Enhanced Fuzzy Elephant Herding Optimization-Based OTSU Segmentation and Deep Learning for Alzheimer’s Disease Diagnosis
Abstract
:1. Introduction
- Initially, the two AD database such as ADNI and AIBL are taken as the input.
- Then, the pre-processing steps such as geometry correction, linear registration and skull-stripping are done.
- Third, EFEHO to OTSU is suggested for segmentation.
- Finally, the findings show that our DA-MIDL technique beats numerous existing techniques regarding reliability and generality.
2. Related Work
2.1. Meta-Heuristic Algorithms
2.2. Machine Learning and Deep Learning Algorithms
2.3. Hybrid Model
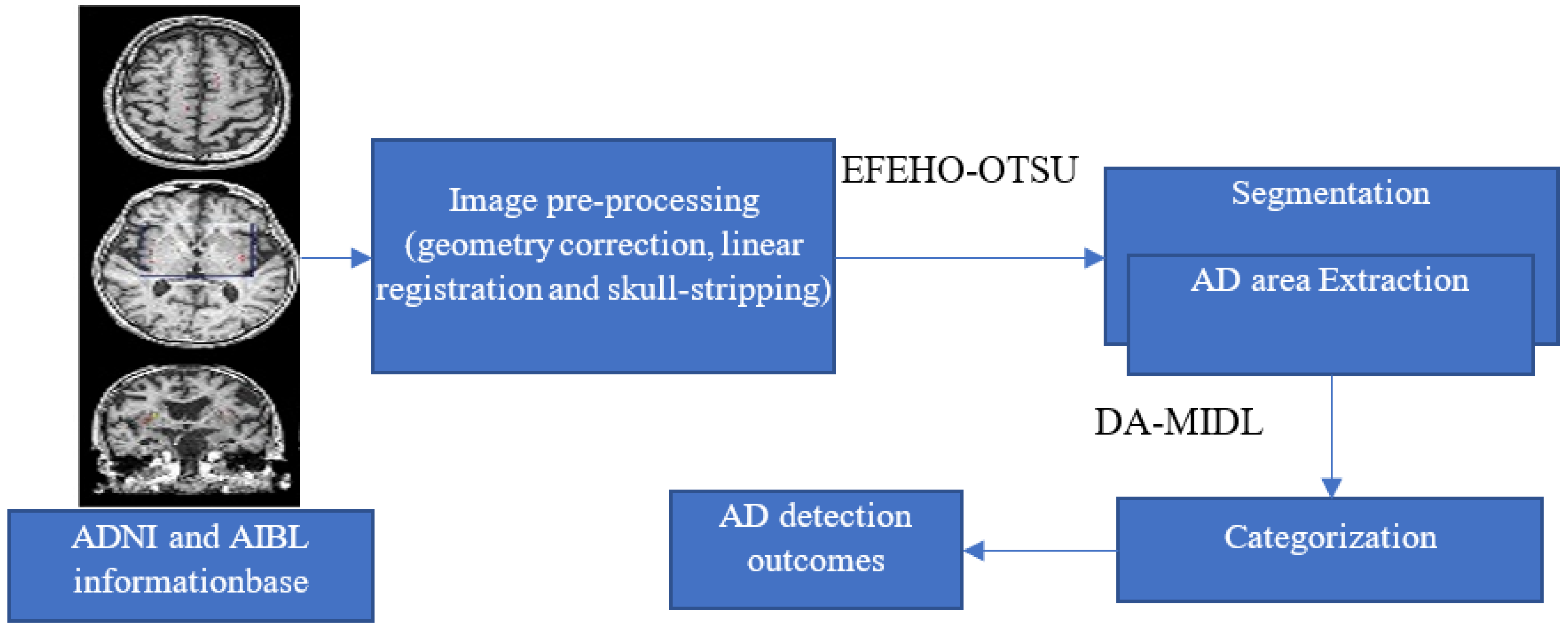
3. Proposed Methodology for AD Detection
3.1. Input Database and Image Pre-Processing
3.2. Segmentation Using EFEHO to OTSU Technique
- (1)
- The image must be retrieved and pre-processed.
- (2)
- Set the elephant’s and clan population’s initial positions at random.
- (3)
- Use fuzzy by determining the scale factor for each particle based on the elephant’s state.
- (4)
- Rank the population based on individual fitness and carry out the upgrading and segregation processes.
- (5)
- Assess the population based on the most recently revised position.
- (6)
- Assess each individual elephant based on its location.
- (7)
- Iteration lengthens.
- (8)
- Complete the procedure and return optimal elephants as an OTSU threshold value.
- (9)
- Image segmentation based on a predefined threshold.
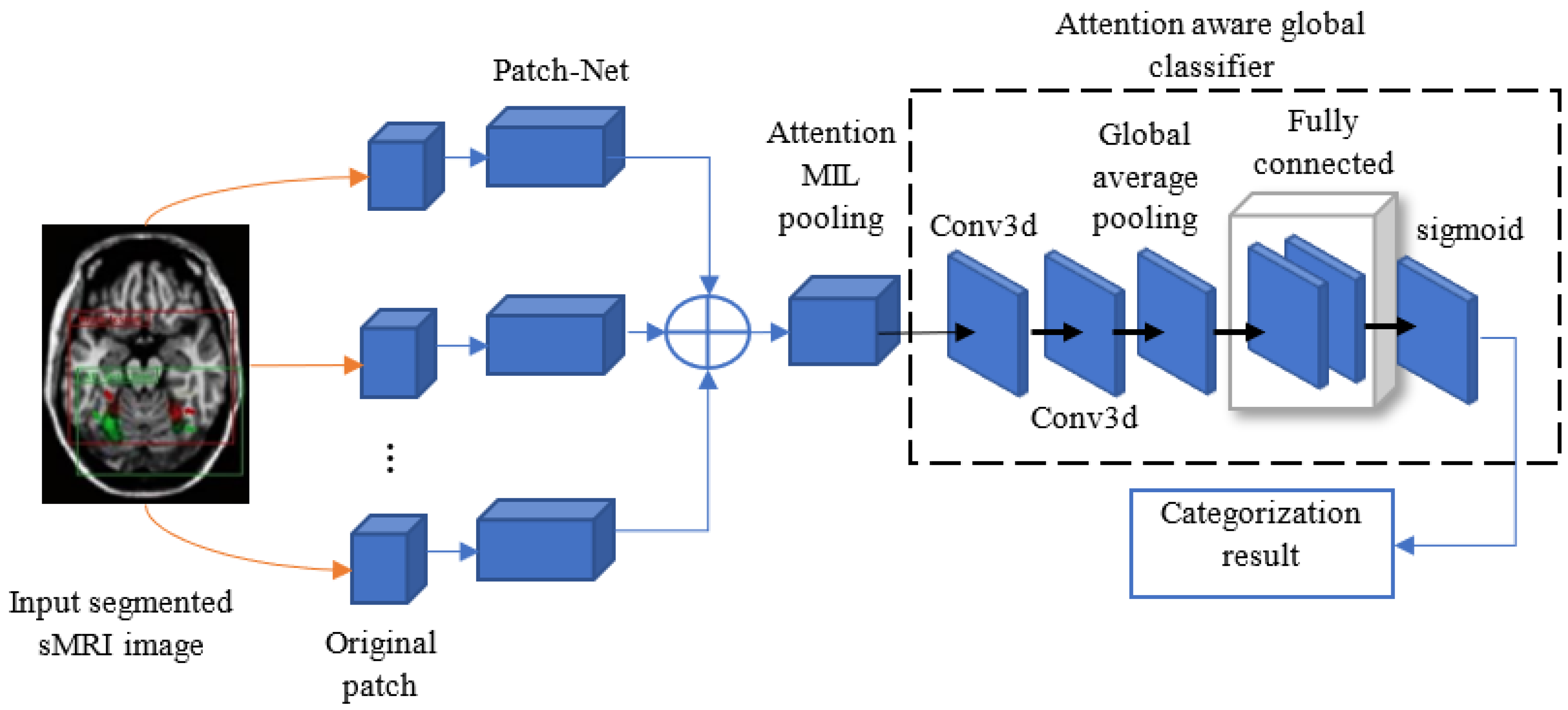
3.3. AD Detection Using DA-MIDL
4. Experimental Outcomes and Discussions
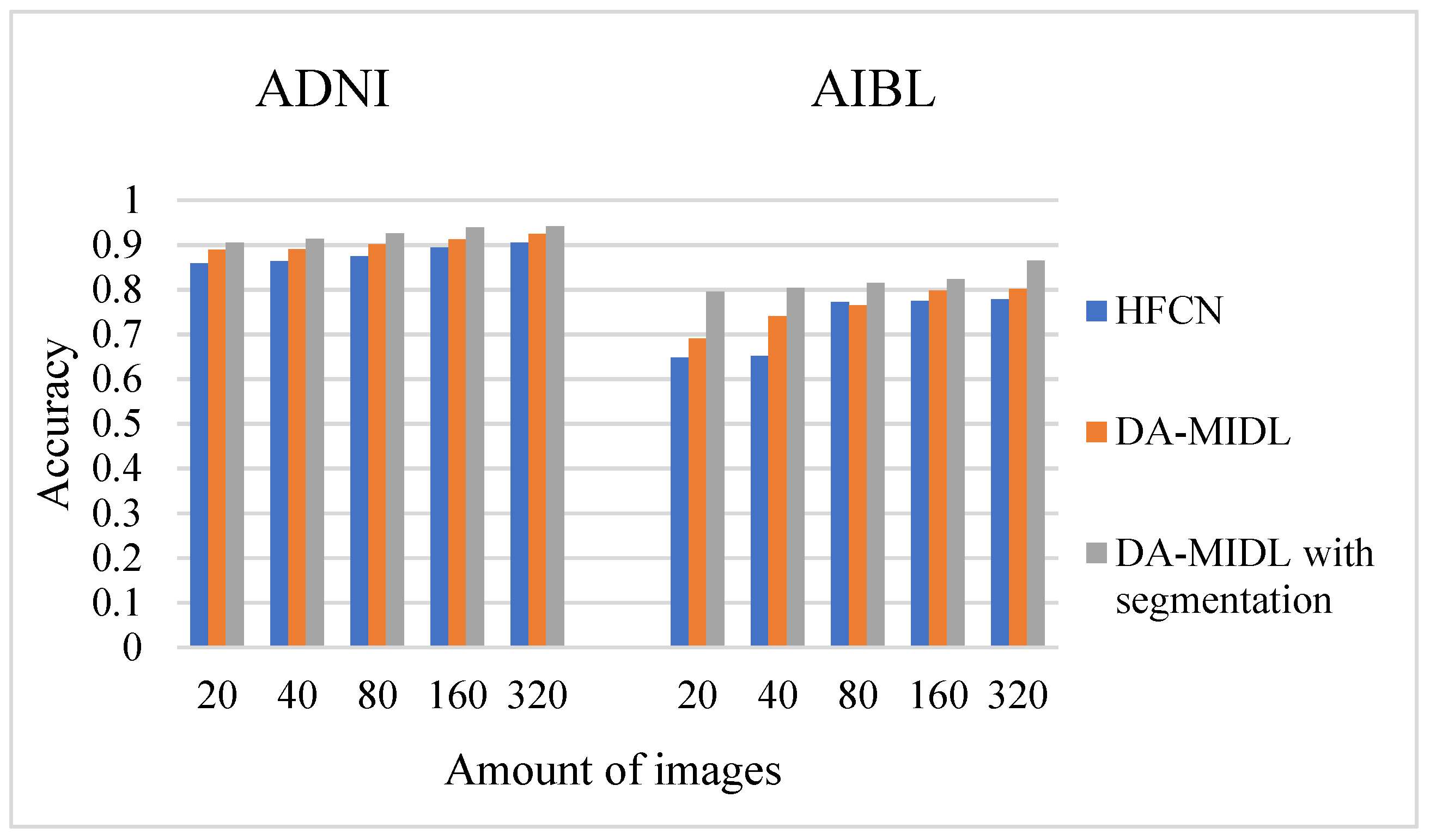
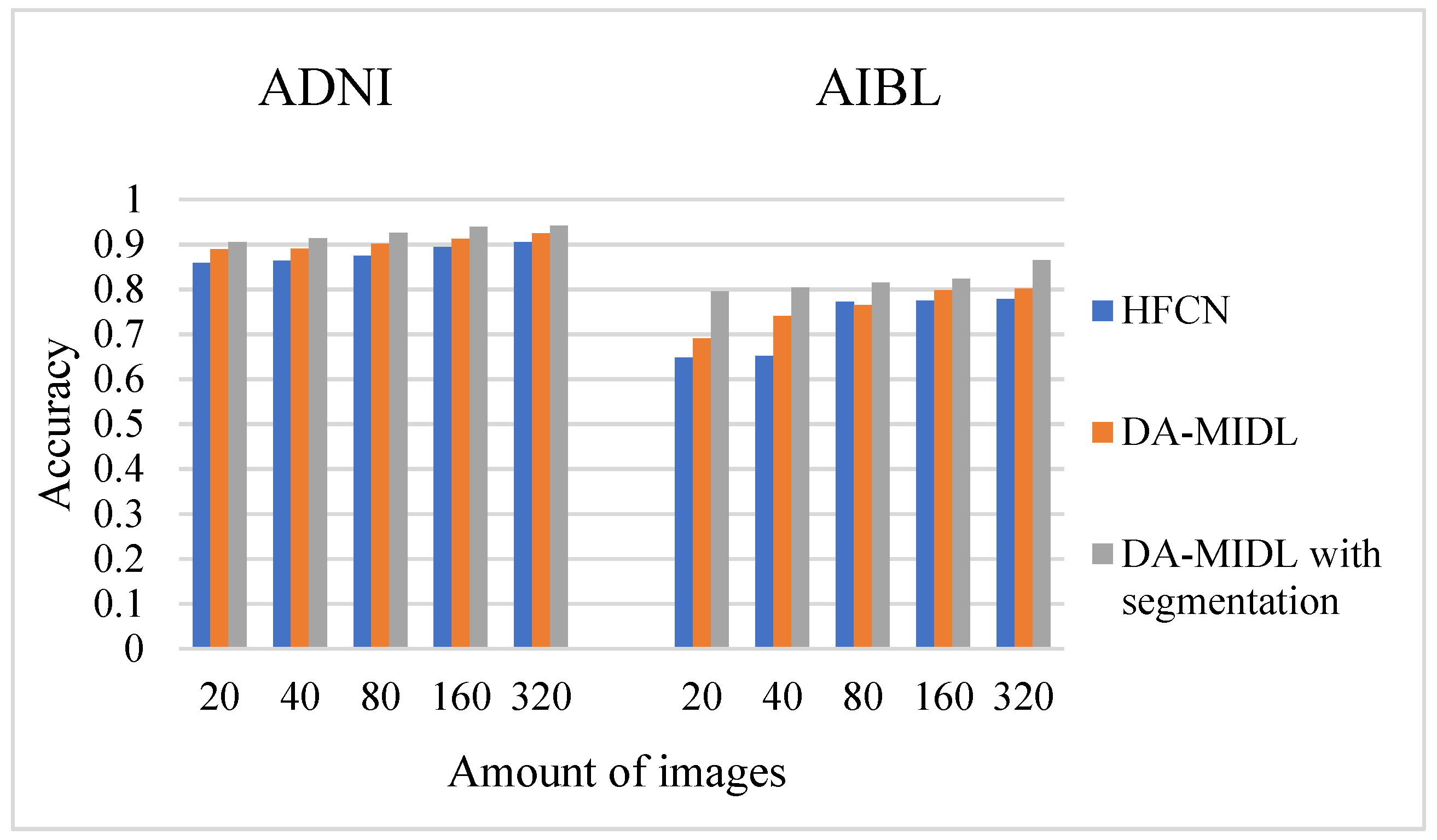
4.1. Accuracy Comparison Outcomes
4.2. Sensitivity Comparison Outcomes
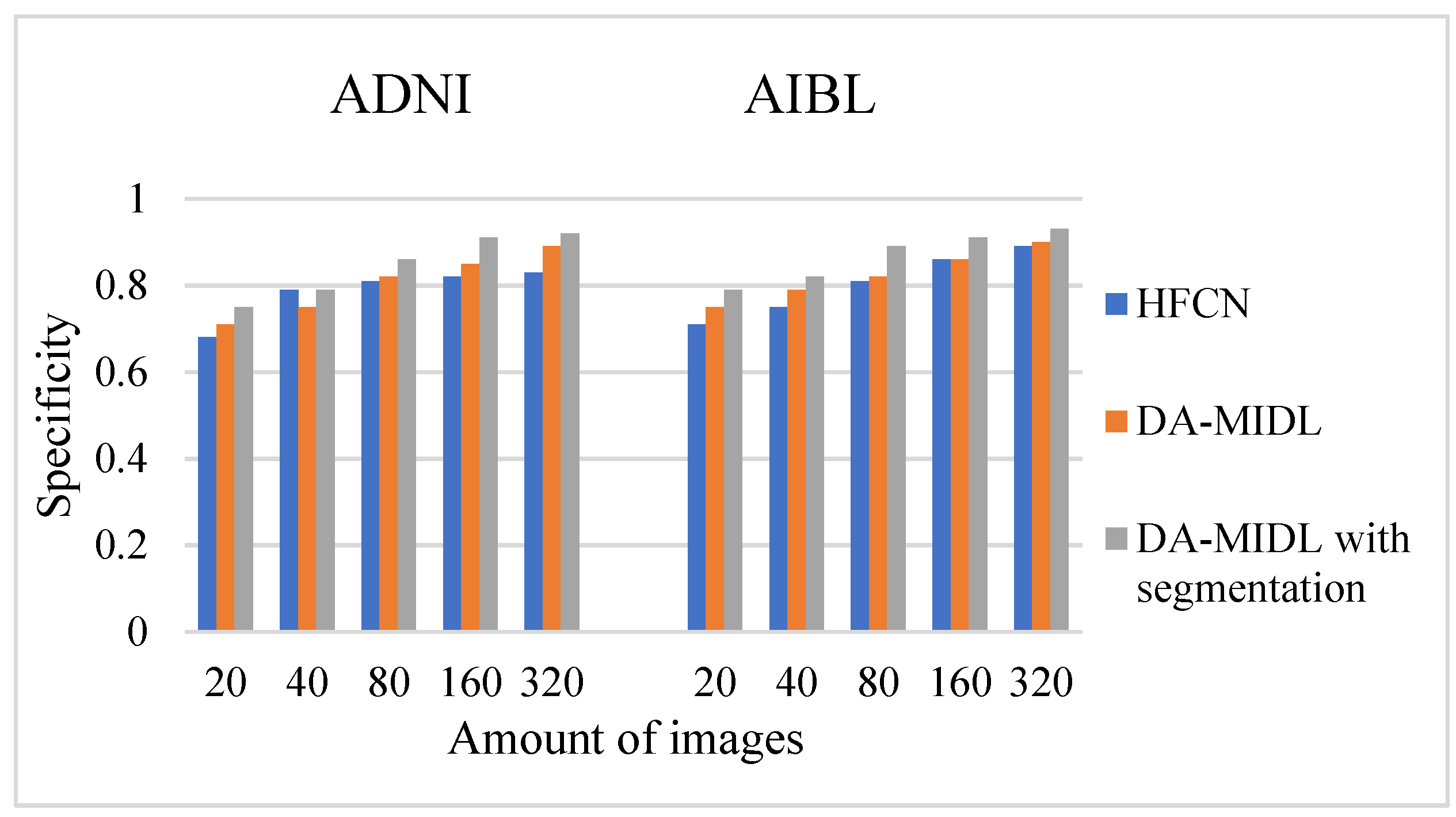
4.3. Specificity Comparison Outcomes
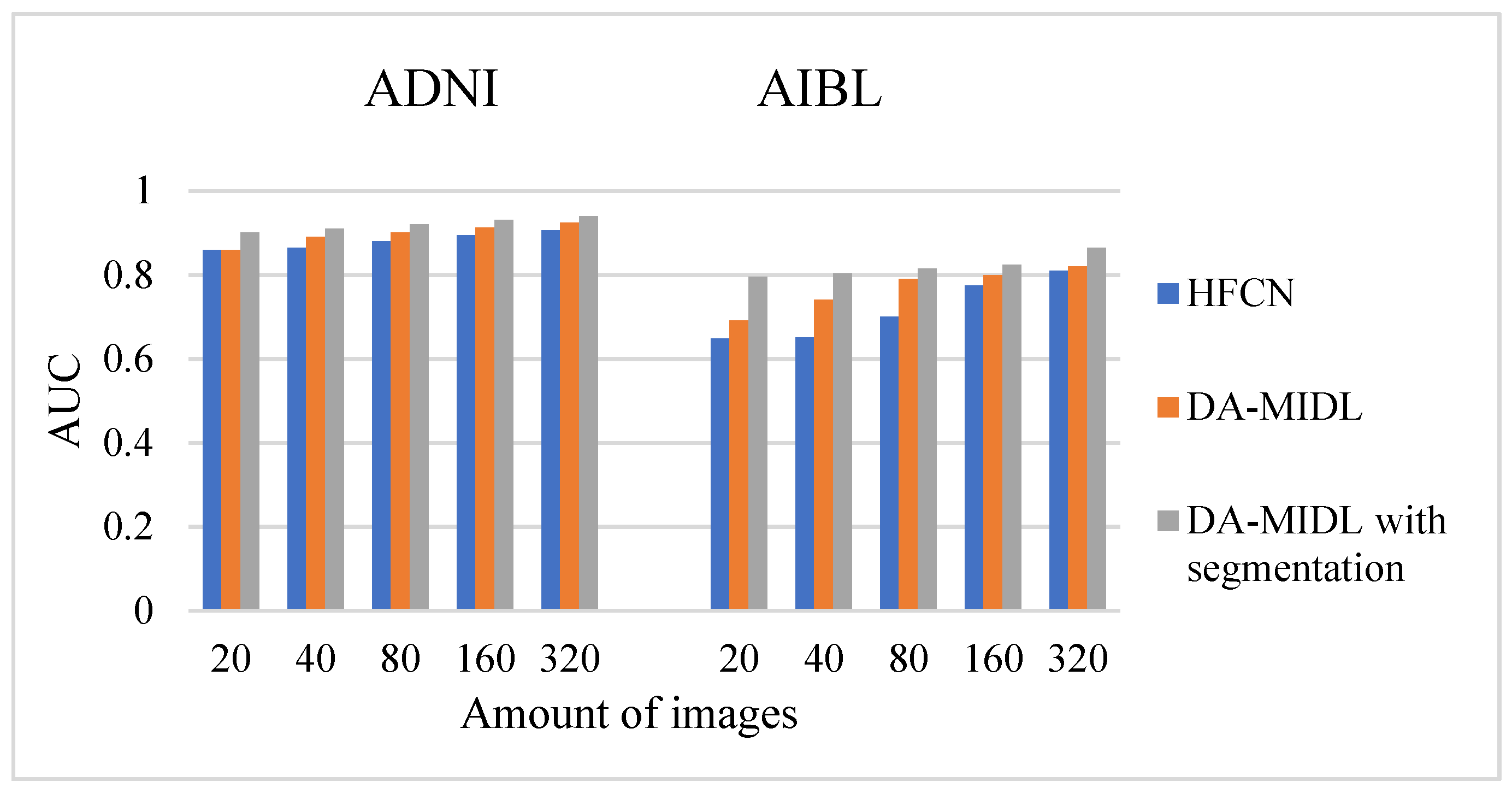
4.4. AUC Comparison Outcomes
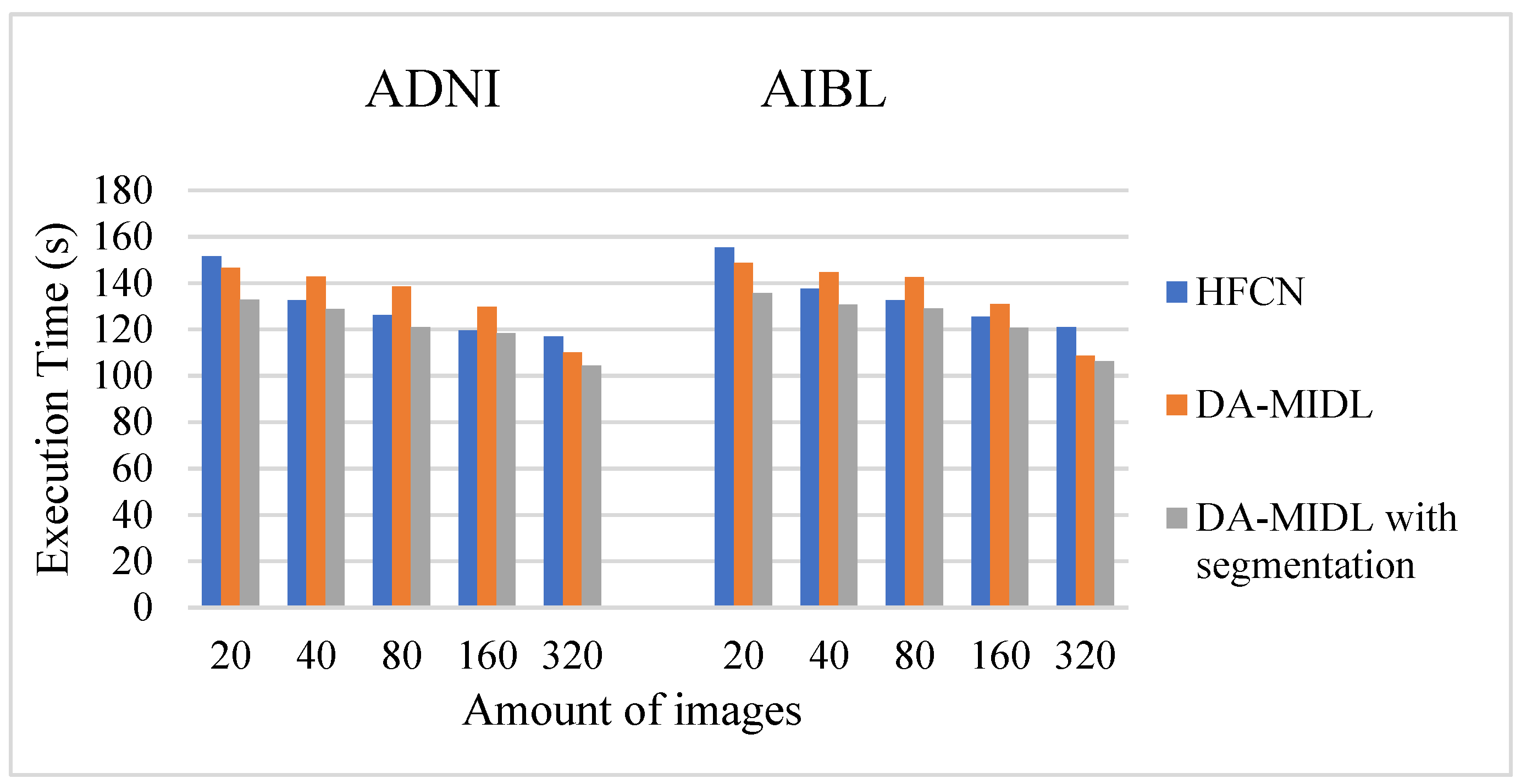
4.5. Execution Time Comparison Outcome
5. Conclusions and Future Work
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosow, K.; Holzapfel, A.; Karlawish, J.H.; Baumgart, M.; Bain, L.J.; Khachaturian, A.S. Countrywide strategic plans on Alzheimer’s disease: Developing the framework for the international battle against Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B. Structural imaging in the clinical diagnosis of Alzheimer’s disease: Problems and tools. J. Neurol. Neurosurg. Psychiatry 2001, 70, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, E.; Pepe, A.; Gaser, C.; Huttunen, H.; Tohka, J. ADNeuroimaging Initiative. Machine learning framework for timely MRI-based Alzheimer’s conversion diagnosis in MCI subjects. Neuroimage 2015, 104, 398–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouzannezhad, P.; Abbaspour, A.; Fang, C.; Cabrerizo, M.; Loewenstein, D.; Duara, R.; Adjouadi, M. A survey on applications and analysis techniques of functional magnetic resonance imaging for Alzheimer’s disease. J. Neurosci. Methods 2019, 317, 121–140. [Google Scholar] [CrossRef]
- Cuingnet, R.; Gerardin, E.; Tessieras, J.; Auzias, G.; Lehéricy, S.; Habert, M.-O.; Chupina, M.; Benali, H.; Colliot, O.; The Alzheimer’s Disease Neuroimaging Initiative1. Automatic categorization of patients with ADfrom structural MRI: Comparing ten techniques using the ADNI database. Neuroimage 2011, 56, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Coupe, P.; Eskildsen, S.F.; Manjon, J.V.; Fonov, V.S.; Pruessner, J.C.; Allard, M.; The Alzheimer’s Disease Neuroimaging Initiative. Scoring by nonlocal image patch estimator for timely detection of Alzheimer’s disease. Neuroimage 2012, 1, 141–152. [Google Scholar] [CrossRef]
- Dukart, J.; Schroeter, M.L.; Mueller, K.; ADNeuroimaging. Age correction in dementia–matching to a healthy brain. PLoS ONE 2011, 6, e22193. [Google Scholar] [CrossRef]
- Tanveer, M.; Richhariya, B.; Khan, R.U.; Rashid, A.H.; Khanna, P.; Prasad, M.; Lin, C.T. Machine learning techniques for the diagnosis of Alzheimer’s disease: A review. ACM Trans. Multimed. Comput. Commun. Appl. 2020, 16, 1–35. [Google Scholar] [CrossRef]
- Noor, M.B.T.; Zenia, N.Z.; Kaiser, M.S.; Al Mamun, S.; Mahmud, M. Application of deep learning in detecting neurological disorders from magnetic resonance images: A survey on detecting Alzheimer’s disease, Parkinson’s disease and schizophrenia. Brain Inform. 2020, 7, 11. [Google Scholar] [CrossRef]
- Lin, W.; Tong, T.; Gao, Q.; Guo, D.; Du, X.; Yang, Y.; Guo, G.; Xiao, M.; Du, M.; Qu, X.; et al. Convolutional neural networks-based MRI image analysis for the ADdiagnosis from mild cognitive impairment. Front. Neurosci. 2018, 12, 777. [Google Scholar] [CrossRef]
- Mirjalili, S.; Mirjalili, S.M.; Lewis, A. Grey wolf optimizer. Adv. Eng. Softw. 2014, 69, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Zamani, H.; Nadimi-Shahraki, M.H.; Gandomi, A.H. Starling murmuration optimizer: A novel bio-inspired algorithm for global and engineering optimization. Comput. Methods Appl. Mech. Eng. 2022, 392, 114616. [Google Scholar] [CrossRef]
- Nadimi-Shahraki, M.H.; Fatahi, A.; Zamani, H.; Mirjalili, S.; Abualigah, L.; Abd Elaziz, M. Migration-based moth-flame optimization algorithm. Processes 2021, 9, 2276. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, F.; Qi, X.; Li, C.; Yao, L. Categorization of AD based on brain MRI and machine learning. Neural Comput. Appl. 2020, 32, 1927–1936. [Google Scholar] [CrossRef]
- Magnin, B.; Mesrob, L.; Kinkingnéhun, S.; Pélégrini-Issac, M.; Colliot, O.; Sarazin, M.; Dubois, B.; Lehéricy, S.; Benali, H. Support vector machine-based categorization of ADfrom whole-brain anatomical MRI. Neuroradiology 2009, 51, 73–83. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.; Shen, D. Relationship induced multi-template learning for diagnosis of ADand mild cognitive impairment. IEEE Trans. Med. Imaging 2016, 35, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Tran, L.; Thung, K.H.; Ji, S.; Shen, D.; Li, J. A robust deep technique for improved categorization of AD/MCI patients. IEEE J. Biomed. Health Inform. 2015, 19, 1610–1616. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, Y.; Zhu, D.C.; Bozoki, A.C.; Li, T. Categorization of Alzheimer’s disease, mild cognitive impairment and normal control subjects using resting-state fMRI based network connectivity analysis. IEEE J. Transl. Eng. Health Med. 2018, 6, 1801009. [Google Scholar] [CrossRef]
- Hazarika, R.A.; Abraham, A.; Kandar, D.; Maji, A.K. An improved LeNet-Deep Neural Network technique for ADcategorization using Brain Magnetic Resonance Images. IEEE Access 2021, 9, 161194–161207. [Google Scholar] [CrossRef]
- Zhu 2021, W.; Sun, L.; Huang, J.; Han, L.; Zhang, D. Dual Attention Multi-Instance Deep Learning for ADDiagnosis with Structural MRI. IEEE Trans. Med. Imaging 2021, 40, 2354–2366. [Google Scholar] [CrossRef]
- Song, X.; Mao, M.; Qian, X. Auto-Metric Graph Neural Network Based on a Meta-learning Strategy for diagnosing Alzheimer’s disease. IEEE J. Biomed. Health Inform. 2021, 25, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Elazab, A.; Yang, P.; Wang, T.; Zhou, F.; Hu, H.; Xiao, X.; Lei, B. Deep learning framework for ADdiagnosis via 3D-CNN and FSBi-LSTM. IEEE Access 2019, 7, 63605–63618. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Adeli, E.; Shen, D. Landmark-based deep multiinstance learning for brain disease diagnosis. Med. Image Anal. 2018, 43, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Liu, M.; Zhang, J.; Shen, D. Hierarchical fully convolutional network for joint atrophy localization and ADdiagnosis using structural MRI. IEEE Trans. Pattern Anal. Mach. Intell. 2020, 42, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Abuhmed, T.; El-Sappagh, S.; Alonso, J.M. Robust hybrid deep learning models for Alzheimer’s progression detection. Knowl. Based Syst. 2021, 213, 106688. [Google Scholar] [CrossRef]
- Basheera, S.; Ram, M.S.S. Convolution neural network–based Alzheimer’s disease classification using hybrid enhanced independent component analysis based segmented gray matter of T2 weighted magnetic resonance imaging with clinical valuation. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 974–986. [Google Scholar] [CrossRef]
- Ellis, K.A.; Bush, A.I.; Darby, D.; De Fazio, D.; Foster, J.; Hudson, P.; AIBL Research Group. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int. Psychogeriatr. 2009, 21, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Murugan, T.M.; Baburaj, E. Comparison of Hybrid Elephant Herding Optimization with Different Evolutionary Optimization Algorithms. ICTACT J. Soft Comput. 2020, 10, 2171–2182. [Google Scholar]
- Tuba, E.; Alihodzic, A.; Tuba, M. Multilevel image thresholding using elephant herding optimization procedure. In Proceedings of the 2017 14th International Conference on the Engineering of Modern Electric Systems (EMES), Oradea, Romania, 1–2 June 2017; pp. 240–243. [Google Scholar]

| SFRBP | Scale Factor | ||
|---|---|---|---|
| Sm | Me | La | |
| Sm | La | Sm | Sm |
| Me | La | Me | Sm |
| La | La | Me | Sm |
| Hyper Parameter | Values |
|---|---|
| Number of features (c) | 512 |
| Learning rate | 0.1 |
| epochs | 60 |
| Patch (k) | 64,000 |
| loss hyper-parameters (weight is λ and temperature parameter is τ) | 0.5 and 1.0, respectively |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhassan, A.M.; The Alzheimer’s Disease Neuroimaging Initiative; The Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Enhanced Fuzzy Elephant Herding Optimization-Based OTSU Segmentation and Deep Learning for Alzheimer’s Disease Diagnosis. Mathematics 2022, 10, 1259. https://doi.org/10.3390/math10081259
Alhassan AM, The Alzheimer’s Disease Neuroimaging Initiative, The Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Enhanced Fuzzy Elephant Herding Optimization-Based OTSU Segmentation and Deep Learning for Alzheimer’s Disease Diagnosis. Mathematics. 2022; 10(8):1259. https://doi.org/10.3390/math10081259
Chicago/Turabian StyleAlhassan, Afnan M., The Alzheimer’s Disease Neuroimaging Initiative, and The Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. 2022. "Enhanced Fuzzy Elephant Herding Optimization-Based OTSU Segmentation and Deep Learning for Alzheimer’s Disease Diagnosis" Mathematics 10, no. 8: 1259. https://doi.org/10.3390/math10081259
APA StyleAlhassan, A. M., The Alzheimer’s Disease Neuroimaging Initiative, & The Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. (2022). Enhanced Fuzzy Elephant Herding Optimization-Based OTSU Segmentation and Deep Learning for Alzheimer’s Disease Diagnosis. Mathematics, 10(8), 1259. https://doi.org/10.3390/math10081259






