Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube–Water Nanofluid under Ha’il City Climatic Conditions
Abstract
1. Introduction
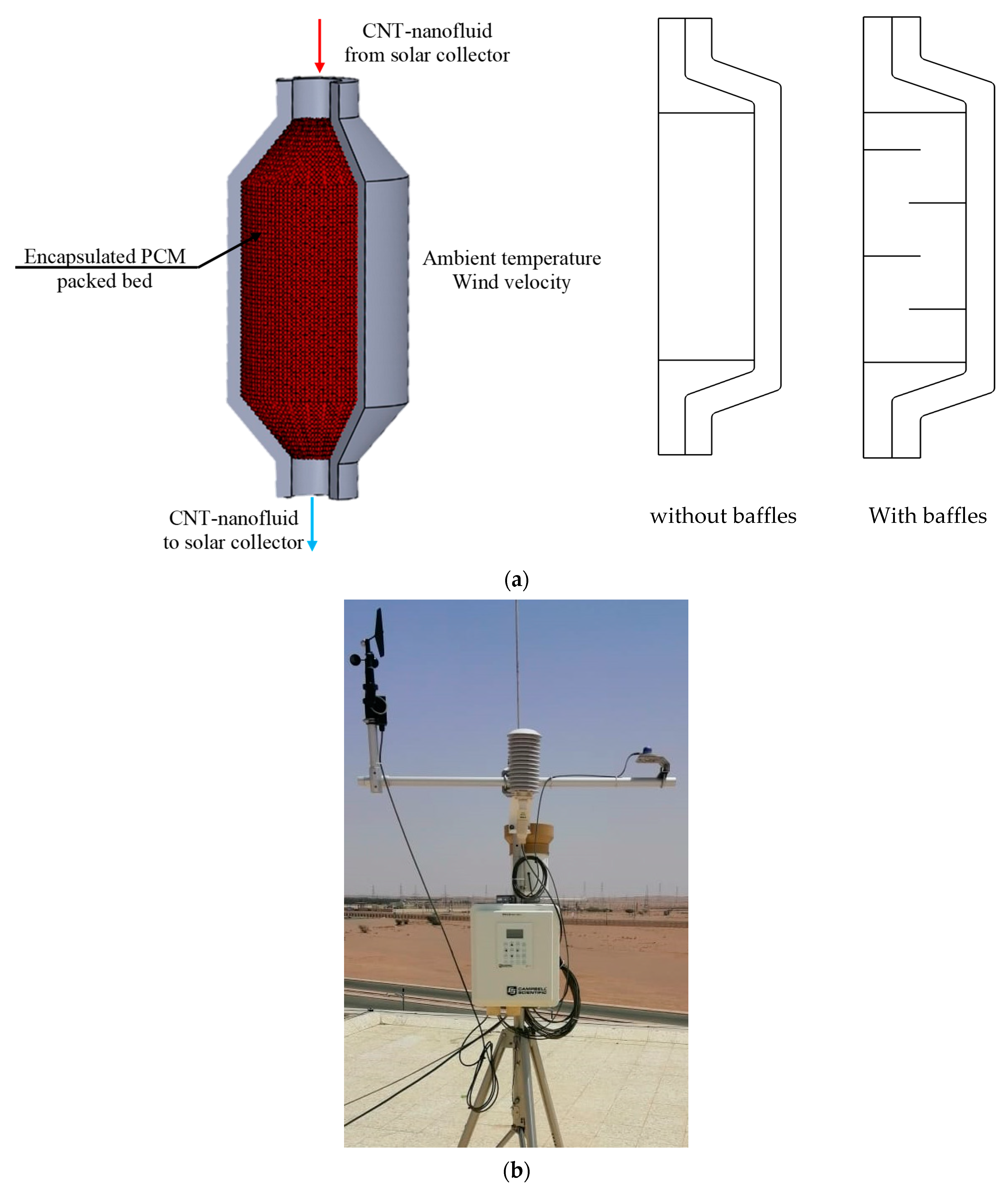
2. Studied Configuration, Mathematical Formulation, and Numerical Procedure
- -
- For the nanofluid region
- -
- For the PCM regiondenotes the Kozeny–Carman permeability
- -
- Density:
- -
- Heat capacitance:
- -
- -
- , are the transverse and longitudinal thermal conductivities;
- = 9.2 nm;
- =1.5 μm; and
- = .
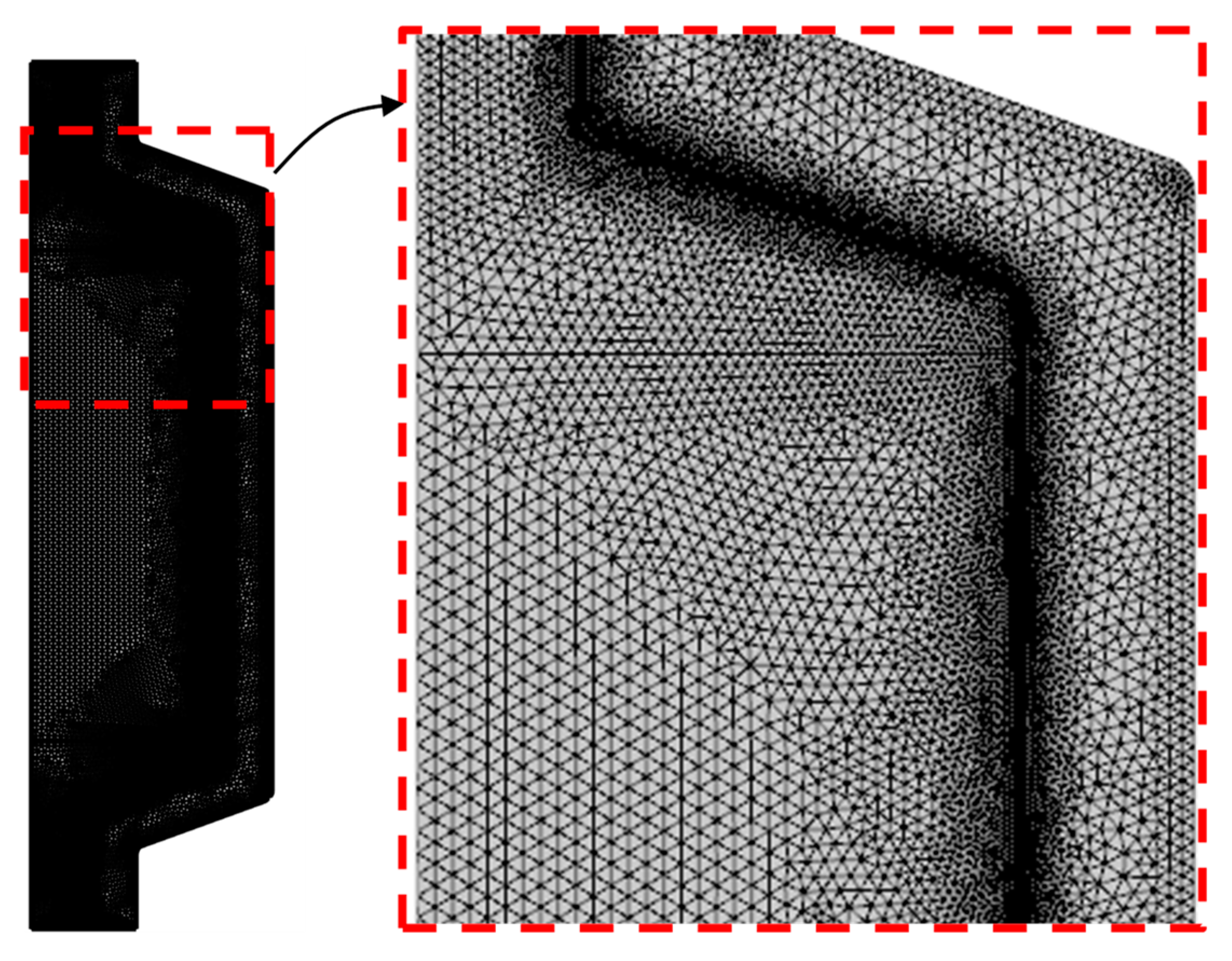
3. Validation and Grid Sensitivity Test
4. Results and Discussion
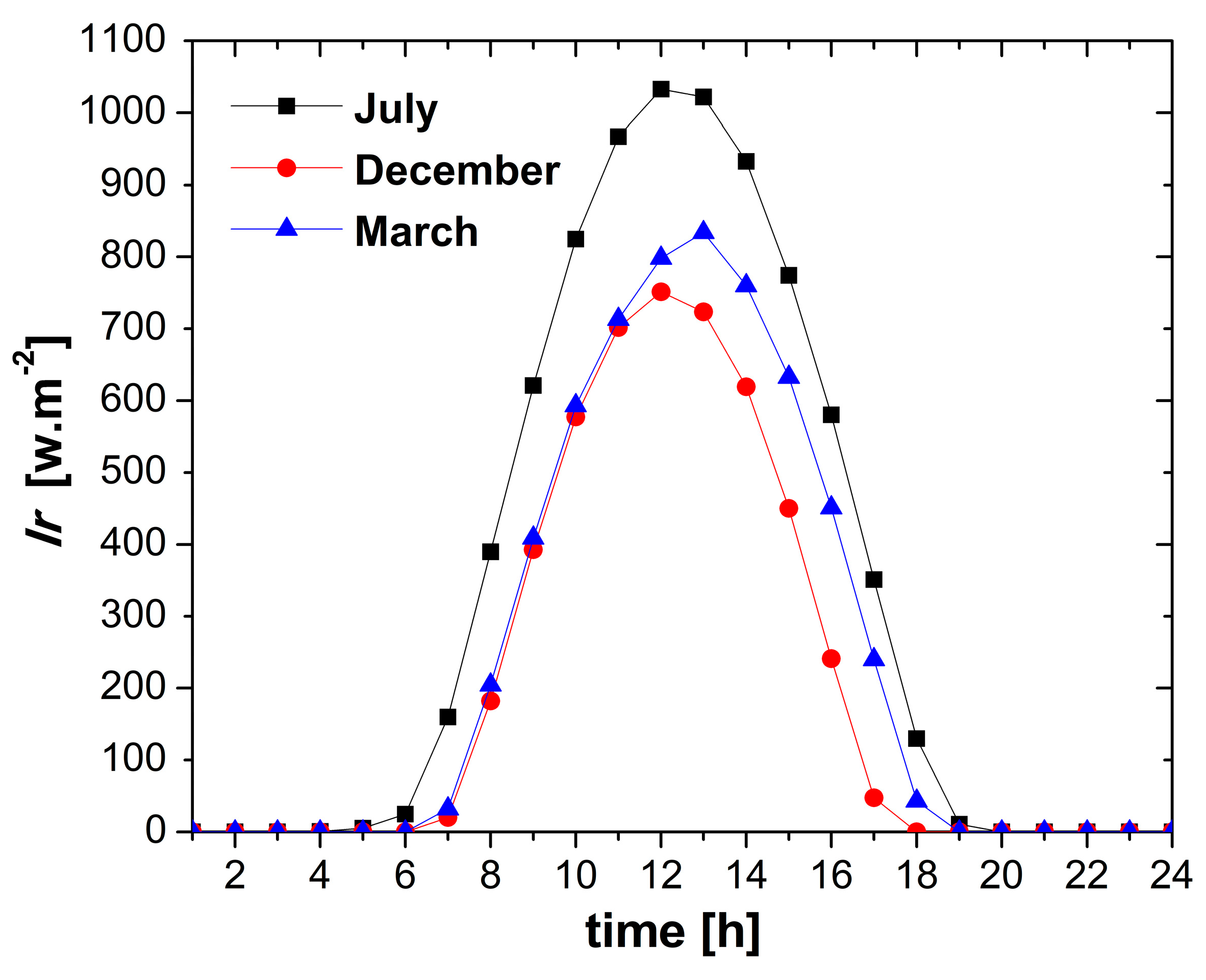
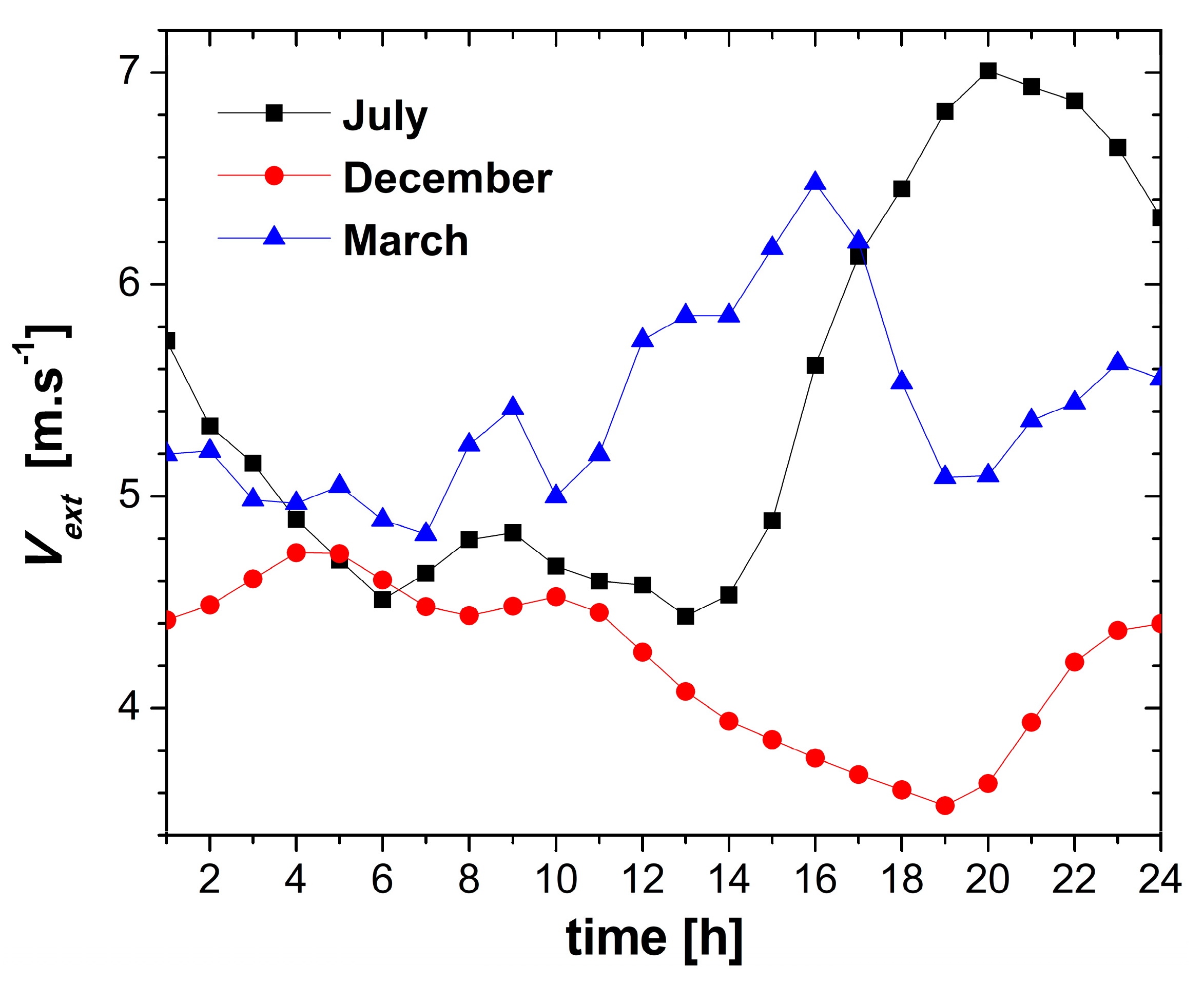
4.1. Weather Conditions
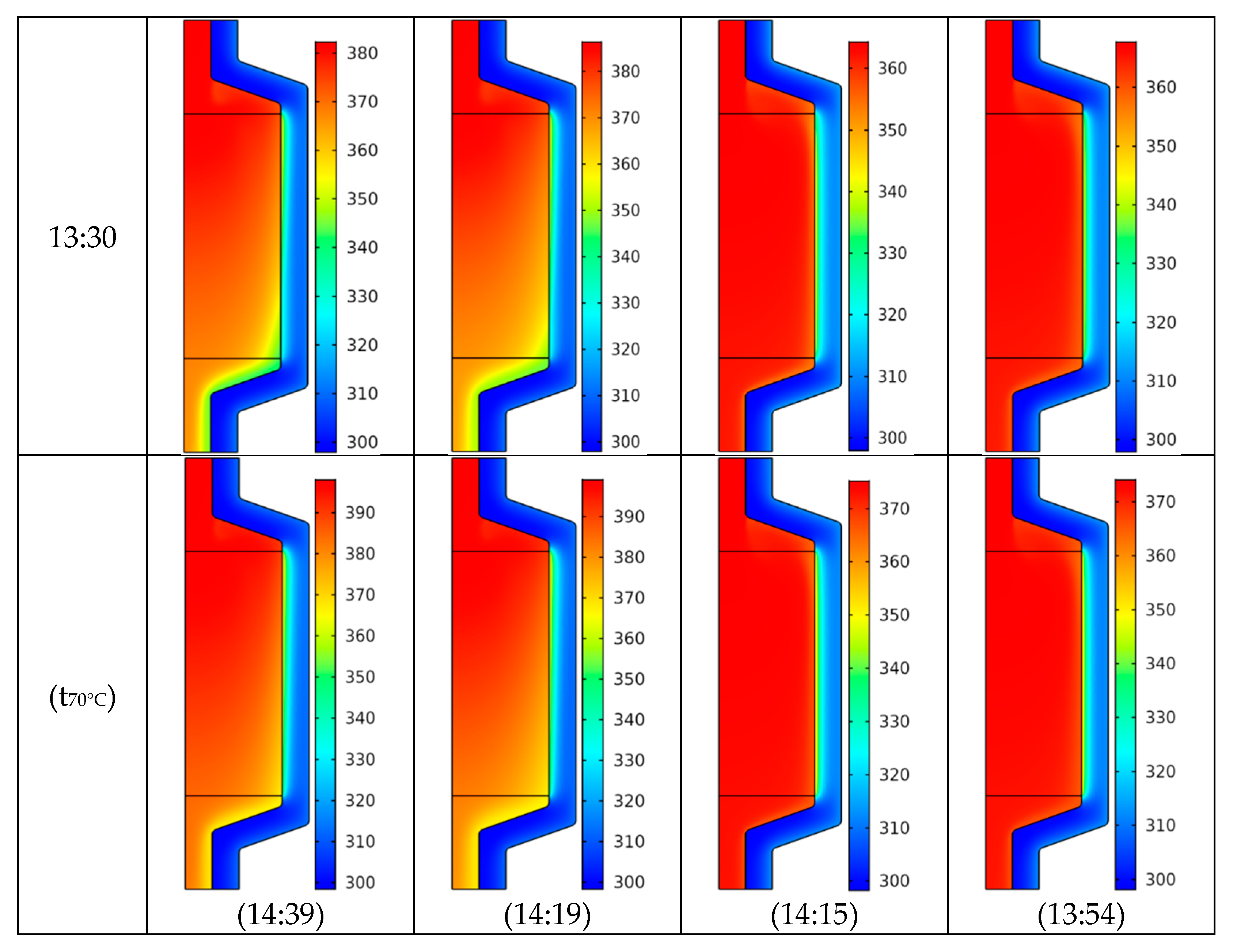
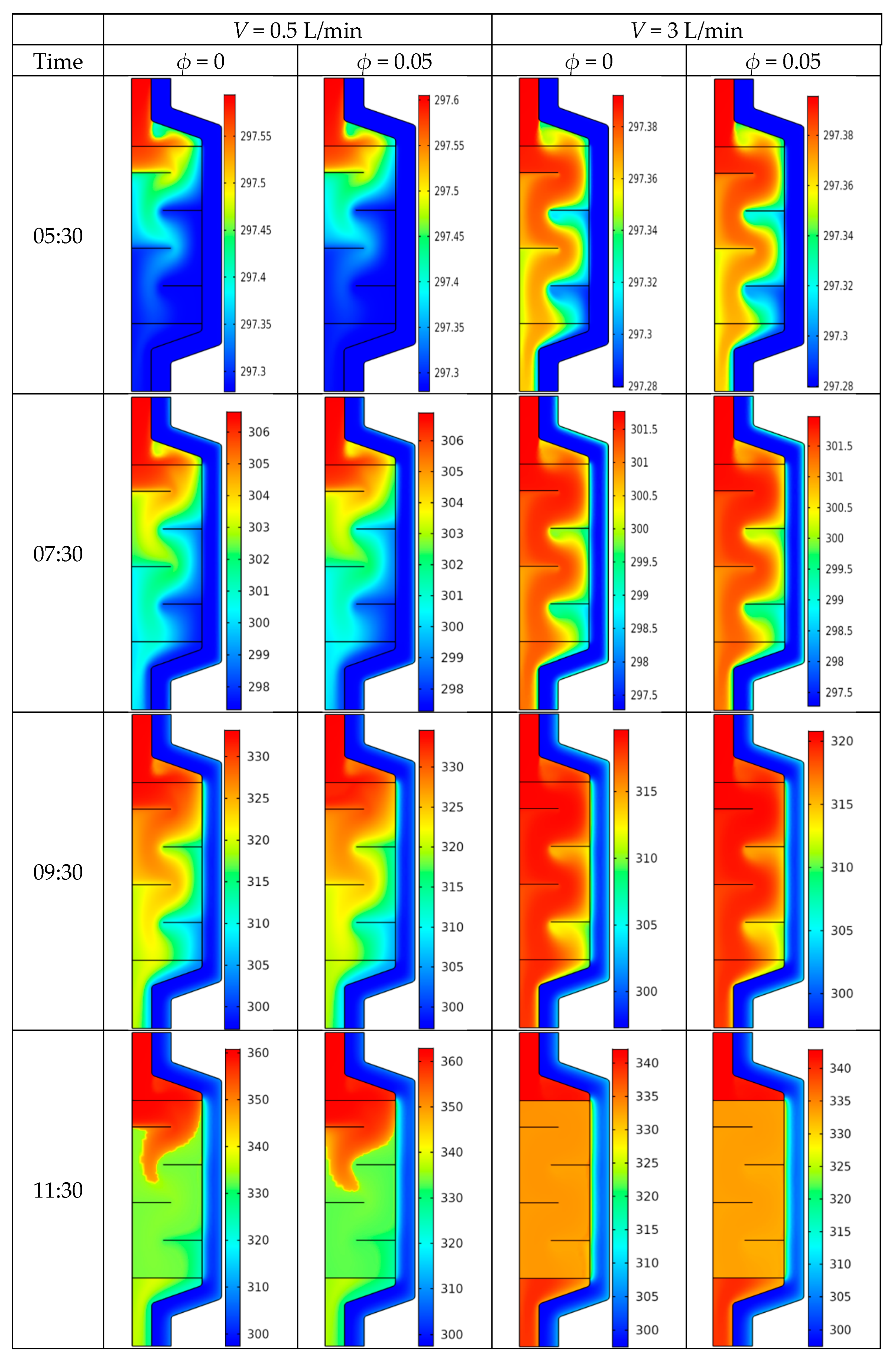
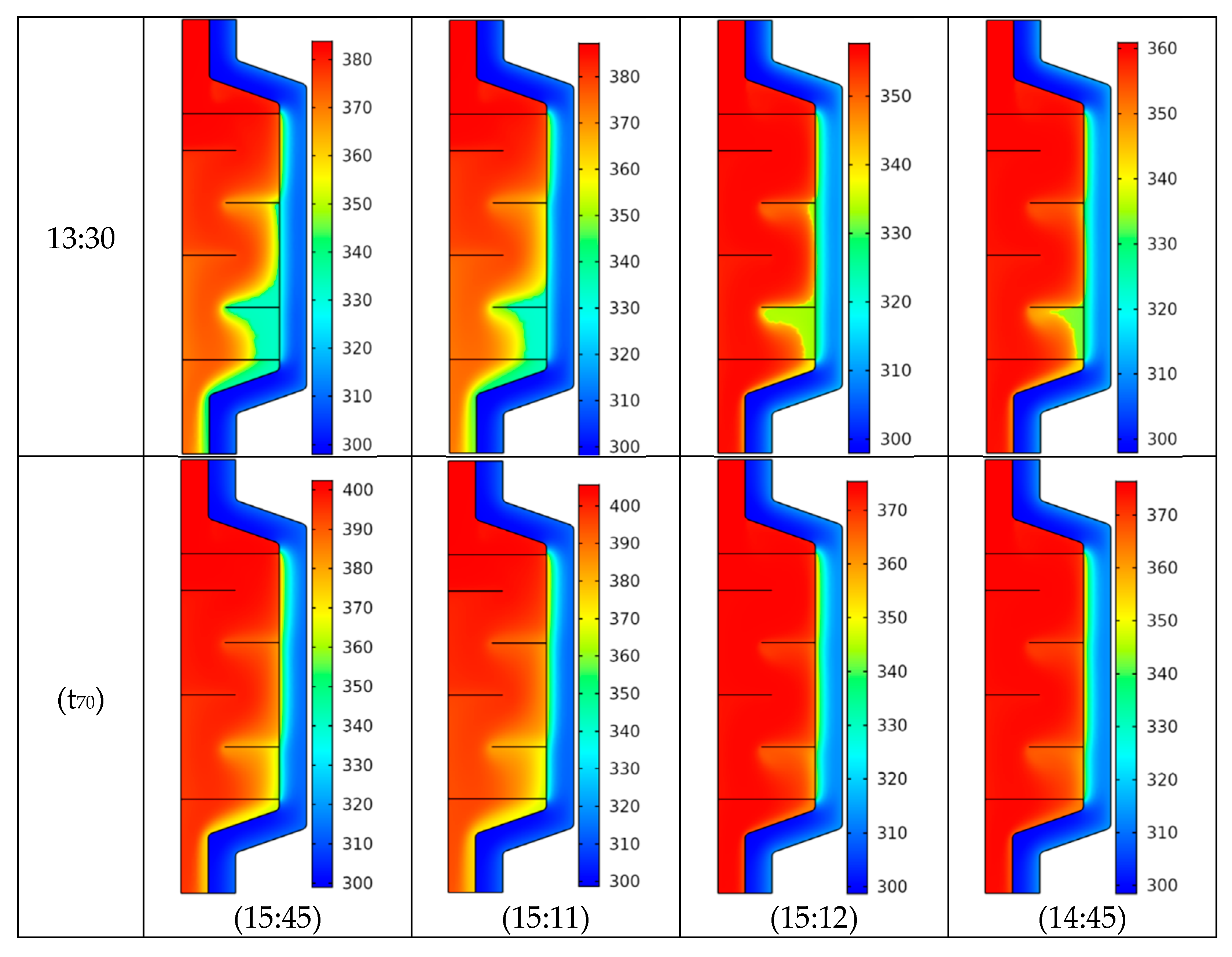
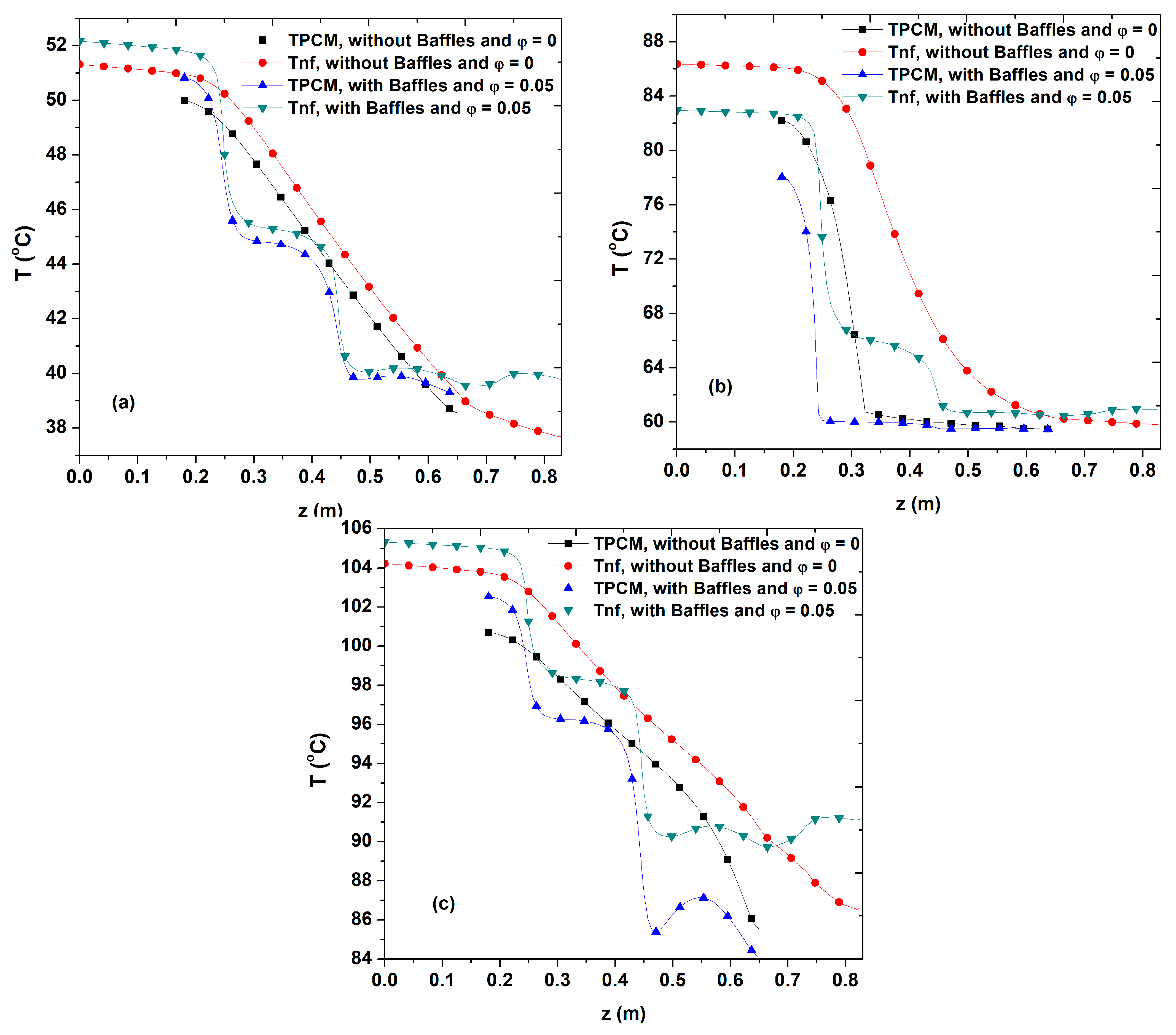
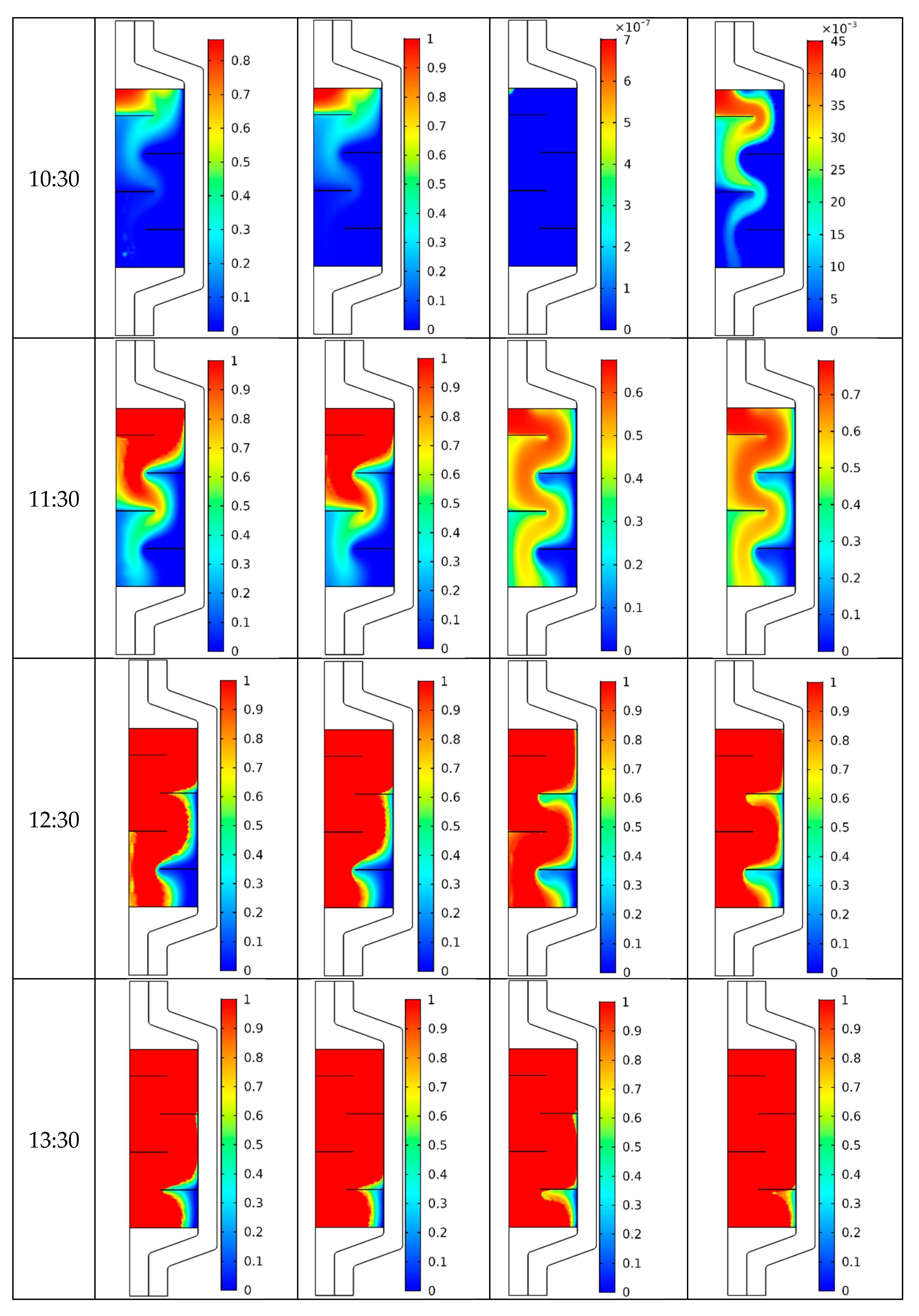
4.2. Temperature Field
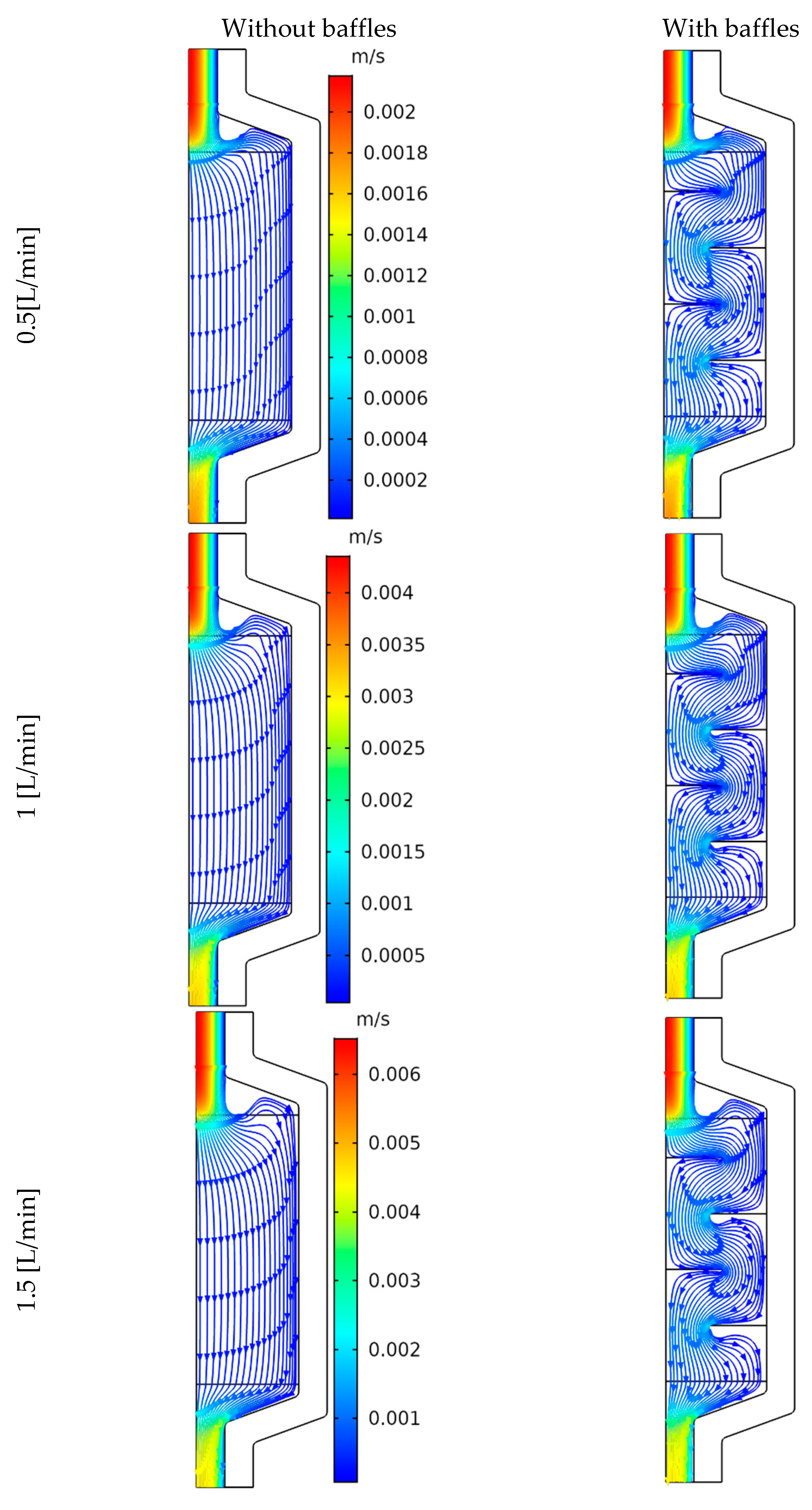
4.3. Flow Structure
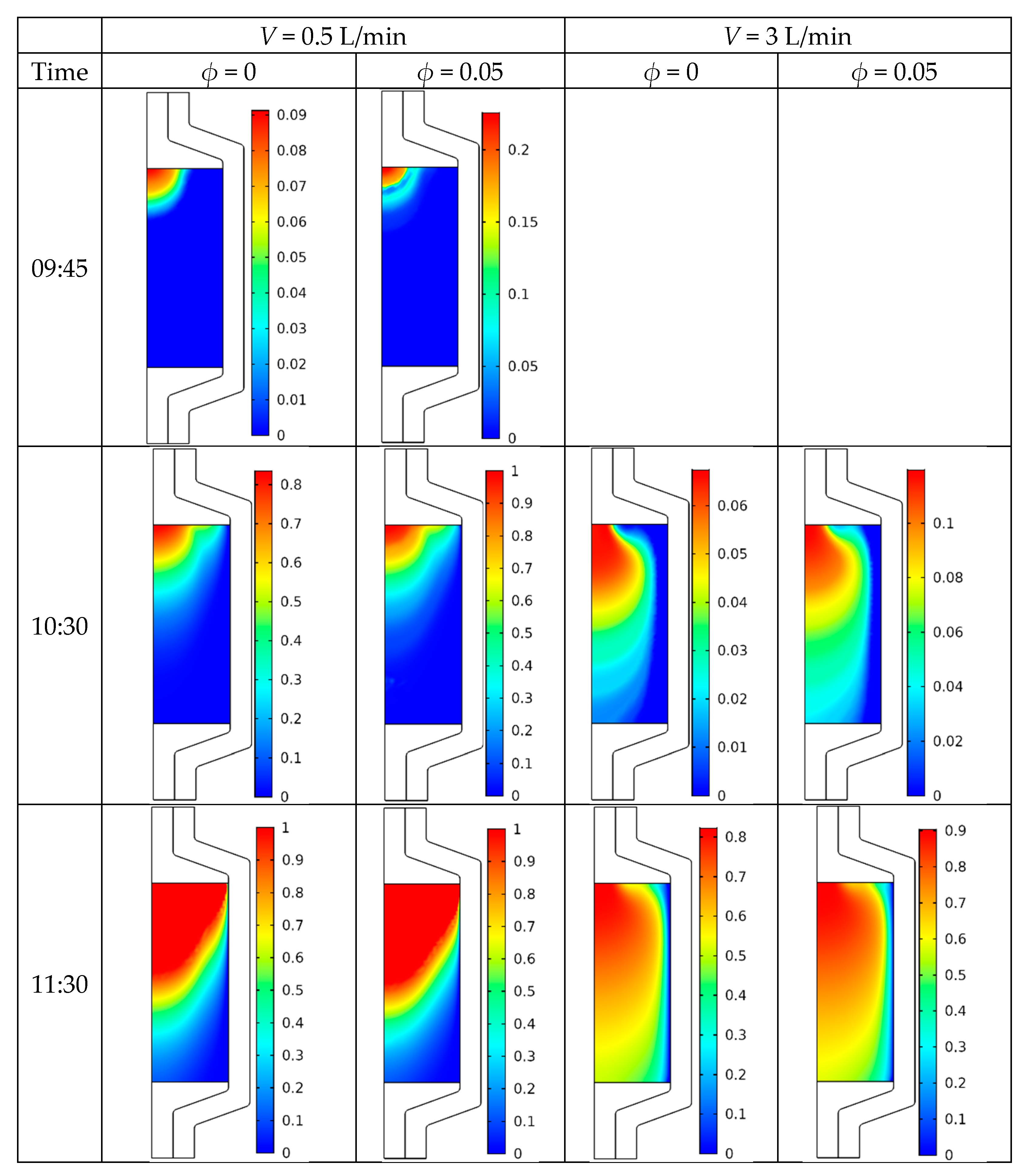
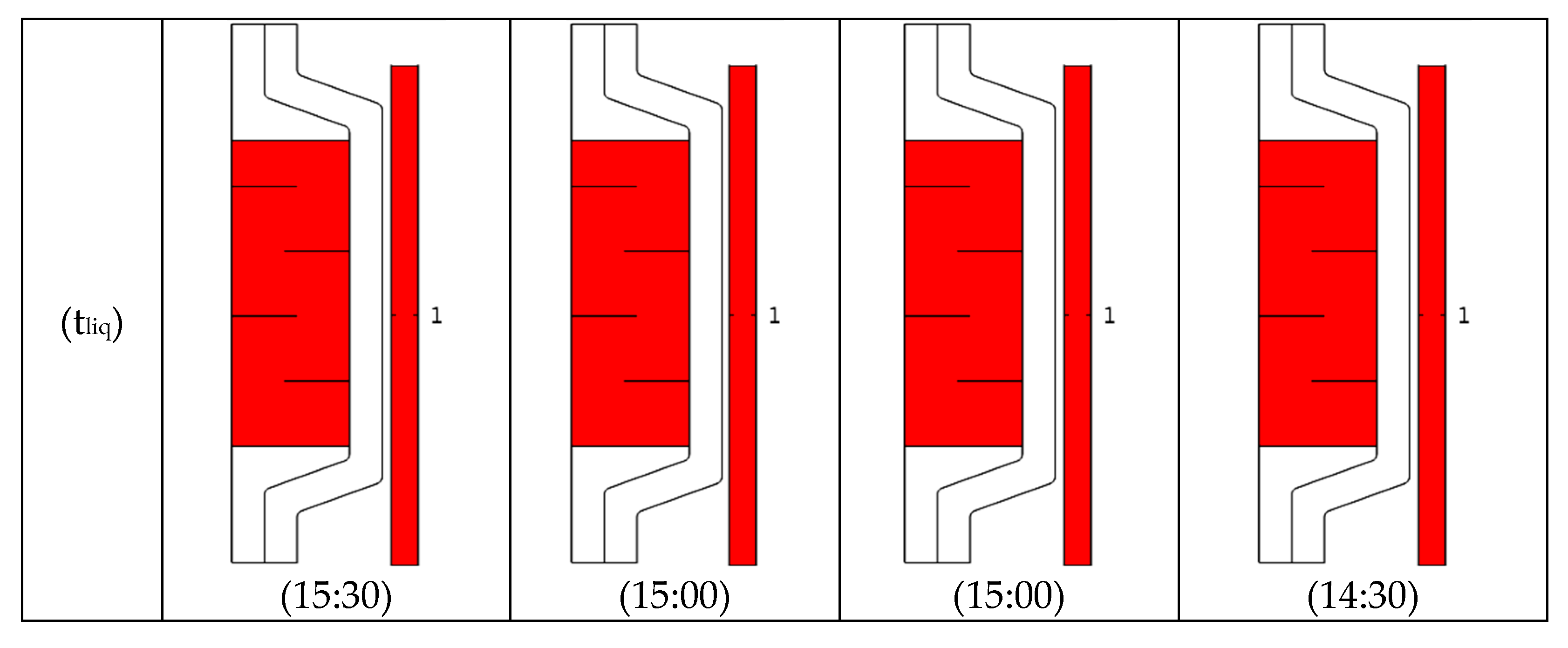
4.4. Melting Process
5. Conclusions
- -
- A similar pattern of the temperature distribution inside the PCM energy storage tank was noticed with and without the use of CNT nanofluid.
- -
- For all the considered cases, the temperature began to increase gradually with time.
- -
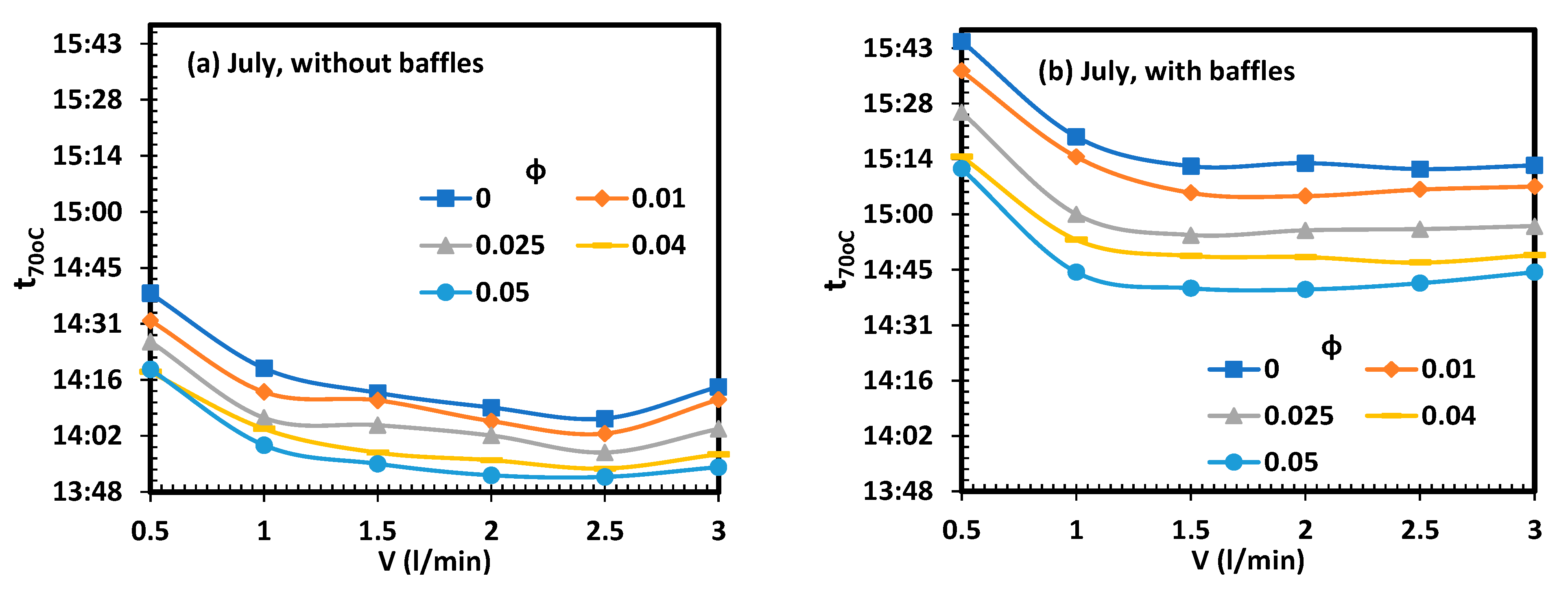
- The increase in the volume flow rate and addition of CNT nanoparticles lead to a reduction in the charging time.
- -
- The time required to completely transform the PCM to the liquid was reduced by using CNT nanoparticles.
- -
- The use of baffles opposes the melting process, especially at the corners.
- -
- The midline nanofluid and PCM temperature profiles was found to decrease along the axial axis.
- -
- The optimum melting process occurred for V = 2.5 L/min when no baffles were used and for V = 2 L/min when baffles were used.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghodbane, M.; Bellos, E.; Said, Z.; Boumeddane, B.; Hussein, A.K.; Kolsi, L. Evaluating Energy efficiency and economic effect of heat transfer in copper tube for small solar linear Fresnel reflector. J. Therm. Anal. Calorim. 2021, 143, 4197–4215. [Google Scholar] [CrossRef]
- Ghodbane, M.; Boumeddane, B.; Hussein, A.K.; Li, D.; Sivasankaran, S. Optical numerical investigation of a solar power plant of parabolic trough collectors. J. Therm. Eng. 2021, 7, 550–569. [Google Scholar] [CrossRef]
- Ben Abderrahmane, A.; Benazza, A.; Hussein, A.K. Heat transfer enhancement analysis of tube receiver for parabolic trough solar collector with central corrugated insert. J. Heat Transf.-Trans. ASME 2020, 142, 062001. [Google Scholar] [CrossRef]
- Hussein, A.K.; Walunj, A.; Kolsi, L. Applications of nanotechnology to enhance the performance of the direct absorption solar collectors. J. Therm. Eng. 2016, 2, 529–540. [Google Scholar] [CrossRef]
- Ghodbane, M.; Boumeddane, B.; Hussein, A.K.; Ali, H.; Li, D. Thermal numerical investigation of a small parabolic trough collector under desert climatic conditions. J. Therm. Eng. 2021, 7, 429–446. [Google Scholar] [CrossRef]
- Riahi, S.; Liu, M.; Jacob, R.; Belusko, M.; Bruno, F. Assessment of exergy delivery of thermal energy storage systems for CSP plants: Cascade PCMs, graphite-PCMs and two-tank sensible heat storage systems. Sustain. Energy Technol. Assess. 2020, 42, 100823. [Google Scholar] [CrossRef]
- Elfeky, K.; Li, X.; Ahmed, N.; Lu, L.; Wang, Q. Optimization of thermal performance in thermocline tank thermal energy storage system with the multilayered PCM(s) for CSP tower plants. Appl. Energy 2019, 243, 175–190. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zheng, Y.; Liu, C.; Hussein, A.K.; Liu, X. Thermal performance of a PCM-filled double-glazing unit with different thermophysical parameters of PCM. Sol. Energy 2016, 133, 207–220. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Li, D.; Ma, T.; Hussein, A.K.; Zhou, Y. Investigation of thermal and optical performance of a phase change material filled double-glazing unit. J. Build. Phys. 2018, 42, 99–119. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, Y. Thermal energy storage performance of a three-PCM cascade tank in a high-temperature packed bed system. Renew. Energy 2020, 152, 110–119. [Google Scholar] [CrossRef]
- Galione, P.; Pérez-Segarra, C.; Rodriguez, I.; Torras, S.; Rigola, J. Numerical evaluation of multi-layered solid-PCM thermocline-like tanks as thermal energy storage systems for CSP applications. Energy Procedia 2015, 69, 832–841. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Ding, Z. Investigation on the energy performance of using air-source heat pump to charge PCM storage tank. J. Energy Storage 2020, 28, 101270. [Google Scholar] [CrossRef]
- Fang, Y.; Niu, J.; Deng, S. An analytical technique for the optimal designs of tube-in-tank thermal energy storage systems using PCM. Int. J. Heat Mass Transf. 2019, 128, 849–859. [Google Scholar] [CrossRef]
- Saw, C.; Al-Kayiem, H.; Aris, M. Experimental investigation on the performance of integrated PCM-flat plate solar collector. J. Appl. Sci. 2012, 12, 2390–2396. [Google Scholar]
- Sharma, S.; Micheli, L.; Chang, W.; Tahir, A.; Reddy, K.; Mallick, T. Nano-enhanced phase change material for thermal management of BICPV. Appl. Energy 2017, 208, 719–733. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Solomon, G.; Kumaresan, V.; Velraj, R. Parametric studies on packed bed storage unit filled with PCM encapsulated spherical containers for low temperature solar air heating applications. Energy Convers. Manag. 2014, 78, 74–80. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Sarafraz, P.; Cotton, J.; Lightstone, M. Heat transfer characteristics of a hybrid thermal energy storage tank with phase change materials (PCMs) during indirect charging using isothermal coil heat exchanger. Sol. Energy 2017, 157, 462–476. [Google Scholar] [CrossRef]
- Delgado, M.; Lazaro, A.; Mazo, J.; Penalosa, C.; Marin, J.; Zalba, B. Experimental analysis of a coiled stirred tank containing a low cost PCM emulsion as a thermal energy storage system. Energy 2017, 138, 590–601. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, P. Experimental and numerical investigation of a tube-in-tank latent thermal energy storage unit using composite PCM. Appl. Energy 2017, 190, 524–539. [Google Scholar] [CrossRef]
- Bouhal, T.; El- Rhafiki, T.; Kousksou, T.; Jamil, A.; Zeraouli, Y. PCM addition inside solar water heaters: Numerical comparative approach. J. Energy Storage 2018, 19, 232–246. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Zhang, H.; Dou, B.; Huang, X.; Liang, H.; Goula, M. An experimental investigation on thermal stratification characteristics with PCMs in solar water tank. Sol. Energy 2019, 177, 8–21. [Google Scholar] [CrossRef]
- Mousa, H.; Naser, J.; Houche, O. Using PCM as energy storage material in water tanks: Theoretical and experimental investigation. J. Energy Storage 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Lee, S.; Gim, J.; Chung, J. Numerical analysis in a full-scale thermal energy storage tank with dual PCM capsules. Energy Build. 2019, 204, 109410. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, Y.; Lin, J.; Zhou, T.; Liu, Y.; Zhao, Q. Improvement of the efficiency of solar thermal energy storage systems by cascading a PCM unit with a water tank. J. Clean. Prod. 2020, 245, 118864. [Google Scholar] [CrossRef]
- Carmona, M.; Rincon, A.; Gulfo, L. Energy and exergy model with parametric study of a hot water storage tank with PCM for domestic applications and experimental validation for multiple operational scenarios. Energy Convers. Manag. 2020, 222, 113189. [Google Scholar] [CrossRef]
- Saw, C.; Al-Kayiem, H.; Owolabi, A. Experimental investigation on the effect of PCM and nano-enhanced PCM of integrated solar collector performance. WIT Trans. Ecol. Environ. 2013, 179, 899–909. [Google Scholar] [CrossRef]
- Al-Kayiem, H.; Lin, S. Performance evaluation of a solar water heater integrated with a PCM nanocomposite TES at various inclinations. Sol. Energy 2014, 109, 82–92. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Experimental and numerical study of heat transfer performance of nitrate/expanded graphite composite PCM for solar energy storage. Energy Convers. Manag. 2015, 105, 272–284. [Google Scholar] [CrossRef]
- Mahdi, J.; Nsofor, E. Solidification of a PCM with nanoparticles in triplex-tube thermal energy storage system. Appl. Therm. Eng. 2016, 108, 596–604. [Google Scholar] [CrossRef]
- Lin, S.; Al-Kayiem, H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Biwole, P. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, S.; Singh, P.; Mishra, S.; Bishwakarma, H.; Choudhry, N.; Nayak, R.; Das, A. Performance investigation of CuO-paraffin wax nanocomposite in solar water heater during night. Thermochim. Acta 2019, 671, 36–42. [Google Scholar] [CrossRef]
- Kumar, P.; Mylsamy, K. A comprehensive study on thermal storage characteristics of nano-CeO2 embedded phase change material and its influence on the performance of evacuated tube solar water heater. Renew. Energy 2020, 162, 662–676. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, S.; Singh, P.; Mishra, S.; Singh, D. Performance investigation of nanocomposite based solar water heater. Energy 2020, 198, 117295. [Google Scholar] [CrossRef]
- Kumar, P.; Mylsamy, K.; Alagar, K.; Sudhakar, K. Investigations on an evacuated tube solar water heater using hybrid-nano based organic phase change material. Int. J. Green Energy 2020, 17, 872–883. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Tasnim, S.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Saw, C.; Al-Kayiem, H. Thermophysical properties of nano-phase change material composition for thermal energy storage. Appl. Mech. Mater. 2012, 232, 127–131. [Google Scholar]
- Owolabi, A.; Al-Kayiem, H.; Baheta, A. Nanoadditives induced enhancement of the thermal properties of paraffin-based nanocomposites for thermal energy storage. Sol. Energy 2016, 135, 644–653. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical simulation for solidification in a LHTESS by means of nano-enhanced PCM. J. Taiwan Inst. Chem. Eng. 2018, 86, 25–41. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Devaraju, A.; Kumar, G.; Kalaiselvam, S. Improved thermal energy storage behavior of a novel nanofluid as phase change material (PCM). Mater. Today Proc. 2019, 9, 410–421. [Google Scholar] [CrossRef]
- Dawood, M.; Nabil, T.; Kabeel, A.; Shehata, A.; Abdalla, A.; Elnaghi, B. Experimental study of productivity progress for a solar still integrated with parabolic trough collectors with a phase change material in the receiver evacuated tubes and in the still. J. Energy Storage 2020, 32, 102007. [Google Scholar] [CrossRef]
- Naghdbishi, A.; Yazdi, M.; Akbari, G. Experimental investigation of the effect of multi-wall carbon nano tube—Water/glycol based nanofluids on a PVT system integrated with PCM covered collector. Appl. Therm. Eng. 2020, 178, 115556. [Google Scholar] [CrossRef]
- Ji, C.; Qin, Z.; Dubey, S.; Choo, F.; Duan, F. Simulation on PCM melting enhancement with double-fin length arrangements in a rectangular enclosure induced by natural convection. Int. J. Heat Mass Transf. 2018, 127, 255–265. [Google Scholar] [CrossRef]
- Gharebaghi, M.; Sezai, I. Enhancement of heat transfer in latent heat storage modules with internal fins. Numer. Heat Transf. Part A Appl. 2007, 53, 749–765. [Google Scholar] [CrossRef]
- Touati, B.; Kerroumi, N.; Virgone, J. Solar thermal energy discharging from a multiple phase change materials storage tank. Appl. Sol. Energy 2017, 53, 185–189. [Google Scholar] [CrossRef]
- Sathe, T.; Dhoble, A. Thermal analysis of an inclined heat sink with finned PCM container for solar applications. Int. J. Heat Mass Transf. 2019, 144, 118679. [Google Scholar] [CrossRef]
- Gürtürk, M.; Kok, B. A new approach in the design of heat transfer fin for melting and solidification of PCM. Int. J. Heat Mass Transf. 2020, 153, 119671. [Google Scholar] [CrossRef]
- Singh, R.; Xu, H.; Kaushik, S.; Rakshit, D.; Romagnoli, A. Effective utilization of natural convection via novel fin design and influence of enhanced viscosity due to carbon nano-particles in a solar cooling thermal storage system. Sol. Energy 2019, 183, 105–119. [Google Scholar] [CrossRef]
- Mousavi, S.; Siavashi, M.; Heyhat, M. Numerical melting performance analysis of a cylindrical thermal energy storage unit using nano-enhanced PCM and multiple horizontal fins. Numer. Heat Transf. Part A Appl. 2019, 75, 560–577. [Google Scholar] [CrossRef]
- Foudhil, W.; Dhifaoui, B.; Jabrallah, S.B.; Dutil, Y.; Rousse, D.R. Numerical simulation of thermal storage by latent and sensible heat in a porous vertical channel: Performance analysis and comparison. Numer. Heat Transf. Part A Appl. 2012, 62, 948–972. [Google Scholar] [CrossRef]
- Nield, D.; Bejan, A. Convection in Porous Media, in Convection Heat Transfer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wakao, N.; Kaguei, S.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to fluid heat transfer coefficients in packed beds: Correlation of Nusselt numbers. Chem. Eng. Sci. 1979, 34, 325–336. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Bandpy, M.; Ashorynejad, H. Lattice Boltzmann method for simulation of magnetic field effect on hydrothermal behavior of nanofluid in a cubic cavity. Phys. A 2015, 432, 58–70. [Google Scholar] [CrossRef]
- Sajjadi, H.; Amiri Delouei, A.; Atashafrooz, M.; Sheikholeslami, M. Double MRT Lattice Boltzmann simulation of 3D MHD natural convection in a cubic cavity with sinusoidal temperature distribution utilizing nanofluid. Int. J. Heat Mass Transf. 2018, 126, 489–503. [Google Scholar] [CrossRef]
- Kolsi, L.; Oztop, H.; Ghachem, K.; Al-Meshaal, M.; Mohammed, H.; Babazadeh, H.; Abu-Hamdeh, N. Numerical study of periodic magnetic field effect on 3D natural convection of MWCNT–water/nanofluid with consideration of aggregation. Processes 2019, 7, 957. [Google Scholar] [CrossRef]
- Reddy, J. An Introduction to the Finite Element Method; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Basak, T.; Roy, S.; Babu, S.K.; Pop, I. Finite element simulations of natural convection flow in an isosceles triangular enclosure filled with a porous medium: Effects of various thermal boundary conditions. Int. J. Heat Mass Transf. 2008, 51, 2733–2741. [Google Scholar] [CrossRef]
- Basak, T.; Ayappa, K.G. Influence of internal convection during microwave thawing of cylinders. AIChE J. 2001, 47, 835–850. [Google Scholar] [CrossRef]
- Nallusamy, N.; Sampath, S.; Velraj, R. Study on performance of a packed bed latent heat thermal energy storage unit integrated with solar water heating system. J. Zhejiang Univ.-Sci. A 2006, 7, 1422–1430. [Google Scholar] [CrossRef]









| Material Property | Solid Paraffin | Liquid Paraffin |
|---|---|---|
| Melting temperature, Tm (°C) | 60 | - |
| Latent heat of fusion, L (kJ kg−1) | 213 | - |
| Heat capacity, Cp (J kg−1·K−1) | 1851 | 2384 |
| Density, (kg.m−3) | 861 | 778 |
| Thermal conductivity, k (W m−1 K−1) | 0.4 | 0.15 |
| Water | CNT | |
|---|---|---|
| Specific heat, Cp (J kg−1 K−1) | 4179 | 796 |
| Density, ρ (kg.m−3) | 997.1 | 1600 |
| Thermal conductivity, λ (W m−1 K−1) | 0.613 | 3000 |
| Thermal expansion coefficient, β (K−1) | 21 × 10−5 | 4.2 × 10−5 |
| Dynamic viscosity, μ (Pa s) | 0.85 × 10−3 | - |
| Number of Elements | t70°C | Variation (%) | Incremental Variation (%) |
|---|---|---|---|
| G1: 7790 | 14:07 | - | - |
| G2: 15502 | 13:59 | 0.85694 | - |
| G3: 36857 | 13:54 | 1.51558 | 0.66433 |
| G4: 63121 | 13:53 | 1.64306 | 0.12944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolsi, L.; Hussein, A.K.; Hassen, W.; Ben Said, L.; Ayadi, B.; Rajhi, W.; Labidi, T.; Shawabkeh, A.; Ramesh, K. Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube–Water Nanofluid under Ha’il City Climatic Conditions. Mathematics 2023, 11, 1057. https://doi.org/10.3390/math11041057
Kolsi L, Hussein AK, Hassen W, Ben Said L, Ayadi B, Rajhi W, Labidi T, Shawabkeh A, Ramesh K. Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube–Water Nanofluid under Ha’il City Climatic Conditions. Mathematics. 2023; 11(4):1057. https://doi.org/10.3390/math11041057
Chicago/Turabian StyleKolsi, Lioua, Ahmed Kadhim Hussein, Walid Hassen, Lotfi Ben Said, Badreddine Ayadi, Wajdi Rajhi, Taher Labidi, Ali Shawabkeh, and Katta Ramesh. 2023. "Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube–Water Nanofluid under Ha’il City Climatic Conditions" Mathematics 11, no. 4: 1057. https://doi.org/10.3390/math11041057
APA StyleKolsi, L., Hussein, A. K., Hassen, W., Ben Said, L., Ayadi, B., Rajhi, W., Labidi, T., Shawabkeh, A., & Ramesh, K. (2023). Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube–Water Nanofluid under Ha’il City Climatic Conditions. Mathematics, 11(4), 1057. https://doi.org/10.3390/math11041057









