Abstract
Mathematical modeling can help the medical community to more fully understand and explore the physiological and pathological processes within the human body and can provide more accurate and reliable medical predictions and diagnoses. Neural network models, machine learning models, and statistical models, among others, have become important tools. The paper details the applications of mathematical modeling in the medical field: by building differential equations to simulate the patient’s cardiovascular system, physicians can gain a deeper understanding of the pathogenesis and treatment of heart disease. With machine learning algorithms, medical images can be better quantified and analyzed, thus improving the precision and accuracy of diagnosis and treatment. In the drug development process, network models can help researchers more quickly screen for potentially active compounds and optimize them for eventual drug launch and application. By mining and analyzing a large number of medical data, more accurate and comprehensive disease risk assessment and prediction results can be obtained, providing the medical community with a more scientific and accurate basis for decision-making. In conclusion, research on medical problems based on mathematical models has become an important part of modern medical research, and great progress has been made in different fields.
MSC:
68W99
1. Introduction
Medical issues are one of the most important problems facing mankind, involving aspects of human health, life, and well-being. With the development and progress of human society, the field of medicine is also rapidly evolving and changing. Modern medicine is no longer simply about treating diseases, but covers a full range of services from prevention, diagnosis, and treatment to rehabilitation at many levels [1]. However, there are still many challenges and difficulties in the research of medical problems. For a long time, the medical community has relied mainly on experimental and clinical observation methods to study the mechanisms and treatments of diseases [2]. This approach has a high degree of credibility and rigor, but it also has some limitations, such as high experimental costs, difficulty in controlling all influencing factors, and individual variability [3]. With the development of science and technology and the continuous accumulation of medical knowledge, the complexity and diversity of medical problems have become more and more obvious as people study them more and more deeply. Therefore, more advanced methods and tools are needed to solve these problems. A mathematical model is a mathematical formal description that expresses the essential characteristics and laws of a phenomenon, problem, or system using mathematical symbols, equations, and graphs to facilitate its study, analysis, and prediction. Medical problems can be described by establishing mathematical formulas and systems of equations in order to analyze and predict medical problems, thus abstracting and formalizing real problems [4,5,6]. Therefore, the study of medical problems based on mathematical models has great potential and development prospects. Figure 1 presents the history of the development of the use of mathematical models to study medical problems.
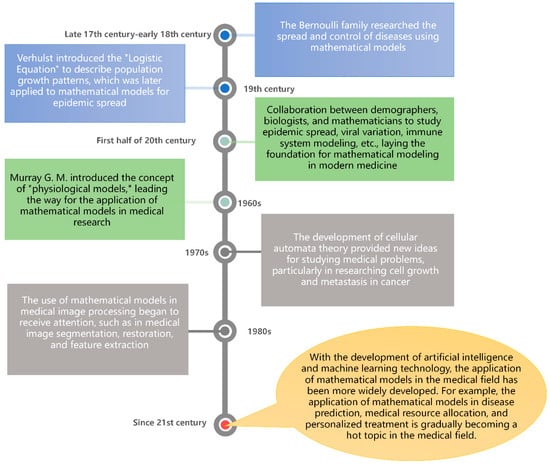
Figure 1.
History of the development of mathematical models applied in the medical field.
In recent years, research on medical problems based on mathematical modeling has received widespread attention, and experts and scholars at home and abroad have conducted a series of studies on specific medical problems: researchers at the Institute of Mathematics and System Sciences of the Chinese Academy of Sciences have conducted a series of mathematical modeling and simulation studies on medical problems such as cancer treatment [7]; Tsinghua University School of Medicine has brought together an interdisciplinary team of researchers dedicated to solving medical challenges through mathematical modeling [8]; researchers at Harvard University in the United States have used mathematical models to study the growth pattern of lung cancer cells, providing new ideas for the treatment of lung cancer [9]; and researchers at the University of Cambridge in the UK have used mathematical modeling to predict which human diseases are caused by different DNA variants and to develop corresponding treatments [10]. Mathematical models can help us understand the nature and patterns of diseases more deeply. By integrating multiple sources of information such as genes and proteins, mathematical models can reveal the mechanism of disease occurrence and provide doctors and researchers with more effective treatment options. Mathematical models can help experimental design and drug screening, thus improving the efficiency and success rate of drug development. At the same time, they can help develop personalized treatment plans, tailoring the best treatment plan for each patient based on the patient’s genetic background, the extent of the disease, and other indicators. Mathematical models can help predict disease epidemic trends and changes in demand, thus optimizing the allocation and management of medical resources [11,12,13]. For example, during an epidemic, mathematical models can help decision-makers determine the best isolation measures and vaccination schedules to better control its spread [14]. Mathematical models can help doctors and patients make more rational and scientific decisions, thus improving the effectiveness of medical treatment and the quality of services. For example, in the process of diagnosis and treatment, mathematical models can help doctors more accurately determine the type and severity of diseases so as to choose the best treatment plan and improve patients’ treatment satisfaction [15]. To sum up, mathematical models have an important meaning and role in the medical field. With the help of mathematical models, we can gain a deeper understanding of the nature and mechanism of diseases, discover new treatments and drugs, improve the prevention and diagnosis of diseases, and have broad application prospects in optimizing the allocation and management of medical resources.
Overall, many medical problems are modeled and analyzed mathematically to probe deeply into the nature and laws of medical problems, to improve the precision and efficiency of medical research, and to provide a scientific basis for medical practice. By building mathematical models, it helps researchers to understand the intrinsic mechanisms of biological systems, optimize diagnosis and treatment plans, predict and prevent the occurrence and spread of diseases, etc. Research on medical problems based on mathematical modeling has made some progress both at home and abroad, and more researchers will join in the future to promote the development of this field.
In this paper, we focus on the application of mathematical models in the medical field, including various aspects such as drug treatment, virus transmission, medical resource allocation, and physiology. First, we make a brief overview of the current situation of domestic and international research and introduce the development history of mathematical models applied in the medical field; in the Section 2, we introduce the basic concepts and methods of mathematical models and then describe the applications of common mathematical models in the study of medical problems from different perspectives; in the Section 3, we introduce in detail the applications of different mathematical models in the medical field, including differential-equation-based models. Finally, Section 4 outlines the problems and challenges of the current application of mathematical models in the medical field and provides an outlook on future research directions and application prospects.
2. Basic Concepts and Methods of Mathematical Modeling
2.1. Definition and Classification of Mathematical Modeling
A mathematical model is a tool for describing real-world or complex systems in mathematical terms. It converts real-world problems into mathematical problems and formalizes them in such a way that they can be analyzed and solved mathematically [16,17], thereby drawing quantitative or qualitative conclusions about practical problems. Table 1 presents the basic concepts used in the mathematical modeling process.

Table 1.
Basic concepts in mathematical modeling process.
Mathematical models can be represented using different mathematical methods and techniques, such as calculus, probability theory, and linear algebra, from the fields of computer science and statistics [18,19]. In engineering computing, a mathematical model is a mathematical expression or set of equations that describes and analyzes an engineering problem and can be used to predict and optimize the performance and efficiency of an engineering system [20,21].
2.2. Common Mathematical Models and Mathematical Modeling Methods
Common mathematical models include linear models, nonlinear models, probabilistic models, and differential equation models. These models are widely used in different fields and applications [22,23]. In the medical field, common mathematical models and mathematical modeling approaches include the following.
- (1)
- Differential equation model [24]: This model is based on the physical laws and kinetic principles of biological processes, such as chemical reactions, cell growth, and signal transmission. By establishing differential equations to describe the changes in these biological processes, it is possible to simulate and predict the behavior of biological systems, such as drug metabolism, tumor growth, nervous system activity, etc. This type of model often requires the use of numerical calculation methods to solve differential equations and the calibration and validation of model parameters.
- (2)
- Statistical model [25]: This model is based on collected medical data, such as patient clinical characteristics, disease incidence, and drug efficacy, and analyzes the relationship between the data through statistical models and hypothesis testing. These models can be used for disease risk assessment, diagnostic accuracy evaluation, and treatment effectiveness evaluation. Common statistical models include linear regression models, logistic regression models, and survival analysis models.
- (3)
- Machine learning model [26]: This model can learn and automatically discover patterns and associations between large-scale medical data. Medical models based on machine learning can be used for tasks such as diagnosis, disease prediction, drug discovery, and image analysis. Common machine learning algorithms include decision tree, support vector machine, neural network, and random forest.
- (4)
- Network science model [27]: This model describes the topological structure and interaction mode of biological system by building a biological network. Networks can represent protein interactions, gene regulation, disease transmission, and more. Medical models based on network science can be used to reveal the pathogenesis of diseases, identify important biomarkers, and predict drug targets. Common network analysis methods include graph theory, complex network analysis, and community detection.
- (5)
- Optimization models [28]: Optimization models are often used to help healthcare organizations manage their resources to obtain the best possible medical outcomes. For example, optimization models can be used to determine how to allocate the work time of doctors and nurses to achieve optimal treatment outcomes.
These different types of medical models play an important role in medical research and clinical practice, and their selection depends on the nature of the specific problem and the characteristics of the available data. At the same time, these models can also be combined and applied comprehensively to better understand and solve medical problems.
3. The Application of Mathematical Models in Medical Problems
The importance of medical issues needs no introduction, as they relate to human growth, reproduction, and health. However, there are many bottlenecks to solving medical problems. The traditional medical approach is mainly based on experience and observation, and this approach, although reliable, is limited by human sensory abilities and cognitive levels, which are subject to misjudgments and limitations [29]. Meanwhile, modern medical technology is constantly improving, leading to the accumulation and explosive growth of data. Understanding to extract valid information from a large number of data and analyze it precisely is a major bottleneck encountered in medical problems [30].
To solve this problem, the introduction of mathematical models has become an effective method. Mathematical modeling allows one to centralize and process a large amount of data and to discover the patterns and trends in them. With the help of the computing power of computers, we can quickly obtain various possible results [31]. In medical problems, mathematical models are widely used in medical image recognition, drug research, genomics, and other fields. By processing images, doctors can diagnose diseases more accurately [32]. In addition, in drug research, the use of mathematical models can predict the effect of drugs and reduce the experimental period and cost [33]. In genomics, mathematical models can help scientists better understand the workings of life and thus develop more precise medical solutions [34].
3.1. Differential-Equation-Based Biomedical Models
Differential-equation-based biomedical models are a common mathematical modeling approach that can be used to describe dynamic changes and interactions in biological systems [35]. These models are usually based on processes such as biochemical reactions, signaling, cell proliferation, and death in living organisms and model these processes by building sets of differential equations. These sets of equations can be used to predict the response and behavior of biological systems and support the control of the system under different conditions [36,37]. For example, the activity of neurons can be described using differential equation models. These models consider ion channels and conductivity in the neuronal membrane, as well as chemical transmission between neuronal synapses [38]. These equations can be used to predict neuronal excitability and inhibition, as well as the effects of different pathways. Differential equation models for cancer treatment can be used to predict the proliferation and death of cancer cells under different treatment conditions, as well as the effects of chemotherapeutic drugs on normal cells. Such models can help physicians to better select appropriate treatment regimens and to optimize doses and treatment duration [39]. Differential equation models have a wide range of applications in the biomedical field, covering everything from basic research to drug design and clinical practice. They can provide medical scientists with powerful tools for understanding the complexity of biological systems and help us to better treat diseases [40]. The application of differential-equation-based mathematical models in biomedicine is specifically described below through growth and development models [41], tumor growth models [42], and cardiovascular system models [43].
3.1.1. Growth and Development Model
A growth and development model is a mathematical model that describes the gradual growth and developmental processes of an organism. These models are usually based on the processes of cell proliferation, cell differentiation, tissue growth, and organ formation in the organism, and they model these processes by creating sets of differential equations. These sets of equations can be used to predict information about the growth rate, energy expenditure, and physiological state of an organism at different stages. Growth and development models are widely used in biomedical fields [44,45,46]; for example, they can be used to study the growth and development of plants, the growth and development of animals, and the growth and development of the human body, etc. Among them, human body growth and development models are one of the most common applications.
The Gompertz model and the Bertalanffy model are two common growth models that are widely used in the field of biology to describe the growth and developmental processes of organisms.
The Gompertz model is a nonlinear mathematical model based on differential equations that can be used to describe changes in the growth rate of an organism during its growth [47]. The model assumes that the growth rate of an organism slows down with time, which can be described using the following differential equation:
where represents the change in biological characteristics such as body length or height with time , A is a constant indicating the maximum body length or height of an individual, and B is a constant indicating the rate of exponential decrease in the growth rate of an organism with increasing time.
The derivation is as follows.
Suppose that the rate of change of height of an individual with time can be described by the growth rate :
Assuming that there is a certain functional relationship between growth rate and height, which can be expressed in terms of , it follows that
Based on empirical data on human growth and development processes, it can be assumed that has the following form:
where and are positive constants.
Substituting the above equation into the expression for yields
This differential equation is nonlinear and cannot be solved directly. However, it can be transformed into a solvable form using the separation of variables method. By multiplying both sides of the equation by at the same time, we obtain
Integrating both sides of the equation gives
The integral on the left side can be solved by the permutation method, and the integral on the right side can be solved by numerical or approximate methods. After calculation, substitution, and simplification, we can finally obtain
where both C and B are constants. Taking both sides of the above equation as an exponential function yields
This is the differential form of the Gompertz model of human growth and development, which describes the gradual slowing down of height-contiguous rate with increasing age over time. It has a wide range of applications in describing biological phenomena such as cell growth and tumor development.
The Bertalanffy model was proposed by Austrian biologist Ludwig von Bertalanffy, and it is a differential-equation-based growth model designed to describe the growth and developmental processes of organisms and organs [48]. The model assumes that the growth rate of an organism varies at different stages and is influenced by several factors during the growth process. The specific differential equations of the Bertalanffy model are as follows:
where denotes the length or volume of the organism or organ, denotes time, is the growth rate constant, and is the final size of the organism or organ. This model is commonly used to describe the growth process of plants and animals and has been widely used in medicine, agriculture, environmental science, and other fields.
Typically, human growth and development models take into account many factors such as genetic, environmental, nutritional, and hormonal influences on physical growth and development. These factors can be modeled by building sets of differential equations to predict physiological indicators such as body height, mass, and proportions at different ages. These models can also be used to study changes in body growth and development under conditions such as nutritional disorders and disease development, helping doctors to better diagnose and treat diseases. In conclusion, growth and development models are a very useful mathematical tool for predicting the growth and development of different organisms in different environments and provide important reference information for medical scientists.
3.1.2. Tumor Growth Model
The tumor growth model is a widely studied mathematical model that can help scientists to better understand the growth and spread process of tumor cells [49]. Among them, the differential-equation-based tumor growth model is one of the most common models, and its core idea is to use differential equations to describe the evolution pattern of tumor cell population over time.
Differential equation-based tumor growth models usually include two equations, one describing the growth rate of the tumor cell population and the other describing the rate of tumor cell spread. Specifically, differential equations of the following form are usually used to describe the change in the number of tumor cells:
where denotes the number of tumor cells, denotes time, denotes the growth rate of tumor cells, and denotes the limit of environmental accommodation. The in the equation can be interpreted as the limiting effect of the environment on the growth of tumor cells.
In addition, a differential equation of the following form can be used to describe the process of tumor cell proliferation:
where denotes the tumor cell density, denotes time, denotes the diffusion coefficient, and denotes the Laplace operator. in the equation describes the conservation of mass in the cell diffusion process. This equation describes the diffusion process of tumor cells along the space, where a larger parameter indicates the greater diffusion ability of tumor cells.
It should be noted that the above two differential equations are independent of each other, but they are related through the number of tumor cells N. Specifically, both the growth rate of tumor cell number r and the maximum volume K are related to the tumor cell concentration C. Therefore, the effects of both aspects need to be considered when building a differential equation-based tumor growth model.
In conclusion, differential-equation-based tumor growth model is a very useful mathematical tool that can help scientists to understand the growth and spread pattern of tumor cells more deeply and provide important theoretical support for studying tumor formation and treatment.
3.1.3. Cardiovascular System Model
A differential-equation-based model of the cardiovascular system is a mathematical model used to describe the dynamic changes within the heart and vascular system. The model uses calculus and differential equations to describe the components of the cardiovascular system and solves these differential equations to predict trends in cardiovascular disease and guide the design of treatment protocols [50,51]
The cardiovascular system consists of the heart, blood vessels, and other components, so it is important to develop an accurate model of the cardiovascular system. For example, for the heart, we can use the continuum equations to represent the changes in pressure and volume due to myocardial contraction and diastole; for the vasculature, we can use the Navier–Stokes equations to describe the flow of blood inside the vessel and the pressure acting on the vessel wall. In addition to the heart and blood vessels, there are a number of differential equation models that can be used to describe other aspects of the cardiovascular system, such as blood transport, myocardial metabolism, and tissue oxygenation. These equations can be combined into a complete model of the cardiovascular system to better predict the interactions between the components within the overall system.
Myocardial contraction and diastole are caused by changes in the concentration of calcium ions within the cardiac muscle cells. According to the theory of elasticity, the ventricular wall is deformed in response to volume and pressure changes. Therefore, the volume and pressure changes in intraventricular blood during myocardial contraction and diastole can be described by the continuum equation, where is the volume of intraventricular blood and is the flow rate:
The stress distribution within the myocardial tissue is described using the stress balance equation where is the stress tensor inside the myocardial tissue at a given moment, is the local body force per unit volume, is the density of the myocardial tissue, and is the acceleration of the myocardial tissue.
For blood vessels, the Navier–Stokes equations describe the flow of blood inside the vessel, where represents the flow rate of blood, represents the pressure of blood, represents the density of blood, 4 represents the viscosity of blood, and 5 represents the force of external forces on blood:
The pressure distribution inside the vessel is described using Poisson’s equation, where represents the radius of the vessel.
The stress distribution acting on the vessel wall is described using the wall force equation, where is the total tangential force acting on the vessel wall, is the stress per unit area, and is the cross-sectional area of the vessel wall:
For transport, the transport equation is used, which describes the transport of substances (e.g., oxygen, carbon dioxide, etc.) in the blood, where denotes the concentration of the substance, denotes time, denotes the flow rate of the blood, and denotes the diffusion coefficient of the substance.
By solving these differential equations, we can obtain information that predicts the interactions between the various parts within the cardiovascular system. This information can help physicians better understand the progression of cardiovascular disease, predict possible problems, and provide guidance for treatment and prevention.
In summary, differential-equation-based models of the cardiovascular system are a very useful tool that can help medical researchers better understand the functioning of the cardiovascular system and design better treatment plans.
3.2. Statistical Medical Model
Statistically based medical models are a method of studying disease correlations based on data and statistical methods. These models can help medical researchers to identify disease causes, evaluate treatment effects, and perform risk assessments on patients [52]. In recent decades, with the development of computer science and data science, statistical medical models have become an integral part of medical research and are widely used in biomedical and clinical medicine [53].
Medical models usually require a large number of clinical data to support their construction and validation. These data usually include patients’ personal information, medical history, biomarkers, and medical images [54]. By combining these data with disease incidence, symptoms, and treatment outcomes, we can determine the relationship between different variables and construct a mathematical model that can predict the risk of future disease occurrence [55]. In addition to risk assessment, it can be used for disease diagnosis and monitoring during treatment [56]. For example, by measuring tumor markers, blood components, and other biomarkers, cancer progression can be monitored and treatment regimens can be adjusted.
Statistically based medical models have a wide range of applications in clinical practice. Some of the key application areas are shown in Table 2 below.

Table 2.
Statistical clinical applications of medical models.
Statistically based medical models are a very important part of medical research, and by collecting and analyzing clinical data, these models can provide physicians with more accurate and efficient disease prediction and treatment options. Although there are challenges, statistical medical models will continue to play an important role and will be further developed and refined in the future.
3.2.1. Survival Analysis Model
Survival analysis modeling is a method used to predict the survival time and prognosis of patients. This model is usually applied to study the prediction of patient survival for specific diseases or clinical situations. It is based on individual patient characteristics and clinical data and can help physicians determine patient survival [57,58].
A typical survival analysis model consists of two components: a survival function and a hazard function. The survival function reflects the probability that an event does not occur before a certain point in time, while the hazard function reflects the probability that an event occurs at a certain point in time [59]. In survival analysis, we usually use Kaplan–Meier survival curves to plot survival functions. Kaplan–Meier survival curves are a nonparametric method for assessing the probability of survival over a specific time period. The method estimates the survival function by dividing the sample into subgroups and plotting the cumulative distribution function of the probability of survival in each subgroup. These subgroups can be divided according to factors such as gender, age, and the severity of disease [60,61]. A Cox proportional risk model was used to construct the hazard function. The Cox proportional risk model is a semiparametric approach to assess the effect of different factors on survival time. The model assumes that the hazard function can be decomposed into a product of a base hazard function and a series of covariates, where the base hazard function is an unknown function and the covariates are the predictor variables (e.g., gender, BMI value, and condition). The mathematical formula of the Cox model is as follows:
where represents the individual’s risk function at time point , is the baseline risk function, is the covariate vector, and is the corresponding regression coefficient.
When constructing a Cox proportional risk model, we usually need to perform pre-processing and variable selection on the data. Pre-processing includes data cleaning, missing value filling, and outlier detection, while variable selection refers to selecting the most relevant variables from multiple predictor variables and incorporating them into the model. This is usually achieved by using techniques such as stepwise regression.
The core idea of a survival analysis model is to describe the survival process of a patient based on a hazard function, which is a density function of the probability of a patient dying at a given time point, representing the risk of the patient dying at different time periods. A higher value of the hazard function indicates a higher risk of patient death. By modeling the patient’s survival time and the hazard function, a mathematical model of predictable survival can be constructed. Figure 2 below shows the flow chart of the survival analysis model.
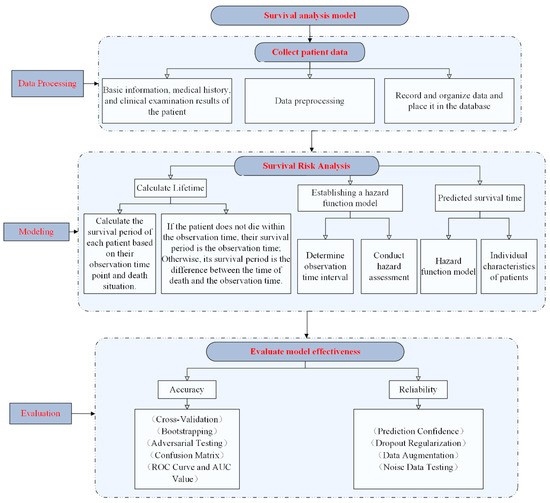
Figure 2.
Survival analysis model flow chart.
Survival risk analysis models have a wide range of applications in medical research and clinical practice. For example, in cancer treatment, the survival risk analysis model can help doctors determine the survival rate and survival period of patients and adjust treatment plans to improve treatment outcomes. In addition, the model can also be used to assess the risk of surgery, predict the probability of heart attack, etc.
3.2.2. Risk-Assessment Model
Statistically based risk-assessment models are a common approach to assessing risk, which is based on various statistical data and probability theory to predict possible risks and events. Such models can be used in many different fields such as finance, insurance, industry, and healthcare [62,63].
In a statistically based risk assessment model, the objective of the assessment needs to be determined first, for example, to predict future medical market trends or to detect whether a patient is at risk for a certain disease. Then, historical data are analyzed to build the model, which involves collecting a large amount of relevant data, analyzing data characteristics and patterns, and using statistical methods to infer possible outcomes. After building the model, data validation and testing are used to determine the accuracy and reliability of the model [64,65]. If the model performs well in these tests, it can be used for risk assessment in real-world situations.
The Framingham Cardiovascular Disease Risk Assessment Model is a statistically based model that predicts a person’s probability of having a cardiovascular event in the next 10 years. The model was originally developed by the Framingham Heart Study in Framingham, Massachusetts, USA, and has become one of the most widely used cardiovascular disease risk assessment models worldwide [66,67]. The model is based on the assessment of age, sex, blood pressure, cholesterol, smoking, and diabetes. Each factor is assigned a different weight, and based on the sum of the weights of these factors, the probability of a person having a future cardiovascular event can be calculated. Specifically, the model evaluates based on the following indicators:
where represents the probability of coronary heart disease (CHD) risk, represents the logarithmic probability of probability (log ods), represents smoking status (usually 0 represents non-smokers and 1 represents smokers), represents total cholesterol level, represents HDL cholesterol level, and represents systolic blood pressure. , , , , , , and are regression coefficients used to measure the contribution of each risk factor to the risk of coronary heart disease. These regression coefficients were obtained through statistical regression analysis of a large amount of data from the Framingham study.
Age: The risk of cardiovascular events increases with age.
Gender: Men are at higher risk than women for developing cardiovascular events.
Blood pressure: Hypertension is one of the major risk factors for cardiovascular disease and is assessed here using the average of systolic and diastolic blood pressure.
Cholesterol: High cholesterol is one of the major risk factors for atherosclerosis and cardiovascular disease. The ratio between total cholesterol and HDL-C (high-density lipoprotein cholesterol) is used here for assessment.
Smoking: Smoking is one of the major risk factors for cardiovascular disease.
Diabetes: Diabetes can lead to many cardiovascular complications, including heart disease and stroke.
The Framingham Cardiovascular Disease Risk-Assessment Model has been widely used in clinical practice to help physicians determine a patient’s risk of future cardiovascular events and develop appropriate preventive measures.
The Charlson comorbidity index (CCI) is a statistically based model for assessing a patient’s risk of death caused by other coexisting conditions during the treatment of a specific disease. The model was originally developed by Mary Charlson and colleagues in 1994 and has been widely used in medicine to assess the burden of chronic disease and predict clinical outcomes [68,69]. The model grades each disease and calculates an overall risk score based on 12 indicators, such as cardiovascular disease, diabetes mellitus, and renal function. The score for each disease is based on its correlation with mortality; for example, certain diseases may lead to a higher risk of death. By summing the scores for each disease, a CCI score can be calculated to assess a patient’s overall health status and risk of death. CCI can help physicians better understand a patient’s overall health status and, therefore, develop more effective treatment plans. For example, for patients with higher CCI scores, physicians may need to consider treatment and medication options more carefully to minimize the risk of adverse events and death. In addition, CCI can be used to assess the efficiency and quality of the healthcare system. By combining CCI scores with indicators such as length of stay and cost of care, the overall treatment capacity and quality level of a hospital or healthcare facility can be measured.
The APACHE II (Acute Physiology and Chronic Health Evaluation II) scoring system is a statistically based assessment model for the evaluation of mortality in patients treated in the ICU (intensive care unit) [70]. The model was originally developed by Knaus et al. in 1985 and has become one of the most widely used tools for the assessment of ICU patients worldwide [71]. The model evaluates patients based on physiological indicators, such as temperature, respiration, blood pressure, and arterial oxygen saturation, as well as factors such as the patient’s age and known health problems. Based on these factors, an APACHE II score can be calculated for each patient to predict his or her risk of death. The APACHE II scoring system consists of 12 physiological parameters and one age parameter. Each parameter has a score that is determined based on the patient’s physiological status. These parameters include blood pressure, heart rate, respiratory rate, temperature, age, mean arterial oxygen saturation, pH, sodium concentration, potassium concentration, creatinine concentration, white blood cell count, hemoglobin concentration, and state of consciousness. The final APACHE II score is obtained by summing the scores of each index. The advantage of the APACHE II scoring system is that it can quickly and accurately assess the risk of death in ICU patients and help physicians plan treatment. By understanding the patient’s vital signs, physicians can adjust the treatment plan in a timely manner, thereby improving the patient’s recovery and survival rates. In addition, the APACHE II scoring system can be used to study and compare the treatment effects at different ICUs.
Hospital readmission is when a patient is discharged from the hospital and then sent back to the hospital for treatment or rehabilitation soon after. It not only increases the pain and burden on patients but also increases the cost and burden on healthcare providers. To predict the risk of hospital readmission that patients may encounter, many researchers have developed statistically based risk-assessment models [72]. One example is the State University of New York’s “LACE” scoring system [73]. This system classifies patients into low-, moderate-, and high-risk groups based on four covariates (number of days in the hospital, number of emergency room visits, patient social factors, and age) using a logistic regression algorithm. This statistically based risk assessment model can help healthcare providers better understand the risk patients may face for readmission and take appropriate preventive measures. For example, high-risk groups may require closer monitoring and attention, as well as better rehabilitation care and referral services.
These are just some of the common types of statistically based risk assessment models, and there are actually many other types of models available, each with their own strengths and limitations. In summary, statistically based risk assessment models are a very useful tool to help companies, investors and decision-makers make informed decisions in a variety of areas. However, these models also have their limitations in that they can only be analyzed based on historical data and probability theory and cannot take into account unknown and human factors that can affect the outcome. Therefore, when these models are used, their reliability needs to be carefully assessed, and possible risks and errors need to be considered.
3.3. Machine-Learning-Based Medical Models
Machine-learning-based medical models are mathematical models that analyze and process medical data by using various machine learning algorithms and techniques to provide predictions, diagnoses, or treatments for diseases. The training of these models relies on a large amount of medical data, including clinical data, genetic data, and medical images [74]. These data are used to design and train machine learning-based models to improve the accuracy, efficiency, and safety of medical decisions. In the medical field, machine-learning-based models can be used in several applications, such as cancer prediction, disease diagnosis, drug discovery, and medical image analysis.
Machine learning models can improve the early detection of cancer by using information such as a patient’s clinical data and genetic data to help doctors determine whether a patient has a probability of having a certain type of cancer [75]. Such models can be used to determine whether a patient has cancer by training a classifier. Classifiers usually use supervised learning algorithms [76], such as support vector machines (SVMs) [77], neural networks [78], and decision trees [79]. These algorithms can learn a model from a large number of cases and predict whether a patient has cancer by inputting information such as the patient’s clinical data and genetic data.
In disease diagnosis, machine-learning-based models can analyze patient symptoms and examination data to assist doctors in making diagnostic and treatment decisions with improved accuracy and efficiency. Such models usually use supervised learning algorithms [80], such as random forest [81], logistic regression [82], and plain Bayesian [83]. These algorithms can learn a model from training data and predict the type of disease a patient may have by inputting the patient’s symptoms and examination data.
In drug discovery, machine learning-based models can analyze large amounts of compounds and known drug data to find new drug combinations or design new drug molecules to support new drug development. Such models usually use unsupervised learning algorithms [84], such as clustering [85] and association rule mining [86]. These algorithms can discover new drug combinations or design new drug molecules by analyzing a large number of compounds and known drug data.
In medical image analysis, machine learning-based models can analyze various medical images, such as X-rays and MRIs (Magnetic Resonance Imaging) to help doctors make diagnostic and treatment decisions. Such models usually use deep learning algorithms such as convolutional neural networks (CNN) [87]. These algorithms can automatically extract key features from images and predict the type of disease a patient may have by inputting medical images.
Machine-learning-based medical models have a wide range of applications and can help the medical industry improve efficiency, reduce costs, and provide more accurate diagnosis and treatment plans, thereby improving patients’ quality of life and extending their lifespan. With the continuous development of and improvement in artificial intelligence technology, machine-learning-based medical models will gradually become widely used and continuously innovated. Figure 3 shows the steps of using machine-learning-based models:
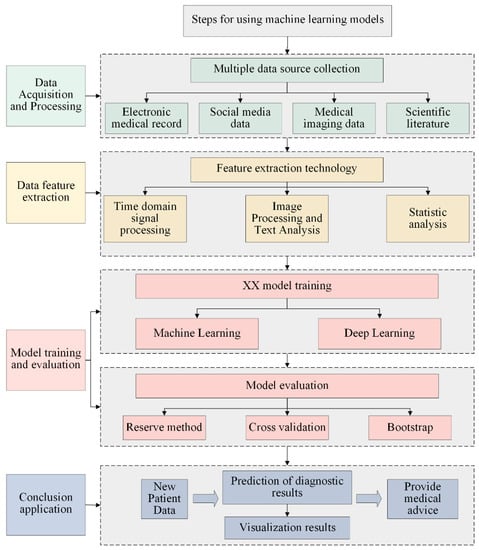
Figure 3.
Flow chart of medical model based on machine learning.
3.3.1. Medical Image Analysis Model
Medical image analysis modeling refers to the processing of medical images using machine learning algorithms and techniques for feature extraction, classification, and recognition to help doctors make accurate diagnosis and treatment decisions [88]. Medical images include various types of images such as X-ray, CT, MRI, and ultrasound images.
Medical images contain rich information, such as color, shape, and texture, which are important for diagnosis and treatment. Therefore, feature extraction is the first step in medical image analysis. Traditional methods usually require the manual selection and design of features, followed by classification using a classifier. However, this approach requires a lot of manual intervention and expertise, and the results are inconsistent. In contrast, machine-learning-based feature extraction methods can automatically discover key features in an image and use these features in the classification process. Feature-extraction methods can be divided into two categories, shallow and deep learning [89]. Shallow learning methods, such as local binary patterns (LBP), gray scale coevolution matrix (GLCM), and Gaussian filters, describe the whole image by extracting local features in the image. Deep learning methods, on the other hand, use deep neural networks for the end-to-end feature extraction and classification of images. One of the most popular is the Convolutional Neural Network (CNN), which automatically extracts high-level semantic features from images and enables their classification [90] and recognition [91].
The CNN model usually consists of a convolution layer, a pooling layer, a full connection layer, and an activation function. The convolutional layer applies filters (convolutional kernels) to input data for feature extraction. Assuming the input is two-dimensional image data, the output of the convolutional layer can be calculated using the following formula:
where represents the pixel value of the output feature map of the convolution layer, represents the pixel value of the input image, is the weight of the convolution kernel, is the offset term, is the activation function, and represents the summation of all elements of the convolution kernel.
The pooling layer is used to reduce the spatial dimensions of feature maps and extract more significant features. The commonly used pooling operation is Max Pooling, which has the following mathematical formula:
where represents the pixel values of the output feature map of the pooling layer and is the pixel values of the input feature map.
The fully connected layer connects the output features of convolutional and pooling layers and performs tasks such as classification or regression. The mathematical formula for fully connected layers can be expressed as
where is the output of the full connection layer, is the weight matrix, is the input vector, is the offset term, and is the activation function.
These formulas describe the calculation process of different layers in the CNN model and update the model parameters through backpropagation algorithms and optimization methods (such as gradient descent) to enable the model to learn and extract useful features from input data, thus completing the tasks of medical image analysis and prediction.
Since the amount of data is usually large, data augmentation and pre-processing of the training data are required to improve the performance and generalization of the model. Data augmentation includes operations such as rotation, flipping, and scaling, which can generate more samples from the limited data to train the model. Pre-processing includes operations such as normalization, denoising, and balancing, which can improve the robustness and reliability of the model.
In addition, issues of model interpretation and transparency need to be taken into account. Due to the complexity of deep neural networks, their decision processes often cannot be understood or explained by humans. Therefore, in order to ensure the reliability and trust of the model, some interpretable techniques, such as gradient CAM, LIME, and SHAP, need to be introduced in the model design phase, which can help us understand the decision process of the model and diagnose errors.
Machine-learning-based medical image analysis models have a wide range of promising applications and can be applied to several fields, such as cancer detection, disease diagnosis, and surgery planning. However, there are some difficulties and challenges with this technology, such as data privacy and security issues, model interpretability, and transparency issues. Therefore, in future development, more technical tools need to be introduced to solve these problems and to combine machine learning technology with human expertise to further promote the development and progress of the medical industry.
3.3.2. Pathology Diagnostic Model
Case diagnosis modeling is a method of automating the analysis and diagnosis of a patient’s condition using artificial intelligence techniques. It is based on the principle of feeding a large amount of medical data into a machine learning algorithm and allowing the algorithm to automatically learn features and predict the diagnosis of a patient [92]. Pathology diagnosis models can use convolutional neural networks (CNNs) to automatically extract features from medical images and to classify and predict conditions.
Lung cancer is a common malignant tumor and one of the cancers causing the highest number of deaths worldwide. Traditional lung cancer diagnosis relies on physicians’ experience and observation, but the misdiagnosis and underdiagnosis rates are high because lung cancer is difficult to detect due to its inconspicuous early symptoms. In recent years, a lung cancer pathology diagnosis model based on machine learning technology has emerged as a new solution to improve the detection, classification and prediction accuracy of lung cancer by automating the analysis and diagnosis of patients’ imaging data, thus providing new tools and methods for clinical medicine. Specifically, the model can accept multiple types of medical image data as input, such as CT scans, X-rays, and pathology slides. The model first pre-processes and enhances the input data, including operations such as denoising, contrast enhancement, and normalization, and then automatically learns the feature information in the images through multi-layer convolution and pooling operations. After that, the features are mapped to the fully connected layer for classification or regression prediction [93]. The following is the processing flow of the lung cancer pathology diagnosis model.
- Data Acquisition and Processing
Machine learning-based pathological diagnosis models for lung cancer require a large amount of medical image data as input, including CT scans, X-rays, and pathology slides. These data sources are usually presented in DICOM (Digital Imaging and Communication in Medicine) format, and DICOM libraries using programming languages such as Python can easily read and process these data.
During data processing, the raw data need to be cleaned and pre-processed to remove extraneous information and noise interference to improve their quality and usability: operations such as normalization, scaling, rotation, and cropping are performed on the images to make them consistent in size and scale; filters can also be used to remove noise and artifacts and adjust image brightness and contrast to make them easier to observe and analyze.
- Feature extraction and selection
After obtaining the processed medical image data, meaningful features need to be extracted from them next as the input to the machine learning model. The pathological diagnosis of lung cancer involves several aspects of features, such as mass size, morphology, texture, density, and location, which have different biological meanings and clinical values, and appropriate features therefore need to be selected for analysis.
Feature extraction of lung CT scan images using convolutional neural networks (CNN) can identify different features such as tumor regions, blood vessels, and other anatomical structures and map them to fully connected layers for classification or regression prediction.
Feature selection needs to consider the complexity and generalization ability of the machine learning model. Generally speaking, the greater the number of features, the higher the complexity of the model and the easier it is to overfit, while too few features may lead to an underfitting of the model, which cannot fully express the characteristics of the data. Therefore, a balance needs to be found in feature selection by selecting those features that have a strong correlation and stability with the target variables.
Commonly used feature-selection methods include filtering, wrapping, and embedding methods. The filtering method refers to evaluating the features first and then selecting the important features based on some criteria (such as correlation coefficient and information gain) and then inputting these features into the machine learning model. This method is suitable for cases with a large number of features and does not require building a machine learning model. The wraparound method, on the other hand, views the feature selection problem as a search problem, and it feeds features as input into the machine learning algorithm through cross-validation or other evaluation methods to arrive at the optimal subset of features. This approach usually works better than the filtering method but also has higher computational complexity. The embedding method refers to integrating feature selection with machine learning model training and adjusting the weights of features by regularization terms or other optimization methods, so as to achieve simultaneous feature selection and model training. This method is usually more efficient than the wrapping and filtering methods, but it also requires repeated training of the model and is more computationally intensive.
- Model Training and Evaluation
After the feature extraction and selection, the next step can be to start building machine learning models and training them using the labeled lung cancer dataset. Commonly used machine learning models include support vector machines (SVMs), random forests (RFs), and neural networks (NNs).
During model training, cross-validation is usually used to evaluate model performance and avoid overfitting. For example, the dataset is divided into several parts, one of which is selected as the test set each time, and the others are used as the training set; the average is repeated several times to calculate the accuracy, recall, F1 score, and other metrics of the model. After the model training and evaluation, the model needs to be optimized and tuned to obtain better generalization ability and performance. Common optimization methods include grid search, random search, Bayesian optimization, etc. In addition, the performance of the model can be further improved by increasing the amount of data, adjusting the model structure, and changing the loss function. In addition, deep learning-based pathology diagnosis models can also combine techniques such as migration learning and augmentation learning to improve the generalization ability and adaptability of the models. For example, migration learning is used to pre-train a generalized feature extractor from an existing large-scale medical image dataset and then fine-tune the model parameters for specific case data to further improve the accuracy and robustness of the model. Meanwhile, augmented learning techniques can help the model autonomously choose the optimal decision strategy, thus improving the model’s decision effectiveness.
3.4. Network-Science-Based Medical Models
Network-science-based medical modeling is a method for constructing and analyzing biologically based complex systems using a network science approach. The core of the approach is to view biological systems as networks composed of many interacting components. By modeling and analyzing these components and their interactions, the importance of the structure, function, and regulation of biological systems can be revealed. The model can view disease as a complex biological network in which molecules, cells, organs, and other elements in the body interact with each other through multiple biological associations [94,95,96]. The disease network model can help us understand the mechanisms of disease onset and progression and provide important guidance for drug development and treatment strategies. Specifically, the disease network model adopts the idea of graph theory to abstract various biological entities such as molecules, proteins, and metabolites in organisms as nodes and multiple biological associations among them as edges to construct a biological network of diseases. This network structure can be used to describe the information about disease-related genes and metabolic pathways, and the nature of the disease can be explored by analyzing indicators such as network topology and node centrality. In addition, the network model can be used to predict new targets and therapeutic strategies and further optimize and improve the model through experimental validation. For example, many diseases involve abnormal changes in certain key signaling pathways or metabolic pathways, which may be described and analyzed using disease network models. The analysis of these key nodes can help us understand the mechanisms of disease onset and provide guidance for developing new therapeutic strategies. In addition, by comparing biological network models among different diseases, commonalities and differences between them can be explored, which can help to gain a deeper understanding of the connections and differences between different diseases. In addition to disease-network models, there are other applications of network science in the medical field. For example, in drug development, researchers can use network science approaches to construct drug target networks, explore the impact of drug targets on the overall biological network, and predict new drug targets and therapeutic strategies. There is also the gene regulatory network model in genomics research. This model constructs gene-regulatory network models by analyzing genomic data, viewing genes as network nodes, and abstracting the interactions between genes as edges to help us understand the interactions of genes within cells and further reveal the links between genes and diseases.
The novel coronavirus has accelerated the pace of innovation in the global pharmaceutical industry. At the same time, the domestic new policies such as the quantitative procurement of drugs and the revision of drug management law this year have also boosted the arrival of changes in the industry, and major domestic and foreign pharmaceutical companies have increased their efforts in new drug research and development. Artificial intelligence technology will also play an active role in promoting the progress of network science toward the development of innovation in the pharmaceutical industry. SensetimeTechnology has announced four major research results that will lay the foundation for innovation in many aspects of new drug development, help the pharmaceutical industry to upgrade intelligently, and further promote the long-term development of the “new medical infrastructure”. The team has creatively trained a set of large-scale Graph Gonvolutional Networks (GCN) network models covering the complex interactions between drugs, diseases, and proteins to accurately predict the relationship between drugs and diseases [97,98]. The model can learn the relationships among key information autonomously without any human intervention and achieve the full automation of the prediction process and the effective prediction of unknown drug indications. On the publicly available dataset of repoDB small molecule drug redirection, the prediction results of this method achieved better results compared to the best published results of the currently used machine learning methods, which improved the performance index (AUC) of the prediction results by 8% (0.792→0.857) for the related research team. The potential effectiveness of an already marketed heart disease drug for breast cancer treatment predicted using the results of this study has now been confirmed in the clinical literature. In the future, the research team hopes to use this approach to fuel a more efficient drug screening process for new indications and to drive clinical progress for new uses of old drugs. Figure 4 below shows the drug redirection analysis framework based on drug, protein, and disease relationship modeling.
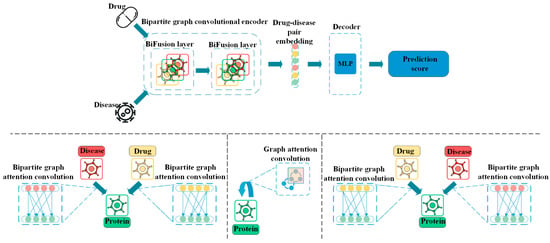
Figure 4.
A Drug Redirection Analysis Framework Based on Modeling the Relationship between Drugs, Proteins, and Diseases.
In summary, network science has a wide range of applications in medicine, contributing to a deeper understanding of the nature of disease, the development of new drugs and treatment strategies, and supporting related fields such as precision medicine.
4. Summary and Outlook
With the continuous development of computer technology and mathematical models, the application of mathematical models in medicine has become more and more widespread. Differential-equation-based biomedical models, statistics-based medical models, machine-learning-based medical models, and network-science-based medical models have become indispensable tools in the medical field. In this paper, a review and outlook of recent years’ medical problems based on mathematical models are presented from the perspective of mathematical models.
Biomedical models are widely used for the early diagnosis of diseases, drug delivery, etc. In cancer treatment, the process of drug delivery between the inner walls of blood vessels and cell membranes can be simulated by building biological transport models to optimize the delivery of drugs and improve their therapeutic effect.
Medical image processing, another method of using machine learning to process medical images, can help doctors diagnose and treat diseases more accurately. In CT scans, machine learning models can be used to enhance and reconstruct images to improve image quality and help doctors better see lesion areas.
Network science approaches have also been widely used in drug research by constructing drug–protein interaction networks to predict potential drug targets, or using drug metabolic pathway networks to predict the structure of drug metabolites, etc. In antibiotic therapy, the distribution and metabolic rate of drugs among different tissues can be predicted to optimize drug dose design and avoid drug abuse and side effects.
4.1. Problems and Challenges
It should be noted that due to the inherently restrictive nature of medical data, these mathematical models may be subject to certain biases and errors. Moreover, although these models can take into account all possible variables as much as possible, certain important factors may still be overlooked, thus affecting the accuracy of the models.
In the future, machine-learning-based medical models need to improve in accuracy and efficiency while taking into account data privacy and security, focus on interpretability and transparency of the models, and combine machine learning technology with medical professional knowledge to further promote the development and progress of the medical industry.
Medical data are inherently complex, and statistical mathematical models usually require significant computational resources for training and validation. The sharing and processing of medical data may also be highly restricted due to issues such as data privacy.
Although the survival risk analysis model has a certain degree of reliability and accuracy, the model can only deal with known risk factors and cannot consider unknown factors; in addition, the model is also susceptible to research bias and requires more detailed data analysis and control. Therefore, when using the survival risk analysis model, its applicability and reliability need to be carefully evaluated and combined with clinical experience and judgment for decision-making.
In summary, the following points also need to be noted when using mathematical models to solve medical problems.
- Data quality: Data quality is critical for building reliable survival analysis models. If historical data are incomplete or inaccurate, the model built may be compromised, leading to inaccurate assessment results.
- Sample size: For building risk assessment models, sample size is also a very important factor. Since statistical methods are based on probability, the sample size determines how much information can be taken into account when the model is built, and thus the accuracy of the model. If the sample is too small, the model will lose some of its predictive power, so it is necessary to ensure that the sample is large enough.
- Model selection: When building a machine learning model, a suitable machine learning algorithm should be selected according to the specific situation. Different types of models are suitable for different types of data sets and problems, so they need to be chosen according to the actual needs to achieve the best prediction results.
- With the continuous development of medical technology, new discoveries and theories may change the understanding of disease mechanisms and treatment methods. It is necessary to timely incorporate new medical knowledge and theories to update the model and ensure that the model reflects the latest scientific insights through continuous learning and attention to the latest developments in medical research.
Mathematical models are a very useful healthcare tool that can help hospitals, patients and other decision-makers make informed decisions in a variety of areas by building predictive models. However, the use of these models also requires attention to their limitations and caveats in order to take full advantage of their benefits and avoid possible errors.
4.2. Future Application Prospects
Looking ahead, the application of mathematical models in the medical field will continue to expand and deepen. Multidisciplinary cross-application will be an important trend in future medical research.
- High-performance computing is a computing technology capable of processing data on a large scale, and it has been widely used in various fields in recent years, such as weather forecasting, climate simulation, and risk assessment. In the medical field, the application of high-performance computing is still relatively small, but in the future, with the improvement in computer processing power, high-performance computing will be able to help medical research explore human physiology and disease mechanisms more deeply and accurately. Large-scale data analysis technology based on high-performance computing can help medical researchers discover potential causes and treatments to improve the diagnosis and treatment of diseases; by analyzing large-scale genetic data sets, medical researchers can discover the mechanisms of cancer occurrence and development and thus develop more effective cancer treatments; through the computing power of high-performance computing, medical researchers can process and analyze image data more rapidly, thus improving the accuracy and speed of medical diagnosis; high-performance computing can also help medical researchers simulate and analyze the functions of human organs and disease mechanisms. Through mathematical models based on high-performance computing, medical researchers can more accurately study human physiology and disease mechanisms and develop more effective treatments.
- Deep learning is a neural-network-based machine learning technique that automatically extracts useful features from massive amounts of data for applications in a variety of fields. In particular, deep learning techniques have started to play an important role in the medical field. Deep learning technology can be applied to the diagnosis and treatment of diseases: deep learning technology can be used to analyze medical images to automatically detect and identify signs of diseases, thus improving the early diagnosis rate and treatment effectiveness of diseases; deep learning can also be applied to genomics and drug development, helping scientists to discover more accurate and effective treatment solutions; and deep learning technology can be used to predict disease epidemics. The use of deep learning technology to predict disease trends can help medical institutions and government departments better respond to the outbreak and spread of diseases, thereby better protecting public health. In addition, with the development of artificial intelligence technology, deep learning technology is also expected to achieve more accurate and personalized treatment in the medical field, making medical services more inclusive and close to people’s needs. In the future, with the continuous development of and improvement in the application of deep learning technology in mathematical models, we can foresee that it will play an even more important role in the medical field.
- Virtual reality is a technology that allows users to interact with and immerse themselves in a computer-generated digital environment. Currently, virtual reality technology is widely used in entertainment and education, and its application in the medical field is starting to receive more and more attention. On the one hand, virtual reality technology can be used for medical simulation, simulating surgical procedures, and operational skills training. Through virtual reality technology, medical professionals can simulate various surgical scenarios, allowing medical students and doctors to operate and practice in a virtual environment, thus improving surgical skills and reducing surgical risks. Virtual reality technology can also be used to simulate and predict disease progression and treatment outcomes, helping doctors make more accurate treatment decisions. On the other hand, virtual reality technology can also be used to treat psychological disorders and pain management. Through virtual reality technology, patients can enter a virtual environment to relieve pain and anxiety through immersion and relaxation. For example, virtual reality technology can be used to relieve symptoms such as chronic pain, post-surgical pain, and nausea and vomiting caused by cancer treatment. In addition, virtual reality technology can be used to treat psychological disorders such as anxiety disorders, post-traumatic stress disorder, and phobias. Virtual reality technology can provide patients with a safe virtual environment in which they can gradually adapt and overcome their psychological disorders. At present, the collection of virtual reality technology and mathematical modeling is not close enough, but it has shown a wide range of application prospects and potential. In the future, virtual reality technology combined with mathematical modeling will play a more important role in the medical field, helping doctors and patients to better treat and recover.
- Gene editing technology is a biotechnology that uses tools such as CRISPR/Cas9 to precisely edit gene sequences. This technology can target specific genes in hereditary diseases and modify them to help patients restore normal function. In addition, gene editing technology can be used to treat other types of diseases, such as cancer, cardiovascular disease, and immune system disorders. As the technology continues to evolve, gene editing technology can also be used in the future to develop more precise mathematical modeling applications. Most current treatments are designed based on average outcomes and cannot be individually tailored to each patient’s unique situation. By using mathematical models and machine learning algorithms, individualized treatment plans can be tailored to achieve the best possible outcome based on each patient’s genetic information, medical history, and other clinical data, which would be an important advance. However, there are some challenges and risks associated with gene editing technology. Some people are concerned that gene editing may lead to unknown side effects and consequences and may even result in the permanent alteration of human genes. Therefore, we need strict ethical review and regulatory mechanisms to ensure the safety and reliability of gene editing technology.
- Blockchain technology is a database technology based on a distributed network of nodes, whose most important features are decentralization and security. The blockchain can distribute the modification and verification of data to multiple nodes throughout the network, ensuring the integrity and trustworthiness of data. The application of blockchain technology in healthcare can make medical data-sharing platforms more secure and reliable and help improve the quality and efficiency of medical services. Blockchain technology combines medical data with mathematical models to better analyze and predict disease occurrence and prevalence trends. The collection and analysis of medical data can help doctors and researchers better understand the development and treatment process of diseases and help improve the accuracy and effectiveness of treatment. At the same time, the analysis of medical data can also provide valuable information to public health departments to help them better control the spread of diseases. In disease prevention and control in the post-epidemic era, blockchain technology can prevent the spread of diseases by tracking the movement routes of infected people. By recording the movement trajectories of infected people on the blockchain, the public can be kept informed of their environment and risks, so they can take appropriate measures to protect themselves. Public health departments can also analyze these data to develop more scientific and precise prevention and control strategies to enhance the control and management of the epidemic.
In summary, research on medical problems based on mathematical models will be driven by various advanced technologies. These technologies can improve the efficiency and accuracy of medical research, thus bringing more opportunities for disease prevention and treatment. It is worth noting that when using these technologies for medical research, we also need to fully consider ethical, legal, and privacy issues to protect the safety of medical data and patients’ rights while promoting the development of technology and medicine.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L.; validation, Y.L., R.W. and A.Y.; formal analysis, R.W.; writing—original draft preparation, Y.L.; writing—review and editing, Yikai Liu, R.W. and A.Y.; supervision, A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Natural Science Foundation of China (NSFC), fund No. 52074126.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (NSFC), fund No. 52074126.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Migliori, G.B.; Kurhasani, X.; van den Boom, M.; Visca, D.; D’Ambrosio, L.; Centis, R.; Tiberi, S. History of prevention, diagnosis, treatment and rehabilitation of pulmonary sequelae of tuberculosis. La Presse Médicale 2022, 51, 104112. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, J.; Collins, S. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Psycho-neuroimmunology and the intestinal microbiota: Clinical observations and basic mechanisms. Clin. Exp. Immunol. 2010, 160, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Usak, M.; Kubiatko, M.; Shabbir, M.S.; Viktorovna Dudnik, O.; Jermsittiparsert, K.; Rajabion, L. Health care service delivery based on the Internet of things: A systematic and comprehensive study. Int. J. Commun. Syst. 2020, 33, e4179. [Google Scholar] [CrossRef]
- Pan, Y.; Fu, M.; Cheng, B.; Tao, X.; Guo, J. Enhanced deep learning assisted convolutional neural network for heart disease prediction on the internet of medical things platform. IEEE Access 2020, 8, 189503–189512. [Google Scholar] [CrossRef]
- Yasnitsky, L.N.; Dumler, A.A.; Poleshchuk, A.N.; Bogdanov, C.V.; Cherepanov, F.M. Artificial neural networks for obtaining new medical knowledge: Diagnostics and prediction of cardiovascular disease progression. Biol. Med. (Aligarh) 2015, 7, 095. [Google Scholar]
- Jang, H.J.; Cho, K.O. Applications of deep learning for the analysis of medical data. Arch. Pharmacal Res. 2019, 42, 492–504. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.; Diggle, P.; Shi, J. Joint modeling of time series measures and recurrent events and analysis of the effects of air quality on respiratory symptoms. J. Am. Stat. Assoc. 2008, 103, 48–60. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, Y.; Tao, L.; Yu, W.; Zhang, Y.; Huo, L.; He, Z. A Chan-Vese model based on the Markov chain for unsupervised medical image segmentation. Tsinghua Sci. Technol. 2021, 26, 833–844. [Google Scholar] [CrossRef]
- Salgia, R.; Mambetsariev, I.; Hewelt, B.; Achuthan, S.; Li, H.; Poroyko, V.; Wang, Y.; Sattler, M. Modeling small cell lung cancer (SCLC) biology through deterministic and stochastic mathematical models. Oncotarget 2018, 9, 26226–26242. [Google Scholar] [CrossRef]
- Li, H.; Slone, J.; Fei, L.; Huang, T. Mitochondrial DNA variants and common diseases: A mathematical model for the diversity of age-related mtDNA mutations. Cells 2019, 8, 608. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D. A dynamic logistics model for medical resources allocation in an epidemic control with demand forecast updating. J. Oper. Res. Soc. 2016, 67, 841–852. [Google Scholar] [CrossRef]
- Ordu, M.; Demir, E.; Tofallis, C.; Gunal, M.M. A novel healthcare resource allocation decision support tool: A forecasting-simulation-optimization approach. J. Oper. Res. Soc. 2021, 72, 485–500. [Google Scholar] [CrossRef]
- McGillen, J.B.; Anderson, S.J.; Dybul, M.R.; Hallett, T.B. Optimum resource allocation to reduce HIV incidence across sub-Saharan Africa: A mathematical modelling study. Lancet HIV 2016, 3, e441–e448. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and non-pharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Albizri, A.; Simsek, S. Artificial intelligence in healthcare operations to enhance treatment outcomes: A framework to predict lung cancer prognosis. Ann. Oper. Res. 2022, 308, 275–305. [Google Scholar] [CrossRef]
- Alsina, Á.; Salgado, M. Understanding early mathematical modelling: First steps in the process of translation between real-world contexts and mathematics. Int. J. Sci. Math. Educ. 2022, 20, 1719–1742. [Google Scholar] [CrossRef]
- Osaba, E.; Villar-Rodriguez, E.; Del Ser, J.; Nebro, A.J.; Molina, D.; LaTorre, A.; Suganthan, P.N.; Coello, C.A.C.; Herrera, F. A tutorial on the design, experimentation and application of metaheuristic algorithms to real-world optimization problems. Swarm Evol. Comput. 2021, 64, 100888. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, A.; Al-Mdallal, Q.M.; Khan, H.; Shah, K.; Khan, A. Fractional order mathematical modeling of COVID-19 transmission. Chaos Solitons Fractals 2020, 139, 110256. [Google Scholar] [CrossRef]
- Pannu, A. Artificial intelligence and its application in different areas. Artif. Intell. 2015, 4, 79–84. [Google Scholar]
- Blum, W. Quality teaching of mathematical modelling: What do we know, what can we do? In Proceedings of the 12th International Congress on Mathematical Education: Intellectual and Attitudinal Challenges; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 73–96. [Google Scholar]
- Bossaerts, P.; Murawski, C. Computational complexity and human decision-making. Trends Cogn. Sci. 2017, 21, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.M.; Hastie, T.J. Statistical models. In Statistical Models; Routledge: Oxfordshire, UK, 2017; pp. 13–44. [Google Scholar]
- Hastie, T.J.; Pregibon, D. Generalized linear models. In tatistical Models in S; Routledge: Oxfordshire, UK, 2017; pp. 195–247. [Google Scholar]
- Kidger, P. On neural differential equations. arXiv 2022, arXiv:2202.02435. [Google Scholar]
- Chen, Y.; Li, N.; Lourenço, J.; Wang, L.; Cazelles, B.; Dong, L.; Li, B.; Liu, Y.; Jit, M.; Bosse, N.I.; et al. Measuring the effects of COVID-19-related disruption on dengue transmission in southeast Asia and Latin America: A statistical modelling study. Lancet Infect. Dis. 2022, 22, 657–667. [Google Scholar] [CrossRef]
- Shehab, M.; Abualigah, L.; Shambour, Q.; Abu-Hashem, M.A.; Shambour, M.K.Y.; Alsalibi, A.I.; Gandomi, A.H. Machine learning in medical applications: A review of state-of-the-art methods. Comput. Biol. Med. 2022, 145, 105458. [Google Scholar] [CrossRef]
- Sridhar, C.; Pareek, P.K.; Kalidoss, R.; Jamal, S.S.; Shukla, P.K.; Nuagah, S.J. Optimal medical image size reduction model creation using recurrent neural network and GenPSOWVQ. J. Healthc. Eng. 2022, 2022, 2354866. [Google Scholar] [CrossRef]
- Hart, W.E.; Laird, C.D.; Watson, J.P.; Woodruff, D.L.; Hackebeil, G.A.; Nicholson, B.L.; Siirola, J.D. Pyomo-Optimization Modeling in Python; Springer: Berlin, Germany, 2017; Volume 67, p. 277. [Google Scholar]
- Koivunen, M.; Saranto, K. Nursing professionals’ experiences of the facilitators and barriers to the use of telehealth applications: A systematic review of qualitative studies. Scand. J. Caring Sci. 2018, 32, 24–44. [Google Scholar] [CrossRef]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 54. [Google Scholar] [CrossRef]
- Beerenwinkel, N.; Schwarz, R.F.; Gerstung, M.; Markowetz, F. Cancer evolution: Mathematical models and computational inference. Syst. Biol. 2015, 64, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Narin, A.; Kaya, C.; Pamuk, Z. Automatic detection of coronavirus disease (COVID-19) using x-ray images and deep convolutional neural networks. Pattern Anal. Appl. 2021, 24, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Katzir, I.; Dekel, E.; Mayo, A.E.; Alon, U. Prediction of multidimensional drug dose responses based on measurements of drug pairs. Proc. Natl. Acad. Sci. USA 2016, 113, 10442–10447. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Sato, Y.; Sinclair, R.; Tsukamoto, K.; Nishida, M. Rapid genome reshaping by multiple-gene loss after whole-genome duplication in teleost fish suggested by mathematical modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 14918–14923. [Google Scholar] [CrossRef]
- Alber, M.; Buganza Tepole, A.; Cannon, W.R.; De, S.; Dura-Bernal, S.; Garikipati, K.; Karniadakis, G.; Lytton, W.W.; Perdikaris, P.; Petzold, L.; et al. Integrating machine learning and multiscale modeling—Perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. NPJ Digit. Med. 2019, 2, 115. [Google Scholar] [CrossRef]
- Ionescu, C.; Lopes, A.; Copot, D.; Machado, J.T.; Bates, J.H. The role of fractional calculus in modeling biological phenomena: A review. Commun. Nonlinear Sci. Numer. Simul. 2017, 51, 141–159. [Google Scholar] [CrossRef]
- Neftci, E.O.; Averbeck, B.B. Reinforcement learning in artificial and biological systems. Nat. Mach. Intell. 2019, 1, 133–143. [Google Scholar] [CrossRef]
- Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017, 20, 340–352. [Google Scholar] [CrossRef]
- Sharma, S.; Samanta, G.P. Analysis of the dynamics of a tumor–immune system with chemotherapy and immunotherapy and quadratic optimal control. Differ. Equ. Dyn. Syst. 2016, 24, 149–171. [Google Scholar] [CrossRef]
- Ibrahim, R.W.; Jalab, H.A.; Karim, F.K.; Alabdulkreem, E.; Ayub, M.N. A medical image enhancement based on generalized class of fractional partial differential equations. Quant. Imaging Med. Surg. 2022, 12, 172. [Google Scholar] [CrossRef]
- Luzyanina, T.Y.B.; Bocharov, G.A. Markov chain Monte Carlo parameter estimation of the ODE compartmental cell growth model. Математическая Биoлoгия И Биoинфoрматика 2018, 13, 376–391. [Google Scholar] [CrossRef]
- Miranville, A.; Rocca, E.; Schimperna, G. On the long time behavior of a tumor growth model. J. Differ. Equ. 2019, 267, 2616–2642. [Google Scholar] [CrossRef]
- Zhang, Y.; Barocas, V.H.; Berceli, S.A.; Clancy, C.E.; Eckmann, D.M.; Garbey, M.; Kassab, G.S.; Lochner, D.R.; McCulloch, A.D.; Tran-Son-Tay, R.; et al. Multi-scale modeling of the cardiovascular system: Disease development, progression, and clinical intervention. Ann. Biomed. Eng. 2016, 44, 2642–2660. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Barucca, G.; Santecchia, E.; Majni, G.; Girardin, E.; Bassoli, E.; Denti, L.; Gatto, A.; Iuliano, L.; Moskalewicz, T.; Mengucci, P. Structural characterization of biomedical Co–Cr–Mo components produced by direct metal laser sintering. Mater. Sci. Eng. C 2015, 48, 263–269. [Google Scholar] [CrossRef]
- Gupta, S.; Sharaff, A.; Nagwani, N.K. Frequent item-set mining and clustering based ranked biomedical text summarization. J. Supercomput. 2023, 79, 139–159. [Google Scholar] [CrossRef]
- Bolton, L.; Cloot, A.H.; Schoombie, S.W.; Slabbert, J.P. A proposed fractional-order Gompertz model and its application to tumour growth data. Math. Med. Biol. A J. IMA 2015, 32, 187–209. [Google Scholar] [CrossRef]
- Kühleitner, M.; Brunner, N.; Nowak, W.G.; Renner-Martin, K.; Scheicher, K. Best fitting tumor growth models of the von Bertalanffy-PütterType. BMC Cancer 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.A.B.F.; Oden, J.T.; Hormuth, D.A.; Yankeelov, T.E.; Almeida, R.C. Selection, calibration, and validation of models of tumor growth. Math. Model. Methods Appl. Sci. 2016, 26, 2341–2368. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular functions and specific roles of circRNAs in the cardiovascular system. Non-Coding RNA Res. 2018, 3, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Femminò, S.; Penna, C.; Margarita, S.; Comità, S.; Brizzi, M.F.; Pagliaro, P. Extracellular vesicles and cardiovascular system: Biomarkers and Cardioprotective Effectors. Vasc. Pharmacol. 2020, 135, 106790. [Google Scholar] [CrossRef]
- Patel, B.; Sengupta, P. Machine learning for predicting cardiac events: What does the future hold? Expert Rev. Cardiovasc. Ther. 2020, 18, 77–84. [Google Scholar] [CrossRef]
- Allenbach, Y.; Saadoun, D.; Maalouf, G.; Vieira, M.; Hellio, A.; Boddaert, J.; Gros, H.; Salem, J.E.; Resche Rigon, M.; Menyssa, C.; et al. Development of a multivariate prediction model of intensive care unit transfer or death: A French prospective cohort study of hospitalized COVID-19 patients. PLoS ONE 2020, 15, e0240711. [Google Scholar] [CrossRef]
- Liu, A.; Wang, Z.; Yang, Y.; Wang, J.; Dai, X.; Wang, L.; Lu, Y.; Xue, F. Preoperative diagnosis of malignant pulmonary nodules in lung cancer screening with a radiomics nomogram. Cancer Commun. 2020, 40, 16–24. [Google Scholar] [CrossRef]
- Zyout, I.; Togneri, R. Empirical mode decomposition of digital mammograms for the statistical based characterization of architectural distortion. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 109–112. [Google Scholar]
- Azbeg, K.; Boudhane, M.; Ouchetto, O.; Jai Andaloussi, S. Diabetes emergency cases identification based on a statistical predictive model. J. Big Data 2022, 9, 31. [Google Scholar] [CrossRef]
- Austin, P.C. A tutorial on multilevel survival analysis: Methods, models and applications. Int. Stat. Rev. 2017, 85, 185–203. [Google Scholar] [CrossRef]
- Jing, B.; Zhang, T.; Wang, Z.; Jin, Y.; Liu, K.; Qiu, W.; Ke, L.; Sun, Y.; He, C.; Hou, D.; et al. A deep survival analysis method based on ranking. Artif. Intell. Med. 2019, 98, 1–9. [Google Scholar] [CrossRef]
- Peláez, R.; Cao, R.; Vilar, J.M. Nonparametric estimation of the conditional survival function with double smoothing. J. Nonparametr. Stat. 2022, 34, 1063–1090. [Google Scholar] [CrossRef]
- Barakat, A.; Mittal, A.; Ricketts, D.; Rogers, B.A. Understanding survival analysis: Actuarial life tables and the Kaplan–Meier plot. Br. J. Hosp. Med. 2019, 80, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Benza, R.L.; Gomberg-Maitland, M.; Elliott, C.G.; Farber, H.W.; Foreman, A.J.; Frost, A.E.; McGoon, M.D.; Pasta, D.J.; Selej, M.; Burger, C.D.; et al. Predicting survival in patients with pulmonary arterial hypertension: The REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019, 156, 323–337. [Google Scholar] [CrossRef]
- Gupta, S.; Provenzale, D.; Llor, X.; Halverson, A.L.; Grady, W.; Chung, D.C.; Haraldsdottir, S.; Markowitz, A.J.; Slavin, T.P., Jr.; Hampel, H.; et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Colorectal, version 2.2019: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Sargent, R.G. Verification and validation of simulation models. In Proceedings of the 2010 IEEE Winter Simulation Conference, Baltimore, MD, USA, 5–8 December 2010; pp. 166–183. [Google Scholar]
- Lwakatare, L.E.; Rånge, E.; Crnkovic, I.; Bosch, J. On the experiences of adopting automated data validation in an industrial machine learning project. In Proceedings of the 2021 IEEE/ACM 43rd International Conference on Software Engineering: Software Engineering in Practice (ICSE-SEIP), Madrid, Spain, 25–28 May 2021; pp. 248–257. [Google Scholar]
- Cybulska, B.; Kłosiewicz-Latoszek, L. Landmark studies in coronary heart disease epidemiology. The Framingham Heart Study after 70 years and the Seven Countries Study after 60 years. Kardiol. Pol. (Pol. Heart J. ) 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Beigmohammadi, M.T.; Amoozadeh, L.; Motlagh, F.R.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2103–2109. [Google Scholar]
- Beigmohammadi, M.T.; Amoozadeh, L.; Rezaei Motlagh, F.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K. Mortality predictive value of APACHE II and SOFA scores in COVID-19 patients in the intensive care unit. Can. Respir. J. 2022, 2022, 5129314. [Google Scholar] [CrossRef]
- Rahmatinejad, Z.; Tohidinezhad, F.; Reihani, H.; Rahmatinejad, F.; Pourmand, A.; Abu-Hanna, A.; Eslami, S. Prognostic utilization of models based on the APACHE II, APACHE IV, and SAPS II scores for predicting in-hospital mortality in emergency department. Am. J. Emerg. Med. 2020, 38, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Linzey, J.R.; Foshee, R.L.; Srinivasan, S.; Fiestan, G.O.; Mossner, J.M.; Gemmete, J.J.; Burke, J.F.; Sheehan, K.M.; Rajajee, V.; Pandey, A.S. The predictive value of the hospital score and Lace Index for an adult neurosurgical population: A prospective analysis. World Neurosurg. 2020, 137, e166–e175. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Mago, V. Role of machine learning in medical research: A survey. Comput. Sci. Rev. 2021, 40, 100370. [Google Scholar] [CrossRef]
- Gopal, V.N.; Al-Turjman, F.; Kumar, R.; Anand, L.; Rajesh, M. Feature selection and classification in breast cancer prediction using IoT and machine learning. Measurement 2021, 178, 109442. [Google Scholar] [CrossRef]
- Sachdev, N.; Rishi, N.; Jain, R. Breast Cancer Prediction Using Supervised Machine Learning Techniques. Int. J. Comput. Biol. Bioinform. 2021, 7, 8–13. [Google Scholar]
- Sun, J.; Yang, Y.; Wang, Y.; Wang, L.; Song, X.; Zhao, X. Survival risk prediction of esophageal cancer based on self-organizing maps clustering and support vector machine ensembles. IEEE Access 2020, 8, 131449–131460. [Google Scholar] [CrossRef]
- Daoud, M.; Mayo, M. A survey of neural network-based cancer prediction models from microarray data. Artif. Intell. Med. 2019, 97, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, S.; Wu, Q.; Azim, R.; Li, W. Predicting potential miRNA-disease associations by combining gradient boosting decision tree with logistic regression. Comput. Biol. Chem. 2020, 85, 107200. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, N.; Shatnawi, I. Comparing supervised and semi-supervised machine learning models on diagnosing breast cancer. Ann. Med. Surg. 2021, 62, 53–64. [Google Scholar] [CrossRef]
- Kabiraj, S.; Raihan, M.; Alvi, N.; Afrin, M.; Akter, L.; Sohagi, S.A.; Podder, E. Breast cancer risk prediction using XGBoost and random forest algorithm. In Proceedings of the 2020 11th International Conference on Computing, Communication and NETWORKING Technologies (ICCCNT), Kharagpur, India, 1–3 July 2020; pp. 1–4. [Google Scholar]
- Morais-Rodrigues, F.; Silv́erio-Machado, R.; Kato, R.B.; Rodrigues, D.L.N.; Valdez-Baez, J.; Fonseca, V.; San, E.J.; Gomes, L.G.R.; Dos Santos, R.G.; Viana, M.V.C.; et al. Analysis of the microarray gene expression for breast cancer progression after the application modified logistic regression. Gene 2020, 726, 144168. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Abdulah, D.; Al-Tuwaijari, J.M. Cancer classification using gaussian naive bayes algorithm. In Proceedings of the 2019 International Engineering Conference (IEC), Erbil, Iraq, 23–25 June 2019; pp. 165–170. [Google Scholar]
- Ray, S. A quick review of machine learning algorithms. In Proceedings of the 2019 International Conference on Machine Learning, Big Data, Cloud and Parallel Computing (COMITCon), Faridabad, India, 14–16 February 2019; pp. 35–39. [Google Scholar]
- Shakeel, P.M.; Burhanuddin, M.A.; Desa, M.I. Lung cancer detection from CT image using improved profuse clustering and deep learning instantaneously trained neural networks. Measurement 2019, 145, 702–712. [Google Scholar] [CrossRef]
- Vougas, K.; Sakellaropoulos, T.; Kotsinas, A.; Foukas, G.R.P.; Ntargaras, A.; Koinis, F.; Polyzos, A.; Myrianthopoulos, V.; Zhou, H.; Narang, S.; et al. Machine learning and data mining frameworks for predicting drug response in cancer: An overview and a novel in silico screening process based on association rule mining. Pharmacol. Ther. 2019, 203, 107395. [Google Scholar] [CrossRef]
- Schneider, L.; Laiouar-Pedari, S.; Kuntz, S.; Krieghoff-Henning, E.; Hekler, A.; Kather, J.N.; Gaiser, T.; Fröhling, S.; Brinker, T.J. Integration of deep learning-based image analysis and genomic data in cancer pathology: A systematic review. Eur. J. Cancer 2022, 160, 80–91. [Google Scholar] [CrossRef]
- Latif, J.; Xiao, C.; Imran, A.; Tu, S. Medical imaging using machine learning and deep learning algorithms: A review. In Proceedings of the 2019 2nd International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 30–31 January 2019; pp. 1–5. [Google Scholar]
- Lundervold, A.S.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Für Med. Phys. 2019, 29, 102–127. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Wu, Q.; Xia, Y. Medical image classification using synergic deep learning. Med. Image Anal. 2019, 54, 10–19. [Google Scholar] [CrossRef]
- Li, Q.; Cai, W.; Wang, X.; Zhou, Y.; Feng, D.D.; Chen, M. Medical image classification with convolutional neural network. In Proceedings of the 2014 IEEE 13th International Conference on Control Automation Robotics & Vision (ICARCV), Singapore, 10–12 December 2014; pp. 844–848. [Google Scholar]
- Nour, M.; Cömert, Z.; Polat, K. A novel medical diagnosis model for COVID-19 infection detection based on deep features and Bayesian optimization. Appl. Soft Comput. 2020, 97, 106580. [Google Scholar] [CrossRef]
- Yadav, S.S.; Jadhav, S.M. Deep convolutional neural network based medical image classification for disease diagnosis. J. Big Data 2019, 6, 113. [Google Scholar] [CrossRef]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef]
- Gysi, D.M.; Valle, Í.D.; Zitnik, M.; Ameli, A.; Gan, X.; Varol, O.; Ghiassian, S.D.; Patten, J.J.; Davey, R.A.; Loscalzo, J.; et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2025581118. [Google Scholar] [CrossRef] [PubMed]
- Mezei, T.; Horváth, A.; Nagy, Z.; Czigléczki, G.; Banczerowski, P.; Báskay, J.; Pollner, P. A Novel Prognostication System for Spinal Metastasis Patients Based on Network Science and Correlation Analysis. Clin. Oncol. 2023, 35, e20–e29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Z.; Jiang, R.; Zhou, M. DeepCDR: A hybrid graph convolutional network for predicting cancer drug response. Bioinformatics 2020, 36 (Suppl. S2), i911–i918. [Google Scholar] [CrossRef]
- Ding, K.; Zhou, M.; Wang, Z.; Liu, Q.; Arnold, C.W.; Zhang, S.; Metaxas, D.N. Graph Convolutional Networks for Multi-modality Medical Imaging: Methods, Architectures, and Clinical Applications. arXiv 2022, arXiv:2202.08916. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).