- Zhao et al. (2023) [62]: The improved ACO model, LACOR, although advantageous for skin lesion, cancer, and melanoma classification, has limitations in adaptability and computational efficiency when applied to extremely large and complex visual datasets. The model may require significant computational resources for training and inference, impacting its practical deployment.
- Dalila et al. (2017) [63]: While the integration of ACO, KNN, and ANN demonstrates promising results in melanoma detection, a critical limitation is the potential for overfitting, especially in scenarios where the diversity of the training dataset does not adequately represent the variance found in real-world conditions.
- Yang et al. (2022) [64]: The Enhanced ACO for Continuous Ranges (EACOR) model faces limitations in dynamic adaptability to new or evolving types of melanoma pathology, potentially requiring frequent retraining or adjustment of its optimization parameters to maintain high levels of effectiveness and performance.
- Mirunalini et al. (2017) [65]: The proposed NN model, despite its learning efficiency, is limited by its relatively lower accuracy rate of approximately 65.8%. This suggests a need for further model optimization or incorporation of additional features to improve its classification performance for skin lesion and melanoma problems.
- Shetty et al. (2022) [41]: The CNN model’s critical limitation lies in its intensive computational demands for training and inference, particularly when processing large datasets of dermoscopic images. This can pose challenges in resource-constrained environments or in real-time applications.
- Attique et al. (2022) [66]: The reliance on Moth–Flame Optimization Algorithm and absence of data augmentation phases may limit the model’s ability to generalize across different skin conditions and variations within the datasets, potentially affecting its robustness in diverse clinical settings.
- Maqsood and Damaševičius (2023) [67]: While the four pre-trained CNN algorithms show high accuracy, their limitation is the potential for model overfitting due to the high capacity of these networks. Balancing model complexity and generalization remains a challenge, especially in multi-class skin lesion classification tasks.
- Shan et al. (2022) [68]: The DenseSFNet-45 model, despite outperforming conventional algorithms, may face limitations in interpretability and transparency, making it challenging for practitioners to understand the basis of its classification decisions, which is crucial in medical diagnostics.
- Tan et al. (2020) [69]: The HLPSO model’s limitation is the potential for increased computational complexity due to the hybrid nature of the optimization process, which combines several algorithms. This complexity can lead to longer training times and may require more computational resources.
- Bi et al. (2017) [70]: The Deep Residual Networks (ResNets) model, while achieving high performance, is limited by its sensitivity to hyperparameter settings and the need for extensive computational resources, which may limit its accessibility for some research or clinical environments.
4.2. WOA with or without NNs to Conduct Accurate SLD Recognition
5. Leveraging ACO, WOA, and NNs for Accurate Skin Lesion Detection
5.1. Addressing Challenges with ACO, WOA, and NNs
5.1.1. Enhanced Accuracy
5.1.2. Automation and Efficiency
5.1.3. Robustness to Variability
5.1.4. Optimization of Resources
5.1.5. Generalization
5.2. Integrating Ant Colony and Whale Optimization with NNs, AI, ML, and DL
5.2.1. Neural Networks (NNs)
5.2.2. Artificial Intelligence (AI)
5.2.3. Machine Learning (ML)
5.2.4. Deep Learning (DL)
5.3. Numerical Results
5.3.1. ACO Model [58]
5.3.2. Hybrid SLICACO Model [59]
5.3.3. ACO Support [60]
5.3.4. ACO-Optimized Edge Detection [48]
5.4. Advantages of Integration
5.4.1. Optimization of Classification Parameters
5.4.2. Improved Accuracy and Efficiency
5.4.3. Automation of Diagnostic Process
5.4.4. Consistent and Reliable Classification
5.4.5. Reduction of Human Error
6. Future Work and Recommendations
- To review other breakthrough concepts and approaches that contributed to considerable upgrading to the numerical ML and DL models’ performance.
- To investigate other technical tactics that can be adopted during the classification procedure to alleviate the adverse effects of massive data dimensionality and computational effort, time, and cost involved in the classification task of visual medical datasets, like SLD.
- To propose hybrid forms of ACP or WOA that can overcome conventional ACO challenges and WOA limitations in classifying different SLDs.
- To conduct a comparative inspection pertaining to the accuracy, robustness, and effectiveness of self-supervised learning (SSL) models compared with ACO and WOA, knowing that SSL models do not need costly and time-consuming data annotation of visual SLD patient datasets.
- To classify other key strengths and other common weaknesses (to handle) in ACO and WOA to upgrade their SLD categorization capability.
- The first recommendation is to use both algorithms together when working with convolutional neural networks and other networks to compensate for any shortcomings of one algorithm with the other. For example, algorithm (ACO) can be used in conjunction with algorithm (WOA), denoted as algorithm (A) and algorithm (B), respectively. If algorithm (A) produces weak prediction accuracy, algorithm (B) can be used to verify the results and adopt the more accurate one.
- The second recommendation is to merge both algorithms to work with deep neural network models and determine if they produce better results.
7. Conclusions
- Both ACO and WOA models, which can be supported (or not) with NNs, have proved their contributory impacts and beneficial practicalities in handling variant difficult-to-identify SLDs that might be also challenging for high-experienced HCPs to achieve.
- Significant computational costs, time, and effort corresponding to the necessary recognition processes of SLDs could be reduced compared with traditional numerical or manual/classification approaches, utilized commonly by most HCPs.
- The rates of accuracy, reliability, potential, and effectiveness of SLD classification procedures could be remarkably upgraded when ACO or WOA are utilized with or without the incorporation of NNs.
- Earlier prediction of the SLD can be flexibly accomplished due to escalated proportions of accuracy and efficiency in identifying the current problem in patients. Responsively, corrective medical actions can be implemented earlier before the SLD may expand, helping alleviate mortality rates.
8. Research Limitations
- There were some peer-reviewed articles with robust findings and noteworthy rationale. Unfortunately, they had no open axis.
- The corresponding number of papers addressing ACO or WOA when combined with other novel approaches is not abundantly available in the literature.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| ACO | Ant Colony Optimization |
| AI | Artificial Intelligence |
| ANNs | Artificial Neural Networks |
| ASDR | Age-Standardized DALY Rate |
| ASIR | Age-standardized Incidence Rate |
| ASMR | Age-Standardized Mortality Rate |
| ASR | Age-Standardized Rates |
| AUCs | The Area under the Curve |
| AUROC | Area Under the Receiver Operating Characteristic |
| BCC | Basal Cell Carcinoma |
| CFCs | Chlorofluorocarbons |
| CNNs | Convolutional Neural Networks |
| DALYs | Disability-Adjusted Life Years |
| D-CNNs | Deep Convolutional Neural Networks |
| DenseNet | Dense Convolutional Network |
| DL | Deep Learning |
| DNNs | Deep Neural Networks |
| DT | Decision Tree |
| EACOR | Enhanced Ant Colony Optimization for Continuous Ranges |
| EAPCs | Estimated Annual Percentage Changes |
| FA | Firefly Algorithm |
| FFNNs | Feed-Forward Neural Networks |
| F-MLP | Fuzzy Multi-Layered Perceptron |
| GA | Genetic Algorithm |
| HFA | Horizontal Flip Augmentation |
| HLPSO | Hybrid Learning Particle Swarm Optimization |
| IEEE | Institute of Electrical and Electronics Engineers |
| IoT | Internet of Things |
| ISBI | International Symposium on Biomedical Imaging |
| ISIC | The International Skin Imaging Collaboration |
| KNN | K-Nearest Neighbor |
| LACOR | An Improved Ant Colony Optimization |
| LNTL | Learning Not to Learn |
| MC | Mutual Congestion |
| MCC | Matthews Correlation Coefficient |
| MC-SVM | Multi-Class Support Vector Machine |
| MELMC | Multiclass Extreme Learning Machine Classifier |
| ML | Machine Learning |
| MLR | Multiple Linear Regression |
| MNNs | Modular Neural Networks |
| MRI | Magnetic Resonance Imaging |
| NB | Naive Bayes |
| NIA | Nature Inspired Algorithm |
| NNs | Neural Networks |
| O-NB | Optimized Naive Bayes |
| OOD | Out-Of-Distribution |
| O-SVM | Optimized Support Vector Machine |
| ResNets | Residual Networks |
| RNN | Recurrent Neural Network |
| SCC | Squamous Cell Carcinoma |
| SCF | Skin Cancer Foundation |
| SE | Squeeze-and-Excitation |
| SL | Supervised Learning |
| SLIC | Simple Linear Iterative Clustering |
| SVM | Support Vector Machine |
| TABE | Turning A Blind Eye |
| TSVM | Transudative Support Vector Machine |
| UMCG | University Medical Center Groningen |
| UTSs | Urban Transportation Systems |
| UV | Ultraviolet |
References
- Ambika, P. Machine learning and deep learning algorithms on the Industrial Internet of Things (IIoT). Adv. Comput. 2020, 117, 321–338. [Google Scholar]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Seeja, R.; Suresh, A. Deep learning based skin lesion segmentation and classification of melanoma using support vector machine (SVM). Asian Pac. J. Cancer Prev. APJCP 2019, 20, 1555. [Google Scholar]
- Tufail, A.B.; Ma, Y.-K.; Kaabar, M.K.; Martínez, F.; Junejo, A.; Ullah, I.; Khan, R. Deep learning in cancer diagnosis and prognosis prediction: A minireview on challenges, recent trends, and future directions. Comput. Math. Methods Med. 2021, 2021, 9025470. [Google Scholar] [CrossRef] [PubMed]
- Khanfari, H.; Mehranfar, S.; Cheki, M.; Mohammadi Sadr, M.; Moniri, S.; Heydarheydari, S.; Rezaeijo, S.M. Exploring the efficacy of multi-flavored feature extraction with radiomics and deep features for prostate cancer grading on mpMRI. BMC Med. Imaging 2023, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Rezaeijo, S.M.; Chegeni, N.; Baghaei Naeini, F.; Makris, D.; Bakas, S. Within-modality synthesis and novel radiomic evaluation of brain MRI scans. Cancers 2023, 15, 3565. [Google Scholar] [CrossRef]
- Khan, M.A.; Akram, T.; Sharif, M.; Saba, T.; Javed, K.; Lali, I.U.; Tanik, U.J.; Rehman, A. Construction of saliency map and hybrid set of features for efficient segmentation and classification of skin lesion. Microsc. Res. Tech. 2019, 82, 741–763. [Google Scholar] [CrossRef]
- Song, L.; Lin, J.; Wang, Z.J.; Wang, H. An end-to-end multi-task deep learning framework for skin lesion analysis. IEEE J. Biomed. Health Inform. 2020, 24, 2912–2921. [Google Scholar] [CrossRef]
- Javed, M.H.; Yu, Z.; Li, T.; Rajeh, T.M.; Rafique, F.; Waqar, S. Hybrid two-stream dynamic CNN for view adaptive human action recognition using ensemble learning. Int. J. Mach. Learn. Cybern. 2022, 13, 1157–1166. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, J.; Xia, Y.; Shen, C. A mutual bootstrapping model for automated skin lesion segmentation and classification. IEEE Trans. Med. Imaging 2020, 39, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Harangi, B. Skin lesion classification with ensembles of deep convolutional neural networks. J. Biomed. Inform. 2018, 86, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Sakr, M.; Abo-Seida, O.M.; Keshk, A.; Chen, H.J. A novel deep-learning model for automatic detection and classification of breast cancer using the transfer-learning technique. IEEE Access 2021, 9, 71194–71209. [Google Scholar] [CrossRef]
- Abbas, Q.; Celebi, M.E. DermoDeep-A classification of melanoma-nevus skin lesions using multi-feature fusion of visual features and deep neural network. Multimed. Tools Appl. 2019, 78, 23559–23580. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; Hosny, K.M.; Damaševičius, R.; Eltoukhy, M.M. Machine learning and deep learning methods for skin lesion classification and diagnosis: A systematic review. Diagnostics 2021, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Aalaei, S.; Shahraki, H.; Rowhanimanesh, A.; Eslami, S. Feature selection using genetic algorithm for breast cancer diagnosis: Experiment on three different datasets. Iran. J. Basic Med. Sci. 2016, 19, 476. [Google Scholar] [PubMed]
- Skin Cancer. 2023. Available online: https://www.skincancer.org/skin-cancer-information/ (accessed on 24 December 2023).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Damarla, A.; Sumathi, D. An approach for optimization of features using gorilla troop optimizer for classification of melanoma. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 275–286. [Google Scholar] [CrossRef]
- Mukherjee, S.; Adhikari, A.; Roy, M. Melanoma Detection From Lesion Images Using Optimized Features Selected by Metaheuristic Algorithms. Int. J. Healthc. Inf. Syst. Inform. (IJHISI) 2021, 16, 1–22. [Google Scholar] [CrossRef]
- Brinker, T.J.; Hekler, A.; Utikal, J.S.; Grabe, N.; Schadendorf, D.; Klode, J.; Berking, C.; Steeb, T.; Enk, A.H.; Von Kalle, C. Skin cancer classification using convolutional neural networks: Systematic review. J. Med. Internet Res. 2018, 20, e11936. [Google Scholar] [CrossRef] [PubMed]
- Mafarja, M.M.; Mirjalili, S. Hybrid whale optimization algorithm with simulated annealing for feature selection. Neurocomputing 2017, 260, 302–312. [Google Scholar] [CrossRef]
- Barata, C.; Ruela, M.; Francisco, M.; Mendonça, T.; Marques, J.S. Two systems for the detection of melanomas in dermoscopy images using texture and color features. IEEE Syst. J. 2013, 8, 965–979. [Google Scholar] [CrossRef]
- Sengupta, S.; Mittal, N.; Modi, M. Improved skin lesion edge detection method using Ant Colony Optimization. Ski. Res. Technol. 2019, 25, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Dorigo, M.; Stützle, T. Ant Colony Optimization: Overview and Recent Advances; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mirjalili, S.; Lewis, A. The whale optimization algorithm. Adv. Eng. Softw. 2016, 95, 51–67. [Google Scholar] [CrossRef]
- Mafarja, M.; Mirjalili, S. Whale optimization approaches for wrapper feature selection. Appl. Soft Comput. 2018, 62, 441–453. [Google Scholar] [CrossRef]
- Nematzadeh, H.; Enayatifar, R.; Mahmud, M.; Akbari, E. Frequency based feature selection method using whale algorithm. Genomics 2019, 111, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.N.; Gomathi, N. WGC: Hybridization of exponential grey wolf optimizer with whale optimization for data clustering. Alex. Eng. J. 2018, 57, 1569–1584. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Niu, T.; Yang, W. A novel hybrid system based on a new proposed algorithm—Multi-Objective Whale Optimization Algorithm for wind speed forecasting. Appl. Energy 2017, 208, 344–360. [Google Scholar] [CrossRef]
- Abd El Aziz, M.; Ewees, A.A.; Hassanien, A.E. Whale optimization algorithm and moth-flame optimization for multilevel thresholding image segmentation. Expert Syst. Appl. 2017, 83, 242–256. [Google Scholar] [CrossRef]
- Eid, H.F. Binary whale optimisation: An effective swarm algorithm for feature selection. Int. J. Metaheuristics 2018, 7, 67–79. [Google Scholar] [CrossRef]
- Reddy, K.S.; Panwar, L.; Panigrahi, B.; Kumar, R. Binary whale optimization algorithm: A new metaheuristic approach for profit-based unit commitment problems in competitive electricity markets. Eng. Optim. 2019, 51, 369–389. [Google Scholar] [CrossRef]
- Hussien, A.G.; Houssein, E.H.; Hassanien, A.E. A binary whale optimization algorithm with hyperbolic tangent fitness function for feature selection. In Proceedings of the 2017 Eighth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt, 5–7 December 2017; pp. 166–172. [Google Scholar]
- Gharehchopogh, F.S.; Gholizadeh, H. A comprehensive survey: Whale Optimization Algorithm and its applications. Swarm Evol. Comput. 2019, 48, 1–24. [Google Scholar] [CrossRef]
- Rana, N.; Latiff, M.S.A.; Abdulhamid, S.i.M.; Chiroma, H. Whale optimization algorithm: A systematic review of contemporary applications, modifications and developments. Neural Comput. Appl. 2020, 32, 16245–16277. [Google Scholar] [CrossRef]
- Kavitha, R.; Jothi, D.K.; Saravanan, K.; Swain, M.P.; Gonzáles, J.L.A.; Bhardwaj, R.J.; Adomako, E. Ant colony optimization-enabled CNN deep learning technique for accurate detection of cervical cancer. BioMed Res. Int. 2023, 2023, 1742891. [Google Scholar] [CrossRef]
- Yan, C.; Kang, X.; Li, M.; Wang, J. A novel feature selection method on mutual information and improved gravitational search algorithm for high dimensional biomedical data. In Proceedings of the 2021 13th International Conference on Computer and Automation Engineering (ICCAE), Melbourne, Australia, 20–22 March 2021; pp. 24–30. [Google Scholar]
- Farid, A.A.; Selim, G.; Khater, H. A Composite Hybrid Feature Selection Learning-Based Optimization of Genetic Algorithm for Breast Cancer Detection. Preprint 2020. [Google Scholar] [CrossRef]
- Shetty, B.; Fernandes, R.; Rodrigues, A.P.; Chengoden, R.; Bhattacharya, S.; Lakshmanna, K. Skin lesion classification of dermoscopic images using machine learning and convolutional neural network. Sci. Rep. 2022, 12, 18134. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.-R.; Li, J.; Kanwal, S.; Yang, G.; Hussain, A.; Jane O’Shea, S. A novel fuzzy multilayer perceptron (F-MLP) for the detection of irregularity in skin lesion border using dermoscopic images. Front. Med. 2020, 7, 297. [Google Scholar] [CrossRef]
- Ibrahim, I.; Abdulazeez, A. The role of machine learning algorithms for diagnosing diseases. J. Appl. Sci. Technol. Trends 2021, 2, 10–19. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, G.; Jiao, S.; Wang, J. Gray Image Denoising Based on Array Stochastic Resonance and Improved Whale Optimization Algorithm. Appl. Sci. 2022, 12, 12084. [Google Scholar] [CrossRef]
- Xu, M.; Cao, L.; Lu, D.; Hu, Z.; Yue, Y. Application of Swarm Intelligence Optimization Algorithms in Image Processing: A Comprehensive Review of Analysis, Synthesis, and Optimization. Biomimetics 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Bhateja, V.; Tripathi, A.; Sharma, A.; Le, B.N.; Satapathy, S.C.; Nguyen, G.N.; Le, D.-N. Ant colony optimization based anisotropic diffusion approach for despeckling of SAR images. In Proceedings of the Integrated Uncertainty in Knowledge Modelling and Decision Making: 5th International Symposium, IUKM 2016, Da Nang, Vietnam, 30 November–2 December 2016; Proceedings 5. pp. 389–396. [Google Scholar]
- Rafsanjani, M.; Varzaneh, Z. Edge detection in digital images using Ant Colony Optimization. Comput. Sci. J. Mold. 2015, 69, 343–359. [Google Scholar]
- Sengupta, S.; Mittal, N.; Modi, M. Improved skin lesions detection using color space and artificial intelligence techniques. J. Dermatol. Treat. 2020, 31, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Park, G.H.; Lim, W.; Kim, M.S.; Na, J.I.; Park, I.; Chang, S.E. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: Automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS ONE 2018, 13, e0191493. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Walia, E.; Babyn, P. Unsupervised and semi-supervised learning with categorical generative adversarial networks assisted by wasserstein distance for dermoscopy image classification. arXiv 2018, arXiv:1804.03700. [Google Scholar]
- Yaqoob, A.; Aziz, R.M.; Verma, N.K.; Lalwani, P.; Makrariya, A.; Kumar, P. A review on nature-inspired algorithms for cancer disease prediction and classification. Mathematics 2023, 11, 1081. [Google Scholar] [CrossRef]
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 60. [Google Scholar] [CrossRef]
- Kumar, N.S.; Hariprasath, K.; Tamilselvi, S.; Kavinya, A.; Kaviyavarshini, N. Detection of stages of melanoma using deep learning. Multimed. Tools Appl. 2021, 80, 18677–18692. [Google Scholar] [CrossRef]
- Umar, S.A.; Tasduq, S.A. Ozone layer depletion and emerging public health concerns-an update on epidemiological perspective of the ambivalent effects of ultraviolet radiation exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahamad, M.A.; Yap, C.H.; Yang, G. A survey, review, and future trends of skin lesion segmentation and classification. Comput. Biol. Med. 2023, 106624. [Google Scholar] [CrossRef]
- Baig, R.; Bibi, M.; Hamid, A.; Kausar, S.; Khalid, S. Deep learning approaches towards skin lesion segmentation and classification from dermoscopic images—A review. Curr. Med. Imaging 2020, 16, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Haenssle, H.A.; Fink, C.; Toberer, F.; Winkler, J.; Stolz, W.; Deinlein, T.; Hofmann-Wellenhof, R.; Lallas, A.; Emmert, S.; Buhl, T.; et al. Man against machine reloaded: Performance of a market-approved convolutional neural network in classifying a broad spectrum of skin lesions in comparison with 96 dermatologists working under less artificial conditions. Ann. Oncol. 2020, 31, 137–143. [Google Scholar] [CrossRef]
- Anjum, M.A.; Amin, J.; Sharif, M.; Khan, H.U.; Malik, M.S.A.; Kadry, S. Deep semantic segmentation and multi-class skin lesion classification based on convolutional neural network. IEEE Access 2020, 8, 129668–129678. [Google Scholar] [CrossRef]
- Singh, L.; Janghel, R.R.; Sahu, S.P. SLICACO: An automated novel hybrid approach for dermatoscopic melanocytic skin lesion segmentation. Int. J. Imaging Syst. Technol. 2021, 31, 1817–1833. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Ema, R.R.; Islam, T. An automated dermatological images segmentation based on a new hybrid intelligent ACO-GA algorithm and diseases identification using TSVM classifier. In Proceedings of the 2019 1st International Conference on Advances in Science, Engineering and Robotics Technology (ICASERT), Dhaka, Bangladesh, 3–5 May 2019; pp. 1–6. [Google Scholar]
- Sarada, B.; Murthy, M.V.; Rani, V.U. Combined secure approach based on whale optimization to improve the data classification for data analytics. Pattern Recognit. Lett. 2021, 152, 327–332. [Google Scholar] [CrossRef]
- Zhao, D.; Qi, A.; Yu, F.; Heidari, A.A.; Chen, H.; Li, Y. Multi-strategy ant colony optimization for multi-level image segmentation: Case study of melanoma. Biomed. Signal Process. Control 2023, 83, 104647. [Google Scholar] [CrossRef]
- Dalila, F.; Zohra, A.; Reda, K.; Hocine, C. Segmentation and classification of melanoma and benign skin lesions. Optik 2017, 140, 749–761. [Google Scholar] [CrossRef]
- Yang, X.; Ye, X.; Zhao, D.; Heidari, A.A.; Xu, Z.; Chen, H.; Li, Y. Multi-threshold image segmentation for melanoma based on Kapur’s entropy using enhanced ant colony optimization. Front. Neuroinformatics 2022, 16, 1041799. [Google Scholar] [CrossRef] [PubMed]
- Mirunalini, P.; Chandrabose, A.; Gokul, V.; Jaisakthi, S. Deep learning for skin lesion classification. arXiv 2017, arXiv:1703.04364. [Google Scholar]
- Attique Khan, M.; Sharif, M.; Akram, T.; Kadry, S.; Hsu, C.H. A two-stream deep neural network-based intelligent system for complex skin cancer types classification. Int. J. Intell. Syst. 2022, 37, 10621–10649. [Google Scholar] [CrossRef]
- Maqsood, S.; Damaševičius, R. Multiclass skin lesion localization and classification using deep learning based features fusion and selection framework for smart healthcare. Neural Netw. 2023, 160, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Fu, C.; Dai, L.; Jia, T.; Tie, M.; Liu, J. Automatic skin lesion classification using a new densely connected convolutional network with an SF module. Med. Biol. Eng. Comput. 2022, 60, 2173–2188. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.; Zhang, L.; Lim, C.P. Adaptive melanoma diagnosis using evolving clustering, ensemble and deep neural networks. Knowl.-Based Syst. 2020, 187, 104807. [Google Scholar] [CrossRef]
- Bi, L.; Kim, J.; Ahn, E.; Feng, D. Automatic skin lesion analysis using large-scale dermoscopy images and deep residual networks. arXiv 2017, arXiv:1703.04197. [Google Scholar]
- Mafarja, M.M.; Mirjalili, S. Hybrid binary ant lion optimizer with rough set and approximate entropy reducts for feature selection. Soft Comput. 2019, 23, 6249–6265. [Google Scholar] [CrossRef]
- Amiriebrahimabadi, M.; Mansouri, N. A comprehensive survey of feature selection techniques based on whale optimization algorithm. Multimed. Tools Appl. 2023, 1–72. [Google Scholar] [CrossRef]
- Sharawi, M.; Zawbaa, H.M.; Emary, E. Feature selection approach based on whale optimization algorithm. In Proceedings of the 2017 Ninth International Conference on Advanced Computational Intelligence (ICACI), Doha, Qatar, 4–6 February 2017; pp. 163–168. [Google Scholar]
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Sci. Data 2018, 5, 180161. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sharif, M.; Akram, T.; Damaševičius, R.; Maskeliūnas, R. Skin lesion segmentation and multiclass classification using deep learning features and improved moth flame optimization. Diagnostics 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shah, M.; Pandya, A.; Sushra, R.; Sushra, R.; Mehta, M.; Patel, K.; Patel, K. A comprehensive study on skin cancer detection using artificial neural network (ANN) and convolutional neural network (CNN). Clin. eHealth 2023, 6, 76–84. [Google Scholar] [CrossRef]
- Strzelecki, M.; Kociołek, M.; Strąkowska, M.; Kozłowski, M.; Grzybowski, A.; Szczypiński, P.M. Artificial Intelligence in the detection of skin cancer: State of the art. Clin. Dermatol. 2024, in press. [Google Scholar] [CrossRef]
- Anupama, C.; Natrayan, L.; Laxmi Lydia, E.; Wahab Sait, A.R.; Escorcia-Gutierrez, J.; Gamarra, M.; Mansour, R.F. Deep learning with backtracking search optimization based skin lesion diagnosis model. Comput. Mater. Contin. 2021, 70, 1297–1313. [Google Scholar] [CrossRef]
- Dinh, H.Q.; Bag, T.; Upadhyay, A.K.; Bandi, R.; Chinnakum, W. On the Structure of Cyclic Codes Over qRS and Applications in Quantum and LCD Codes Constructions. IEEE Access 2020, 8, 18902–18914. [Google Scholar] [CrossRef]
- Dhruv, B.; Mittal, N.; Modi, M. Early and precise detection of pancreatic tumor by hybrid approach with edge detection and artificial intelligence techniques. EAI Endorsed Trans. Pervasive Health Technol. 2021, 7, e1. [Google Scholar] [CrossRef]
- Xiao, X.; Geyer, V.F.; Bowne-Anderson, H.; Howard, J.; Sbalzarini, I.F. Automatic optimal filament segmentation with sub-pixel accuracy using generalized linear models and B-spline level-sets. Med. Image Anal. 2016, 32, 157–172. [Google Scholar] [CrossRef]
- Hosny, K.M.; Elshora, D.; Mohamed, E.R.; Vrochidou, E.; Papakostas, G.A. Deep Learning and Optimization-Based Methods for Skin Lesions Segmentation: A Review. IEEE Access 2023, 11, 85467–85488. [Google Scholar] [CrossRef]
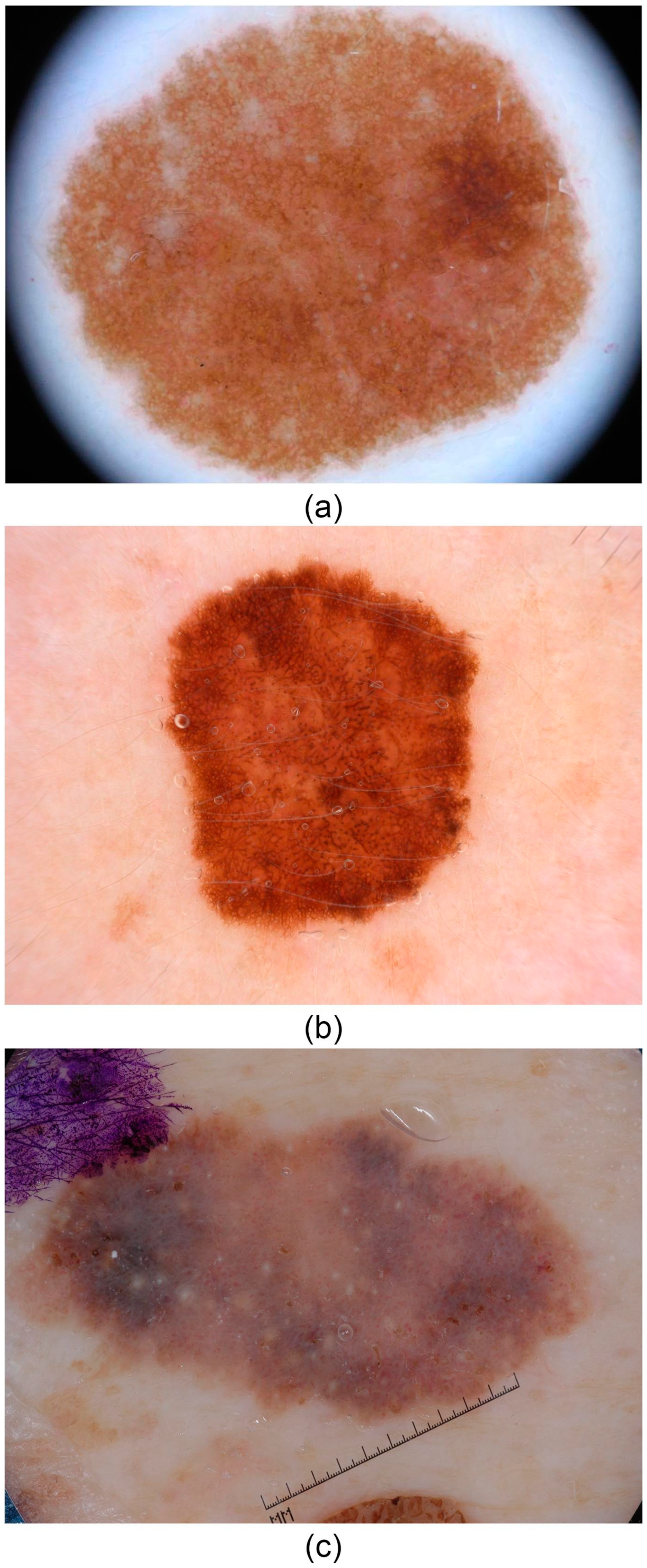
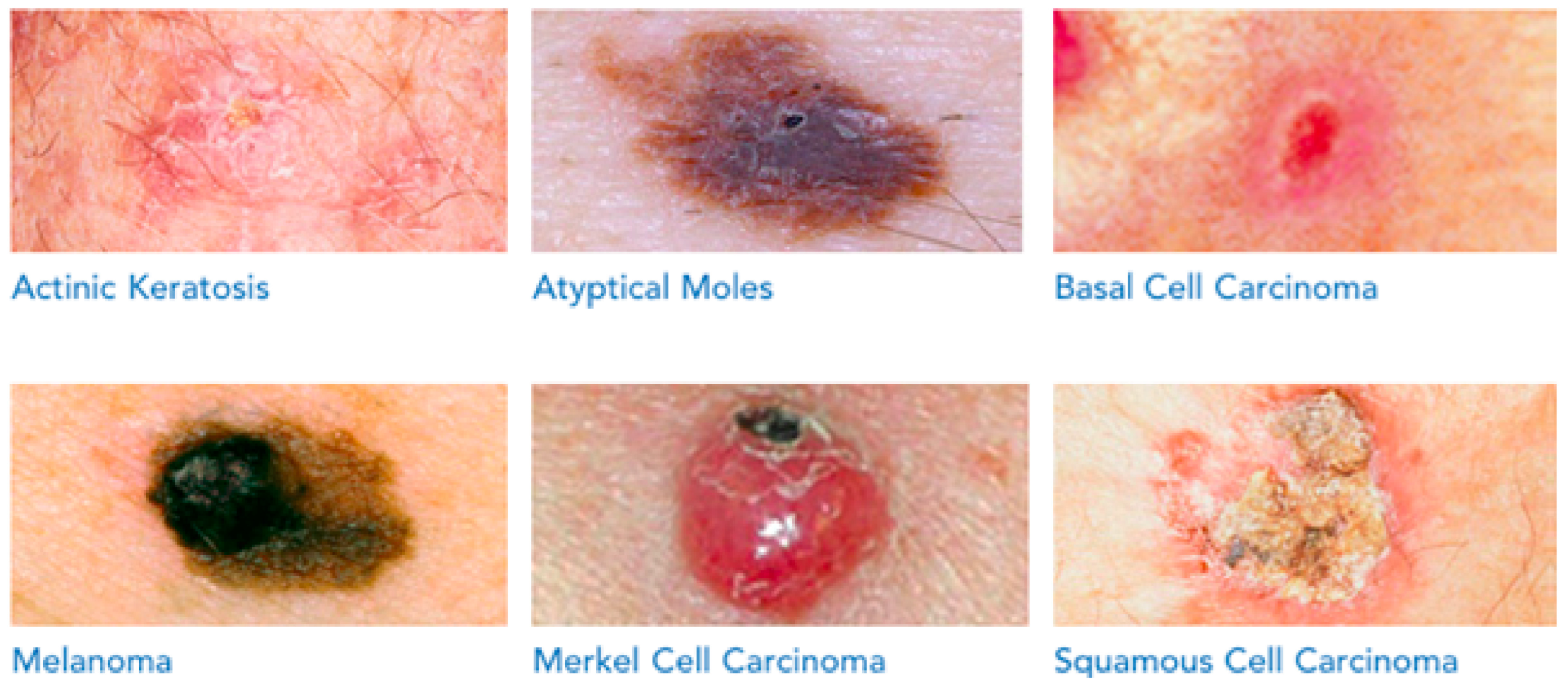

| No. | Name of the Dataset | Overall Number of Photos | Photo Category | Number of Skin Defects | Format of Images | Year Issued | Crucial Relevance |
|---|---|---|---|---|---|---|---|
| 1 | MED-NODE | 170 | Macroscopic | Two | .jpg | 2015 | To identify skin cancer problems |
| 2 | HAM-10,000 | 10,015 | Dermatological | Eight | .jpg | 2018 | To consider the improper variety in datasets and their small size |
| 3 | PH2 | 200 | Dermatological | Three | .bmp | 2013 | To provide active segmentation and classification process of melanoma |
| 4 | Derm7pt | 2000 | Dermatological Structured Database | Fifteen | .jpg | 2018 | To actively detect skin lesion problems for a seven-point malignant analysis |
| 5 | BCN-20,000 | 19,424 | Dermatological | Nine | .jpg | 2019 | To help therapists identify challenging skin defects in difficult areas, like mucous membranes and nails |
| 6 | ISIC Archive | More than 13,000 | Dermatological | Nine | DICOM and .jpg | From 2016 to 2020 | To boost the adoption of numerical skin lesion detection and enhance treatment efficiency and outcomes |
| No. | Paper (Researcher(s) and Year) | Kind of ML/DL Model Analyzed and Its Use | Dataset Classification | Contributory Findings |
|---|---|---|---|---|
| 1 | Sengupta et al. (2019) [25] | ACO Model | Skin Lesion Visual RGB Dataset | The introduction of ACO has enhanced efficiency levels. |
| 2 | Anjum et al. (2020) [58] | ACO, ResNet-18, tinyYOLOv2, Optimized Support Vector Machine (O-SVM) and Optimized Naive Bayes (O-NB) | Skin Lesion MICCAI ISIC 2017, 2018, and 2019 Datasets | DL principles, optimization algorithms, active classifiers, and ACO-driven feature selection enhanced accuracy in localizing, segmenting, and classifying skin lesion defects in visual data. |
| 3 | Singh et al. (2021) [59] | A Novel Hybrid mode (SLICACO) from (A) the Simple Linear Iterative Clustering (SLIC) and (B) ACO Models | Visual Skin Lesion Benchmark Dermatoscopic PH2 Archive Dataset | Simulation results from the novel hybrid model incorporating ACO indicate a classification accuracy rate of approximately 95.9% for multiple skin lesion images. |
| 4 | Ahmed et al. (2019) [60] | Transductive Support Vector Machine (TSVM) Identification with the Suppiort of ACO and GA as Clustering Optimizers | Dermatological Visual Dataset Comprising Skin Lesion Disorders, Skin Cancer, and Other Skin and Dermatological Deficiencies | The TSVM model facilitated an active and efficient classification of various skin and dermatological problems, categorizing 24 different patterns with an elevated accuracy of approximately 95%. |
| 5 | Sengupta et al. (2020) [48] | Nature Inspired Algorithm (NIA) and ACO, ACO-Prewitt, Edge Smoothing Color Space, and ACO-Sobel | Skin Lesion Visual Dataset Consisting of RGB Dermoscopy Images of Skin Lesion Issues | Their proposed method demonstrates more robust performance and higher accuracy percentages compared to other optimization algorithms such as Edge Smoothing-Color Space, ACO-Sobel, and ACO-Prewitt models. |
| 6 | Sarada et al. (2021) [61] | A Hybrid Multi-Layer Algorithms of (I) K-Means and (II) WOA | Dermatological Dataset of Visual Information Containing Different Skin Lesion, Skin Cancer, and Other Skin Defects | Numerical results demonstrate that integrating AI and DL models with firefly optimization procedures can decrease the early stage detection error rate. |
| 7 | Zhao et al. (2023) [62] | An improved ACO (LACOR) | Visual BSDS500 Dataset Comprising Skin Defects, particularly Skin Cancer and Melanoma | The LACOR model, alongside other ML and DL models, facilitates active segmentation and high-performance classification processes for skin lesions and melanoma disorders, offering significantly higher quality rates compared to other algorithms. |
| 8 | Dalila et al. (2017) [63] | ACO, KNN, and Artificial Neural Networks (ANNs) | Visual Skin Lesion Dataset Containing Melanoma Problems and Dermoscopic Images | ANNs classified skin lesion defects with an accuracy of 93.60%, surpassing the 86.60% achieved by traditional techniques. |
| 9 | Yang et al. (2022) [64] | Enhanced ACO for Continuous Ranges (EACOR) | Visual Melanoma Pathology Dataset | Their proposed model could significantly contribute to the high-quality analysis and classification of visual datasets related to melanoma pathology detection and investigation. |
| 10 | Mirunalini et al. (2017) [65] | NN Model | The proposed NN model efficiently learned, aiding in the categorization of visual datasets related to melanoma and skin lesion issues during training, leading to improved levels of accuracy. | |
| 11 | Shetty et al. (2022) [41] | CNN Model | HAM-10000 Visual Dataset consisting of 10,015 images of Various Dermoscopic and Skin Lesion Issues | Numerical simulations revealed that utilizing the CNN model enhanced the accuracy of skin lesion classification. |
| 12 | Attique et al. (2022) [66] | Moth–Flame Optimization Algorithm, MobileNetV2, Multiclass Extreme Learning Machine Classifier (MELMC) | Three imbalanced Visual Skin Datasets, (I) HAM10000, (II) ISBI2018, (III) ISIC2019 | A comparison with current ML and DL techniques demonstrated the enhanced efficiency of the suggested paradigm. |
| 13 | Maqsood and Damaševičius (2023) [67] | Four pre-trained CNN algorithms, namely (A) Xception, (B) ResNet-50, (C) ResNet-101, and (D) VGG16, besides Multi-Class SVM (MC-SVM) | (1) HAM10000, (2) ISIC2018, (3) ISIC2019, (4) PH2 datasets | The accuracy for the four investigated and analyzed datasets, respectively, reached values of 98.57%, 98.62%, 93.47%, and 98.98% in skin lesion classification. |
| 14 | Shan et al. (2022) [68] | DenseSFNet-45, Dense Convolutional Network (DenseNet), Squeeze-and-Excitation (SE) Block | Three public Visual Skin Lesion Datasets: (i) ISBI 2017 Skin Lesion Analysis Towards Melanoma Detection Challenge dataset (ISBI-skin-2017), (ii) ISBI 2018 Skin Lesion Analysis Towards Melanoma Detection Challenge dataset (ISBI-skin-2018), and (iii) PH2 dataset | The proposed method surpassed conventional machine learning algorithms, current classical model classification schemes, and state-of-the-art techniques. |
| 15 | Tan et al. (2020) [69] | Hybrid Learning Particle Swarm Optimization (HLPSO), D-CNN, and Firefly Algorithm (FA), KM Clustering Algorithm | (I) Dermofit Image Library, (II) PH2, (III) ISIC 2017 Dataset | The simulation investigation revealed that the proposed HLPSO model outperformed both advanced and classical search models in managing skin lesion classification, based on benchmark activities related to mathematical landscapes and recognition tasks associated with the complex CEC 2014 testing suite. |
| 16 | Bi et al. (2017) [70] | Deep Residual Networks (ResNets) | Visual Skin Lesion ISIC 2017 Dataset | The ResNets model significantly enhanced performance and accuracy in classifying skin lesions, achieving an average AUC of 91.50%, surpassing that of other conventional ML models. |
| Paper (Researcher(s) and Year) | Kind of WOA (with/without NNs) | Dataset Classification | Critical Limitations | Contributory Findings |
|---|---|---|---|---|
| Mirjalili and Lewis [27] | Standard WOA (without NNs) | Not specific | Potential for premature convergence and sensitivity to parameter settings. | Introduced the Whale Optimization Algorithm, demonstrating its effectiveness in solving optimization problems. |
| Mafarja and Mirjalili [23] | Hybrid WOA with simulated annealing | Not specific | May require careful tuning of hybrid algorithm parameters to ensure a balance between exploration and exploitation. | Proposed a hybrid approach combining WOA and simulated annealing for improved feature selection in machine learning. |
| Mafarja and Mirjalili [71] | WOA for feature selection (without NNs) | Eighteen UCI datasets were used in the experiments | Risk of not adequately exploring the search space in high-dimensional datasets. | Explored various whale optimization approaches for wrapper feature selection, enhancing the selection process in machine learning models. |
| Amiriebrahimabadi and Mansouri [72] | WOA for feature selection (without NNs) | Genomics | Limited by the complexity and high dimensionality of genomic data. | Utilized WOA for frequency-based feature selection in genomics, showcasing the algorithm’s applicability in bioinformatics. |
| Sharawi and Zawbaa [73] | WOA for feature selection (without NNs) | The data set is provided by a power enterprise, including the electricity consumption data of 11,860 high-voltage users who have had arrears | Dependency on the quality of the dataset and the risk of overfitting. | Demonstrated the effectiveness of WOA in feature selection, improving the performance of computational intelligence systems. |
| Sarada and Murthy [61] | WOA for data classification (without NNs) | Generated Data Analytics | Challenges in adapting WOA to diverse and dynamic data analytics environments. | Applied WOA to improve data classification for analytics, highlighting the algorithm’s versatility in handling various data types. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhlif, Y.A.; Ramaha, N.T.A.; Hameed, A.A.; Salman, M.; Yon, D.K.; Fitriyani, N.L.; Syafrudin, M.; Lee, S.W. Ant Colony and Whale Optimization Algorithms Aided by Neural Networks for Optimum Skin Lesion Diagnosis: A Thorough Review. Mathematics 2024, 12, 1049. https://doi.org/10.3390/math12071049
Mukhlif YA, Ramaha NTA, Hameed AA, Salman M, Yon DK, Fitriyani NL, Syafrudin M, Lee SW. Ant Colony and Whale Optimization Algorithms Aided by Neural Networks for Optimum Skin Lesion Diagnosis: A Thorough Review. Mathematics. 2024; 12(7):1049. https://doi.org/10.3390/math12071049
Chicago/Turabian StyleMukhlif, Yasir Adil, Nehad T. A. Ramaha, Alaa Ali Hameed, Mohammad Salman, Dong Keon Yon, Norma Latif Fitriyani, Muhammad Syafrudin, and Seung Won Lee. 2024. "Ant Colony and Whale Optimization Algorithms Aided by Neural Networks for Optimum Skin Lesion Diagnosis: A Thorough Review" Mathematics 12, no. 7: 1049. https://doi.org/10.3390/math12071049
APA StyleMukhlif, Y. A., Ramaha, N. T. A., Hameed, A. A., Salman, M., Yon, D. K., Fitriyani, N. L., Syafrudin, M., & Lee, S. W. (2024). Ant Colony and Whale Optimization Algorithms Aided by Neural Networks for Optimum Skin Lesion Diagnosis: A Thorough Review. Mathematics, 12(7), 1049. https://doi.org/10.3390/math12071049








