Global Dynamics of Viral Infection with Two Distinct Populations of Antibodies
Abstract
1. Introduction
2. Model with Two Antibodies
2.1. Preliminary Results
2.2. Global Stability
- (i)
- The uninfected equilibrium is globally asymptotically stable (G.A.S) in Ξ if ,
- (ii)
- is unstable if .
- (i)
- DefineWe observe that for all and . Calculating along the solutions of (5)–(9) as:Using , and , we obtainIf , then , for all . Moreover, when , , and for all t. The solutions of system (5)–(9) converge to [48]. The set has elements satisfying , , and . We find from Equation (7) that
- (ii)
- The Jacobian matrix of system (5)–(9) is calculated as:Then, the characteristic equation at the equilibrium is given bywhere is the eigenvalue andClearly if , then and Equation (20) has a positive root, and hence, is unstable.
3. Model with Latency
3.1. Preliminary Results
3.2. Global Stability
- (i)
- If then the uninfected equilibrium of system (21)–(26) is G.A.S in ,
- (ii)
- if , then is unstable.
- (i)
- DefineObserve that for all and Calculating along the solutions of (21)–(26) as:Using , and we obtainTherefore, if , then for all . Moreover, when , , and for all t. The solutions of system (21)–(26) converge to , which contains elements that satisfy , , and . It follows from Equation (24) thatFurthermore, from Equation (23) we haveHence, and L-LAS theorem provides that is G.A.S in .
- (ii)
- The Jacobian matrix of system (21)–(26) is calculated as:Then, the characteristic equation at the equilibrium is given bywhere is the eigenvalue andClearly, if , then and Equation (37) has a positive root, and hence, is unstable.
4. Numerical Simulations
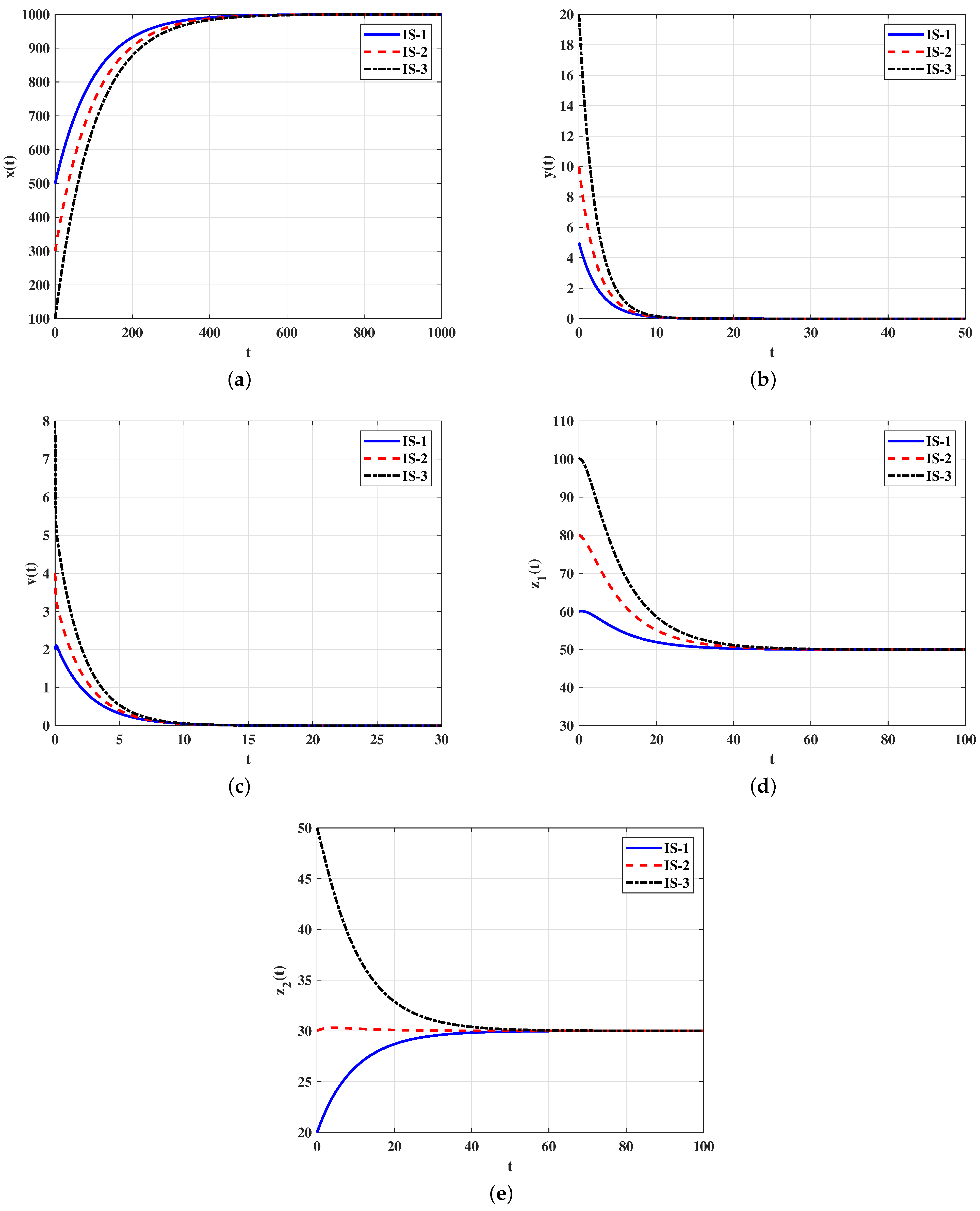
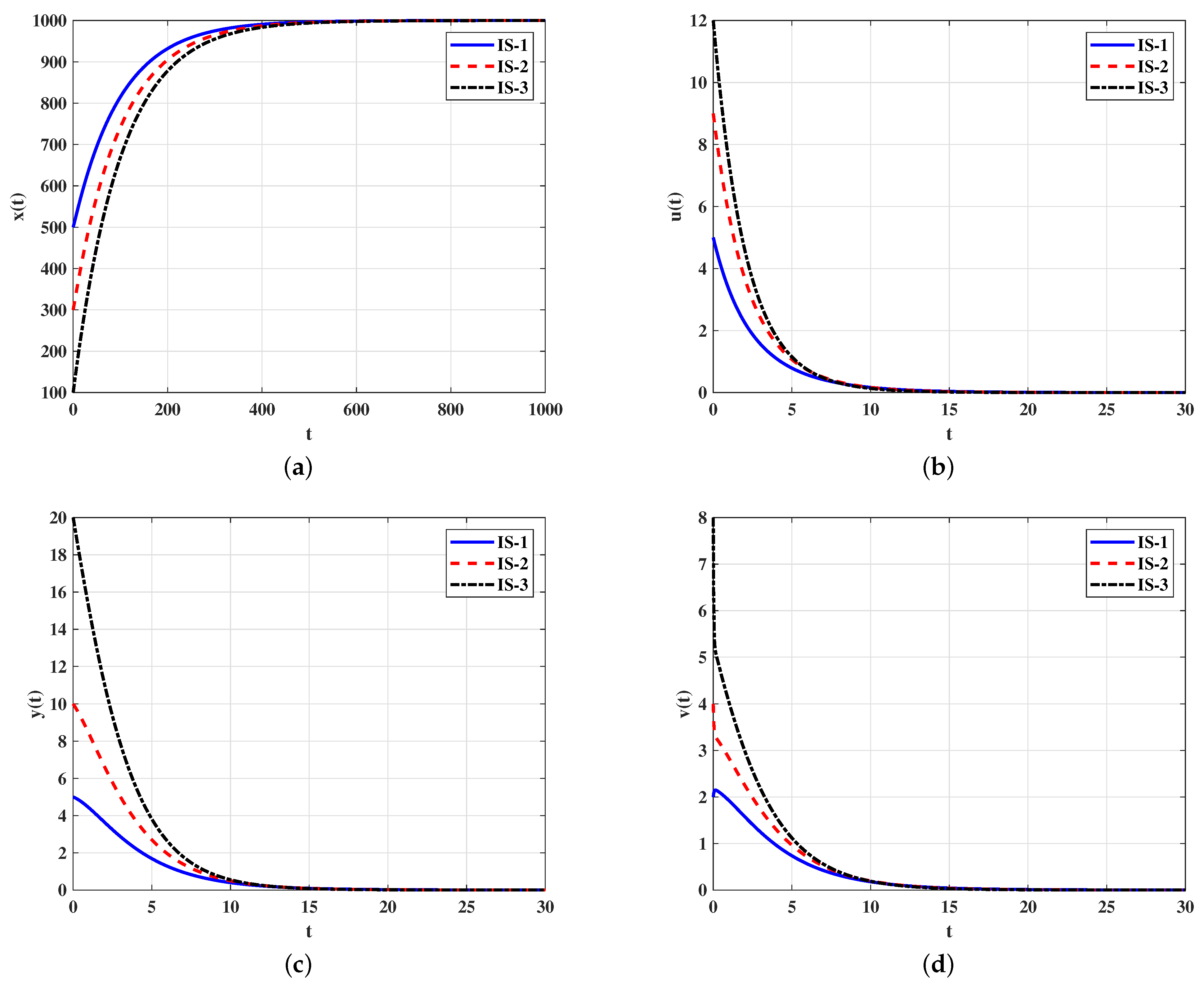
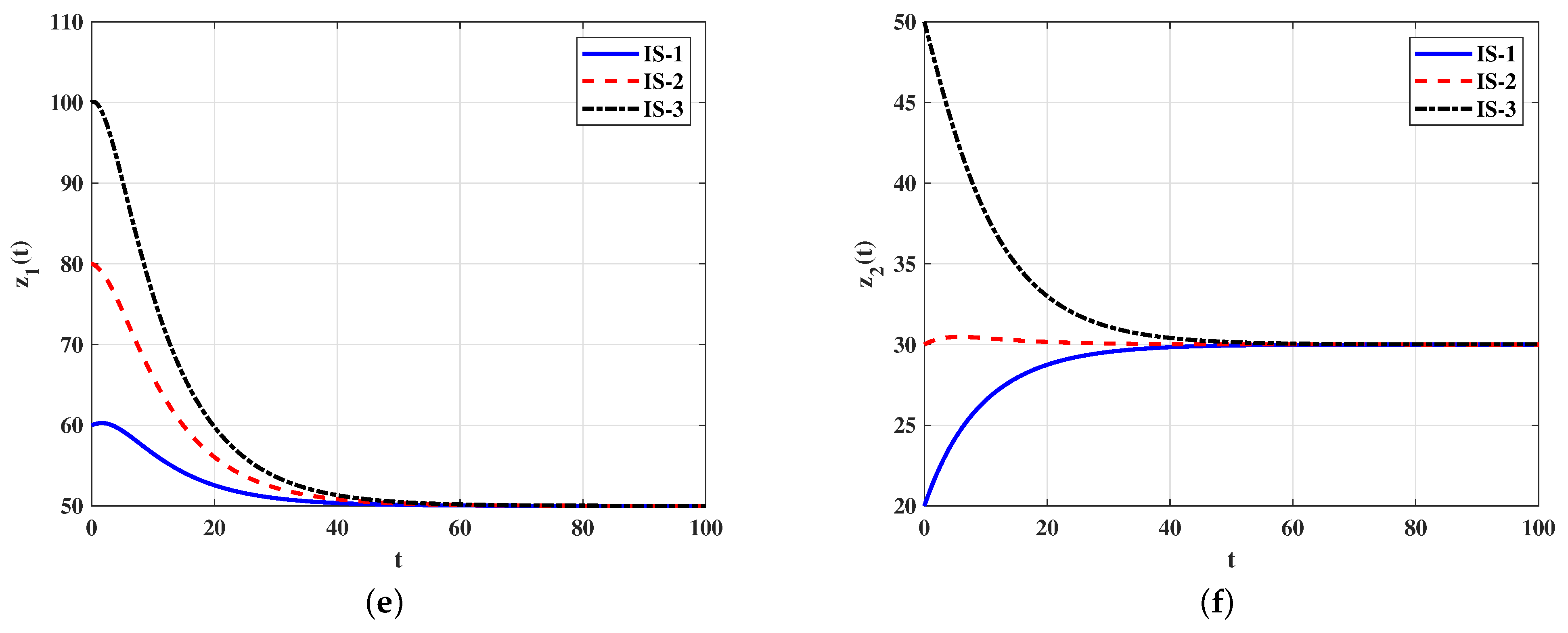
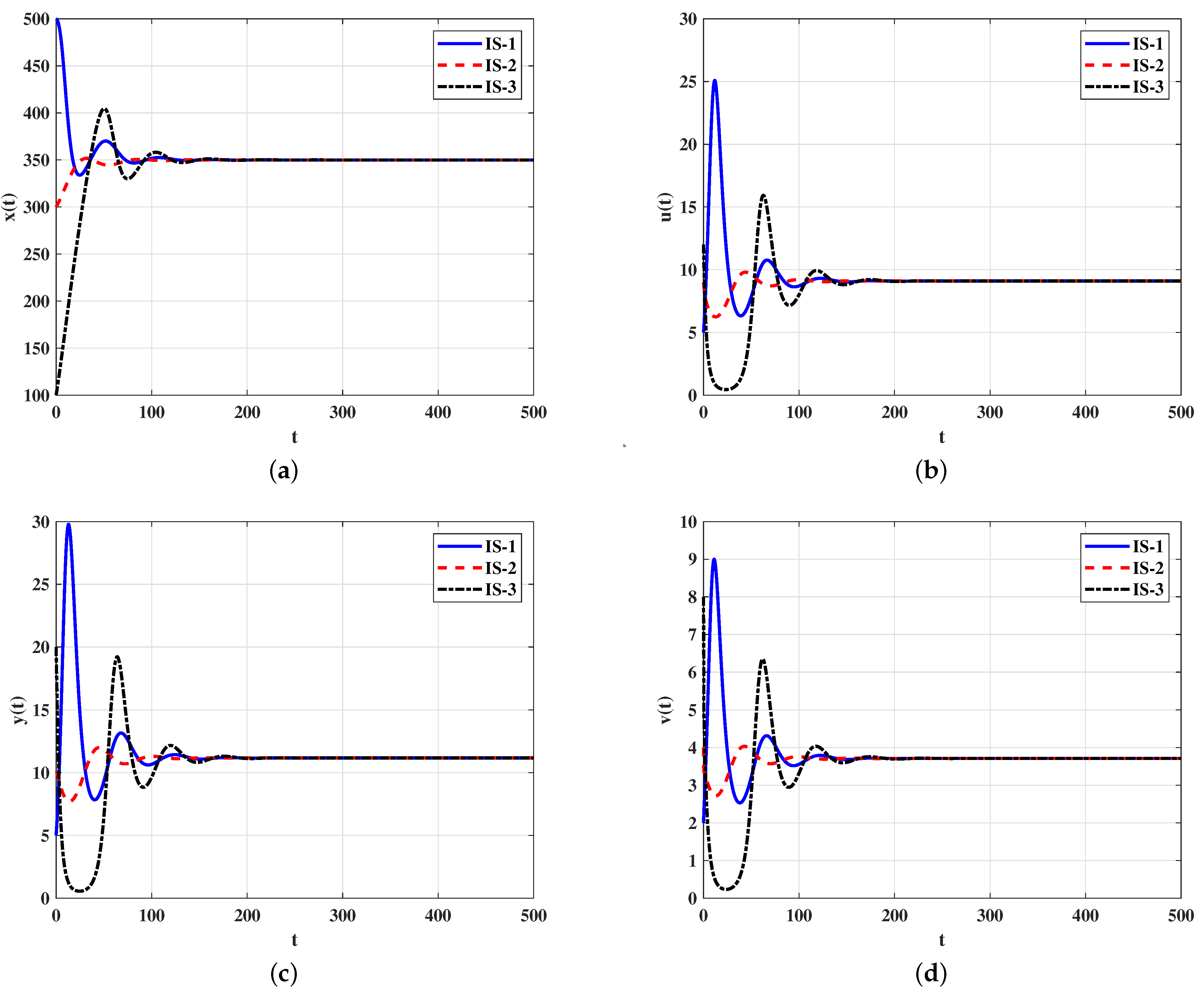
4.1. Numerical Simulations for Model (5)–(9)
Stability of Equilibria
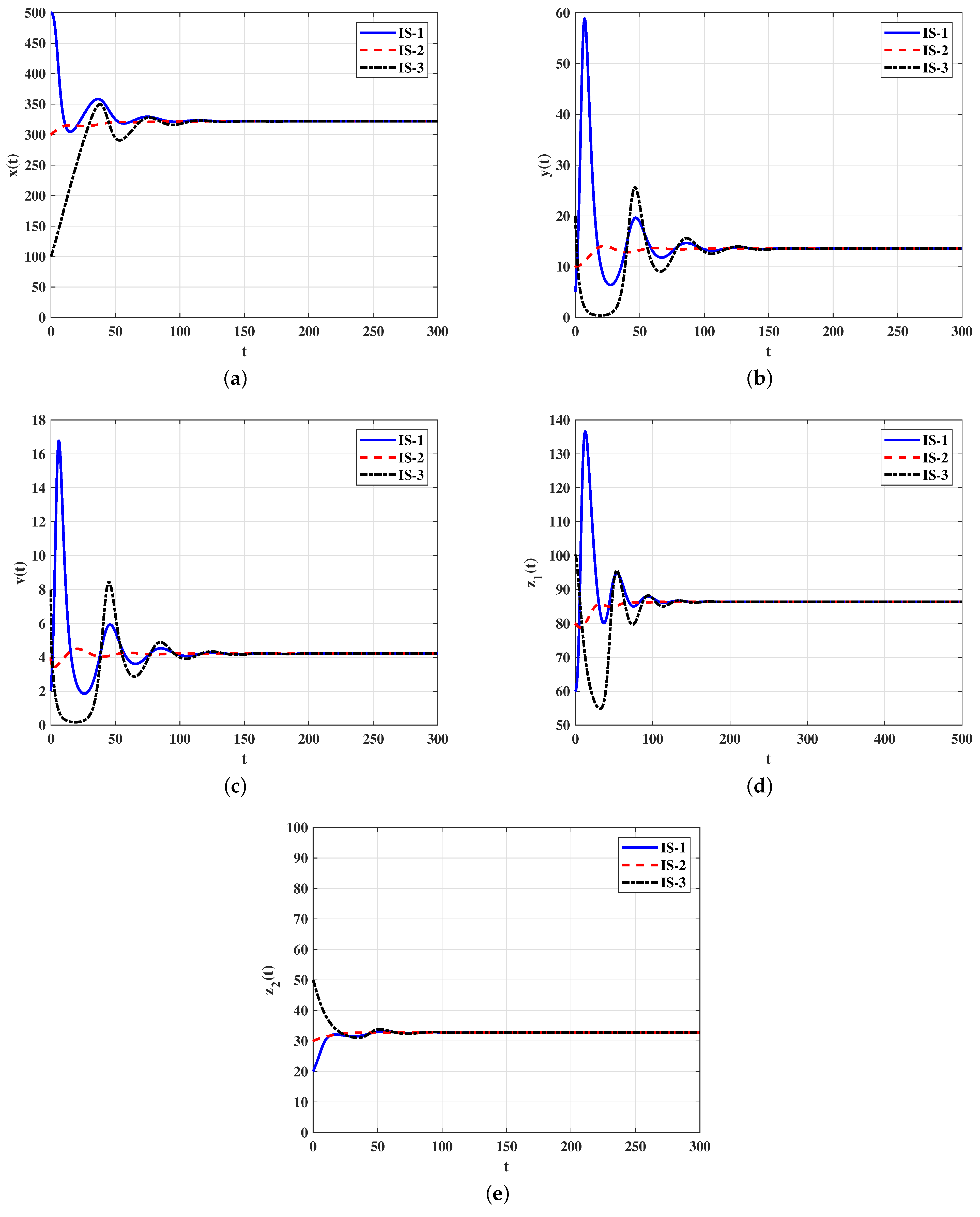
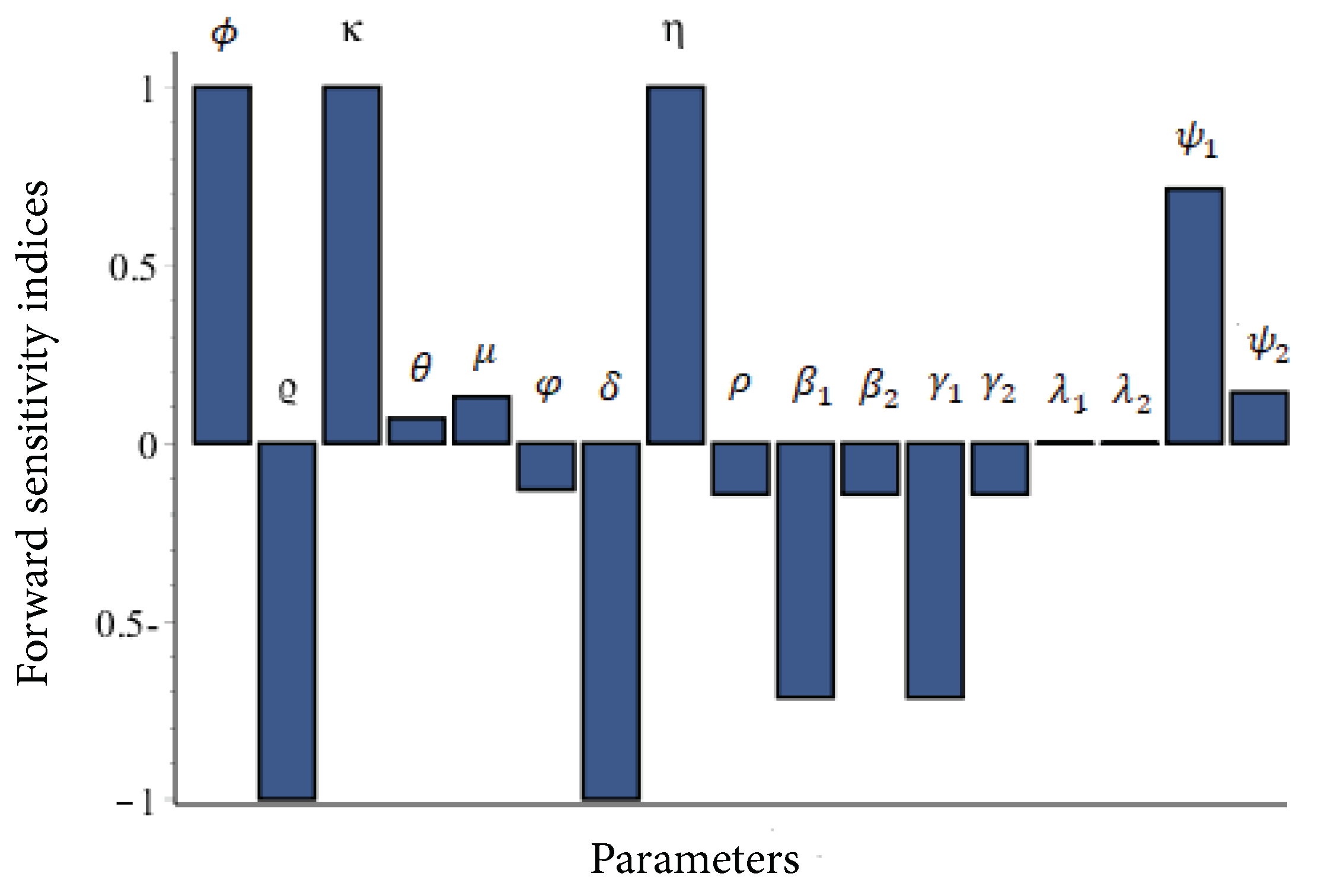
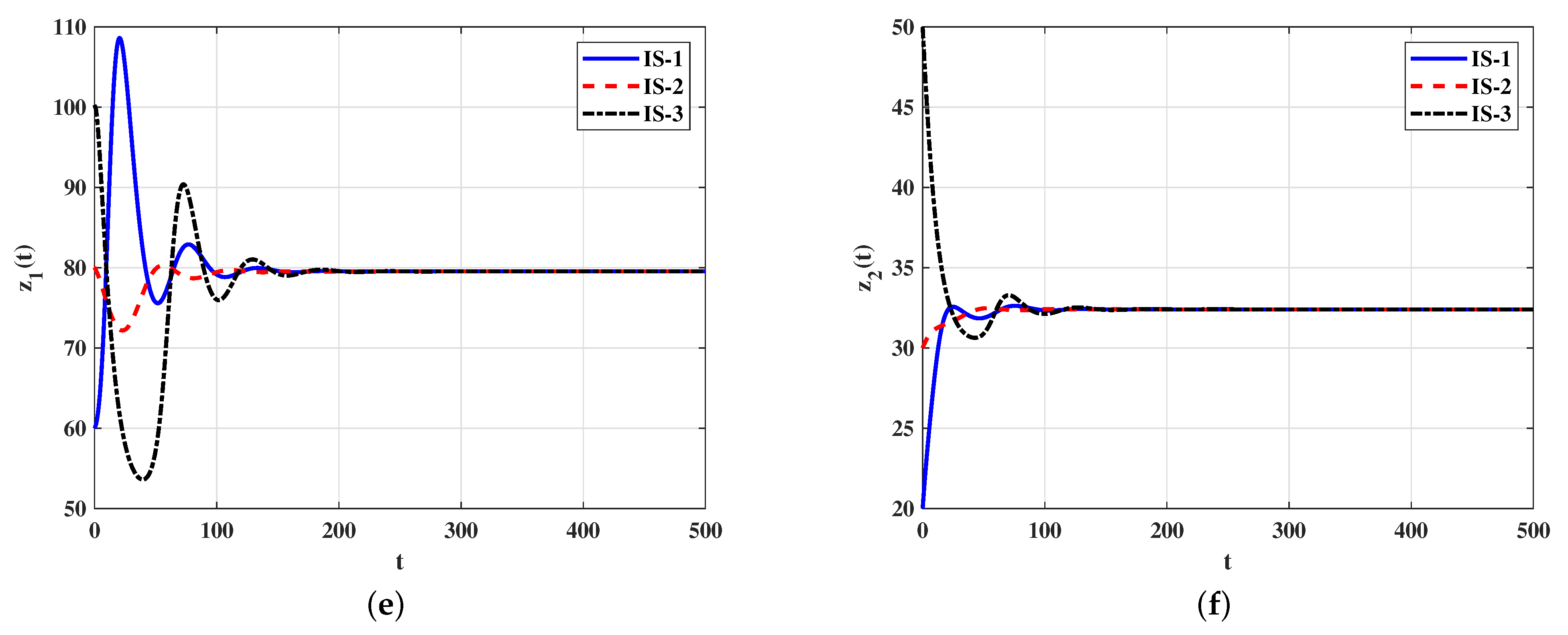
4.2. Numerical Simulations for Model (21)–(26)
4.2.1. Sensitivity Analysis
4.2.2. Stability of Equilibria
5. Discussions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitagawa, K.; Kuniya, T.; Nakaoka, S.; Asai, Y.; Watashi, K.; Iwami, S. Mathematical analysis of a transformed ODE from a PDE multiscale model of hepatitis C virus infection. Bull. Math. 2019, 81, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Li, Y.; Zheng, D. Global dynamics of an age-dependent multiscale hepatitis C virus model. J. Math. 2022, 85, 21. [Google Scholar] [CrossRef]
- Ciupe, S.M.; Ribeiro, R.M.; Nelson, P.W.; Perelson, A.S. Modeling the mechanisms of acute hepatitis B virus infection. J. Theor. Biol. 2007, 247, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chenar, F.F.; Kyrychko, Y.N.; Blyuss, K.B. Mathematical model of immune response to hepatitis B. J. Theor. Biol. 2018, 447, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Stilianakis, N.I.; Seydel, J. Modeling the T-cell dynamics and pathogenesis of HTLV-I infection. Bull. Math. Biol. 1999, 61, 935–947. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Shflot, A.S.; Hobiny, A.D. Global stability of delayed SARS-CoV-2 and HTLV-I coinfection models within a host. Mathematics 2022, 10, 4756. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Li, Y.; Xu, D. Complete dynamical analysis for a nonlinear HTLV-I infection model with distributed delay, CTL response and immune impairment. Discret. Contin. Dyn. Ser. B 2020, 25, 917–933. [Google Scholar] [CrossRef]
- Nowak, M.A.; Bangham, C.R.M. Population dynamics of immune responses to persistent viruses. Science 1996, 272, 74–79. [Google Scholar] [CrossRef]
- Culshaw, R.V.; Ruan, S. A delay-differential equation model of HIV infection of CD4+ T-cells. Math. Biosci. 2000, 165, 27–39. [Google Scholar] [CrossRef]
- Nowak, M.A.; May, R.M. Virus Dynamics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Hernandez-Vargas, E.A.; Velasco-Hernandez, J.X. In-host mathematical modelling of COVID-19 in humans. Annu. Rev. Control 2020, 50, 448–456. [Google Scholar] [CrossRef]
- Wang, S.; Pan, Y.; Wang, Q.; Miao, H.; Brown, A.N.; Rong, L. Modeling the viral dynamics of SARS-CoV-2 infection. Math. Biosci. 2020, 328, 108438. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, N.; Burini, D.; Outada, N. Multiscale models of Covid-19 with mutations and variants. Netw. Heterog. Media 2022, 17, 293–310. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alsulami, R.S.; Hobiny, A.D. Modeling and stability analysis of within-host IAV/SARS-CoV-2 coinfection with antibody immunity. Mathematics 2022, 10, 4382. [Google Scholar] [CrossRef]
- Song, H.; Yuan, Z.; Liu, S.; Jin, Z.; Sun, G. Mathematical modeling the dynamics of SARS-CoV-2 infection with antibody-dependent enhancement. Nonlinear Dyn. 2023, 111, 2943–2958. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alsaedi, A.J.; Hobiny, A.D.; Aly, S.A. Global properties of a diffusive SARS-CoV-2 infection model with antibody and cytotoxic T-lymphocyte immune responses. Mathematics 2022, 11, 190. [Google Scholar] [CrossRef]
- Chen, M.X.; Wu, R.C.; Zheng, Q.Q. Qualitative analysis of a diffusive COVID-19 model with non-monotone incident rate. J. Appl. Anal. Comput. 2023, 1–21. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alsaedi, A.J.; Agha, A.D.A.; Hobiny, A.D. Global stability of a humoral immunity COVID-19 model with logistic growth and delays. Mathematics 2022, 10, 1857. [Google Scholar] [CrossRef]
- Baccam, P.; Beauchemin, C.; Macken, C.A.; Hayden, F.G.; Perelson, A.S. Kinetics of influenza A virus infection in humans. J. Virol. 2006, 80, 7590–7599. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.; Liao, L.E.; Beauchemin, C.A. Progress and trends in mathematical modelling of influenza A virus infections. Curr. Opin. Syst. Biol. 2018, 12, 30–36. [Google Scholar] [CrossRef]
- Nuraini, N.; Tasman, H.; Soewono, E.; Sidarto, K.A. A with-in host dengue infection model with immune response. Math. Comput. Model. 2009, 49, 1148–1155. [Google Scholar] [CrossRef]
- Comez, M.C.; Yang, H.M. Mathematical model of the immune response to dengue virus. J. Appl. Math. Comput. 2020, 63, 455–478. [Google Scholar]
- Wang, Y.; Liu, X. Stability and Hopf bifurcation of a within-host chikungunya virus infection model with two delays. Math. Comput. Simul. 2017, 138, 31–48. [Google Scholar] [CrossRef]
- Best, K.; Perelson, A.S. Mathematical modeling of within-host Zika virus dynamics. Immunol. Rev. 2018, 285, 81–96. [Google Scholar] [CrossRef]
- Wodarz, D.; May, R.M.; Nowak, M.A. The role of antigen-independent persistence of memory cytotoxic T lymphocytes. Int. Immunol. 2000, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zou, D. Global stability of in host viral models with humoral immunity and intracellular delays. Appl. Math. 2012, 36, 1313–1322. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Li, Y.; Yang, Y. Global dynamics of a intracellular infection model with delays and humoral immunity. Math. Methods Appl. Sci. 2016, 39, 5427–5435. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Kang, C.; Muhammadhaji, A. Stability analysis of a virus infection model with humoral immunity response and two time delays. Math. Methods Appl. Sci. 2016, 39, 3434–3449. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Elnahary, E.K. Analysis of general humoral immunity HIV dynamics model with HAART and distributed delays. Mathematics 2019, 7, 157. [Google Scholar] [CrossRef]
- Lin, J.; Xu, R.; Tian, X. Threshold dynamics of an HIV-1 model with both viral and cellular infections, cell-mediated and humoral immune responses. Math. Biosci. Eng. 2018, 16, 292–319. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; AlShamrani, N.H. Global stability of humoral immunity virus dynamics models with nonlinear infection rate and removal. Nonlinear Anal. Real World Appl. 2015, 26, 161–190. [Google Scholar] [CrossRef]
- Duan, X.; Yuan, S. Global dynamics of an age-structured virus model with saturation effects. Math. Methods Appl. Sci. 2017, 40, 1851–1864. [Google Scholar] [CrossRef]
- Kajiwara, T.; Sasaki, T.; Otani, Y. Global stability for an age-structured multistrain virus dynamics model with humoral immunity. J. Appl. Math. Comput. 2020, 62, 239–279. [Google Scholar] [CrossRef]
- Avila-Vales, E.; Pérez, Á.G. Global properties of an age-structured virus model with saturated antibody-immune response, multi-target cells, and general incidence rate. Boletín Soc. Mat. Mex. 2021, 27, 26. [Google Scholar] [CrossRef]
- Inoue, T.; Kajiwara, T.; Sasaki, T. Global stability of models of humoral immunity against multiple viral strains. J. Biol. Dyn. 2010, 4, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Samaddar, S.; Bhattacharya, P. Modeling the effect of non-cytolytic immune response on viral infection dynamics in the presence of humoral immunity. Nonlinear Dyn. 2019, 98, 637–655. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alofi, A.S. Global dynamics of secondary DENV infection with diffusion. J. Math. 2021, 2021, 5585175. [Google Scholar] [CrossRef]
- Raezah, A.A. Dynamical analysis of secondary dengue viral infection with multiple target cells and diffusion by mathematical model. Discret. Dyn. Nat. Soc. 2022, 2022, 2106910. [Google Scholar] [CrossRef]
- Navarro-Sanchez, E.; Despres, P.; Cedillo-Barreon, L. Innate immune responses to dengue virus. Archchives Med. Res. 2005, 36, 425–435. [Google Scholar] [CrossRef]
- Gujarati, T.P.; Ambika, G. Virus antibody dynamics in primary and secondary dengue infections. J. Math. Biol. 2014, 69, 1773–1800. [Google Scholar] [CrossRef]
- Camargo, F.d.A.; Adimy, M.; Esteva, L.; Métayer, C.; Ferreira, C.P. Modeling the relationship between antibody-dependent enhancement and disease severity in secondary dengue infection. Bull. Math. Biol. 2021, 83, 85. [Google Scholar] [CrossRef]
- Nikin-Beers, R.; Ciupe, S.M. Modelling original antigenic sin in dengue viral infection. Math. Med. Biol. 2018, 35, 257–272. [Google Scholar] [CrossRef]
- Smith, H.L.; Waltman, P. The Theory of the Chemostat: Dynamics of Microbial Competition; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Korobeinikov, A. Global properties of basic virus dynamics models. Bull. Math. Biol. 2004, 66, 879–883. [Google Scholar] [CrossRef]
- Korobeinikov, A.; Wake, G.C. Lyapunov functions and global stability for SIR, SIRS, and SIS epidemiological models. Appl. Math. Lett. 2002, 15, 955–960. [Google Scholar] [CrossRef]
- Elaiw, A.M. Global properties of a class of HIV models. Nonlinear Anal. Real World Appl. 2010, 11, 2253–2263. [Google Scholar] [CrossRef]
- Beckenbach, E.F.; Bellman, R. Inequalities; Springer: Berlin, Germany; New York, NY, USA, 1971. [Google Scholar]
- Hale, J.K.; Lunel, S.V. Introduction to Functional Differential Equations; Springer: New York, NY, USA, 1993. [Google Scholar]
- Barbashin, E.A. Introduction to the Theory of Stability; Wolters-Noordhoff: Groningen, The Netherlands, 1970. [Google Scholar]
- LaSalle, J.P. The Stability of Dynamical Systems; SIAM: Philadelphia, PA, USA, 1976. [Google Scholar]
- Lyapunov, A.M. The General Problem of the Stability of Motion; Taylor & Francis, Ltd.: London, UK, 1992. [Google Scholar]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zarin, R.; Hussain, G.; Ahmad, N.A.; Mohd, M.H.; Yusuf, A. Stability analysis and optimal control of covid-19 with convex incidence rate in Khyber Pakhtunkhawa (Pakistan). Results Phys. 2021, 20, 103703. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, L.; Elaiw, A.M.; Alghamdi, M.A.; Althiabi, A.M. Heterogeneous population dynamics of active particles: Progression, mutations, and selection dynamics. Math. Model. Methods Appl. Sci. 2017, 27, 617–640. [Google Scholar] [CrossRef]
- Chen, M.X.; Hu, Z.Y.; Zheng, Q.Q.; Srivastava, H.M. Dynamics analysis of a spatiotemporal SI model. Alex. Eng. J. 2023, 74, 705–714. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Agha, A.D.A. Global Stability of a reaction–diffusion malaria/COVID-19 coinfection dynamics model. Mathematics 2022, 10, 4390. [Google Scholar] [CrossRef]
- Bellomo, N.; Outada, N.; Soler, J.; Tao, Y.; Winkler, M. Chemotaxis and cross diffusion models in complex environments: Modeling towards a multiscale vision. Math. Model. Methods Appl. Sci. 2022, 32, 713–792. [Google Scholar] [CrossRef]
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| 10 | 3 | 0.01 | |||
| 0.3 | 0.002 | ||||
| Varied | 0.1 | 0.1 | |||
| 0.5 | 5 | 0.1 | |||
| 10 | 3 |
| Parameter | Value of | Parameter | Value of | Parameter | Value of |
|---|---|---|---|---|---|
| 1 | |||||
| 1 | 0 | ||||
| 1 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaiw, A.M.; Raezah, A.A.; Alshaikh, M.A. Global Dynamics of Viral Infection with Two Distinct Populations of Antibodies. Mathematics 2023, 11, 3138. https://doi.org/10.3390/math11143138
Elaiw AM, Raezah AA, Alshaikh MA. Global Dynamics of Viral Infection with Two Distinct Populations of Antibodies. Mathematics. 2023; 11(14):3138. https://doi.org/10.3390/math11143138
Chicago/Turabian StyleElaiw, Ahmed M., Aeshah A. Raezah, and Matuka A. Alshaikh. 2023. "Global Dynamics of Viral Infection with Two Distinct Populations of Antibodies" Mathematics 11, no. 14: 3138. https://doi.org/10.3390/math11143138
APA StyleElaiw, A. M., Raezah, A. A., & Alshaikh, M. A. (2023). Global Dynamics of Viral Infection with Two Distinct Populations of Antibodies. Mathematics, 11(14), 3138. https://doi.org/10.3390/math11143138





