Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters
Abstract
:1. Introduction
2. Methods
2.1. Design
2.2. Study Population
2.3. Variables and Measurement Instruments
2.3.1. Measurement of the Anthropometric Parameters
2.3.2. Vitamin Intake
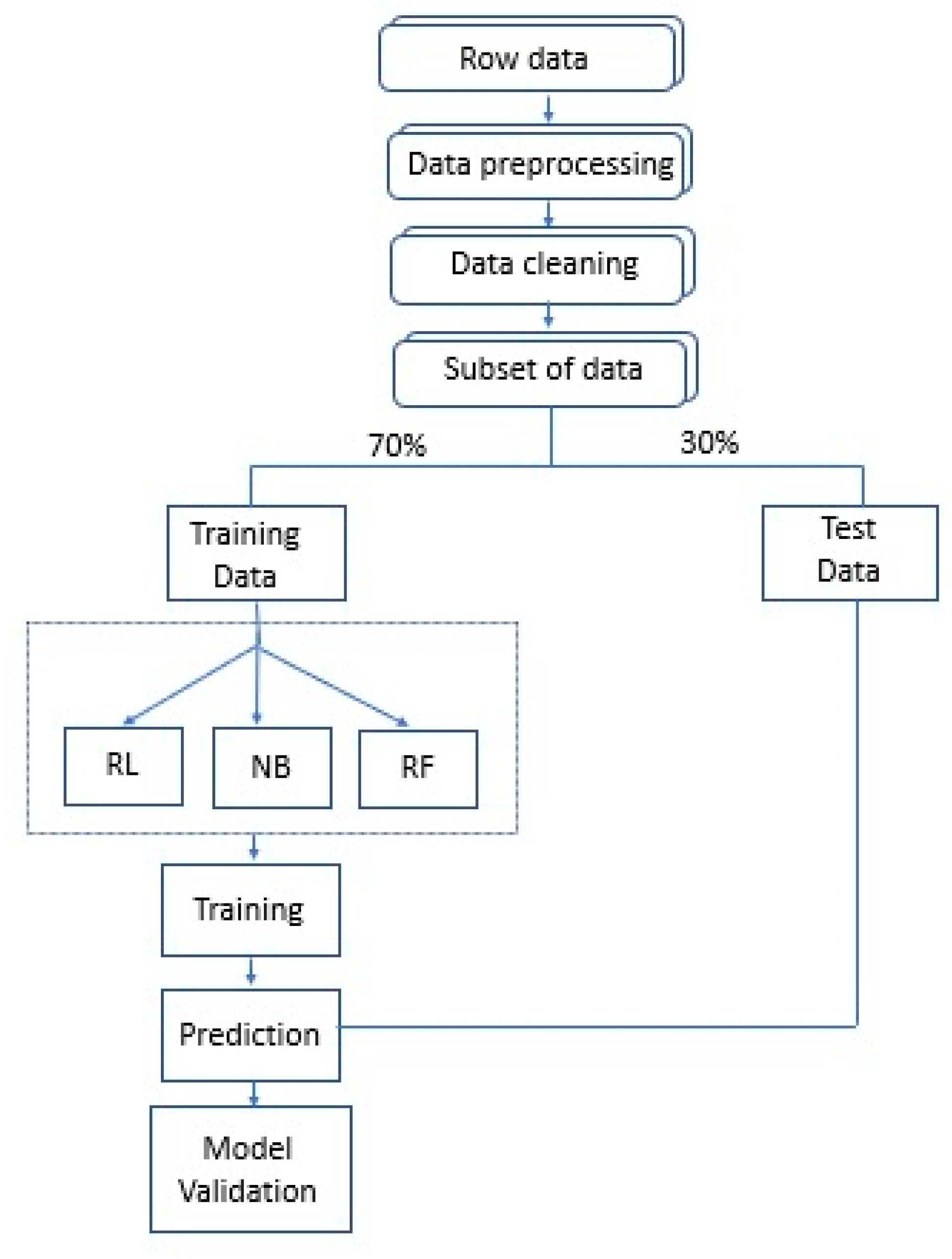
2.4. Statistical Analysis
2.4.1. Machine Learning Techniques: LR, NB, RF
2.4.2. Logistic Regression
2.4.3. Naïve Bayes
- X < as an instance (vector of random variables denoting observed attribute values);
- x < x < as a particular instance;
- C as a random variable denoting the class of an instance;
- c represents the value that C takes.
2.4.4. Random Forest
3. Results
3.1. Characteristics of the Population
3.2. Association of the Anthropometric Parameters with Vitamin D
3.3. Comparing the Performance of Data-Mining Algorithms in the Prediction of Vitamin D Deficiency
4. Discussion and Conclusions
4.1. Limitations of the Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassatne, A.; Chakhtoura, M.; Saad, R.; Fuleihan, G. Vitamin D supplementation in obesity and during weight loss: A review of randomized controlled trials. Metabolism 2019, 92, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.; Santos, A.; Bernardes, M.; Ramalho, A.; Martins, M. Vitamin D metabolism in human adipose tissue: Could it explain low vitamin D status in obesity? Horm. Mol. Biol. Clin. Investig. 2017, 33. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.; Lindberg, F.; Hexeberg, S.; Moan, J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Orces, C. The Association between Body Mass Index and Vitamin D Supplement Use among Adults in the United States. Cureus 2019, 11, e5721. [Google Scholar] [CrossRef]
- Camozzi, V.; Frigo, A.C.; Zaninotto, M.; Sanguin, F.; Plebani, M.; Boscaro, M.; Schiavon, L.; Luisetto, G. 25-hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporos. Int. 2016, 27, 2593–2602. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Alexander, L.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Casey, D.; Coates, M.M.; Delwiche, K.; Estep, K.; Frostad, J.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Ashwell, M.; Cole, T.J.; Dixon, A.K. Ratio of waist circumference to height is strong predictor of intraabdominal fat. BMJ 1996, 313, 559–560. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Peralta, M.; Holick, M.F.; Borrayo-Sánchez, G.; Madrid-Miller, A.; Ramírez-Árias, E.; Arizmendi-Uribe, E. Efectos inmunometabólicos disfuncionales de la deficiencia de vitamina D y aumento de riesgo cardiometabólico. Potencial alerta epidemiológica en América? Endocrinol. Diabetes y Nutr. 2017, 64, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Bertoldo, F.; Braga, V.; Fracassi, E.; Gatti, D.; Gandolini, G.; Minisola, S.; Battista Rini, G. 25-hydroxy vitamin D levels in healthy premenopausal women: Association with bone turnover markers and bone mineral density. Bone 2009, 45, 423–426. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Danik, J.S.; Manson, J.A.E. Vitamin D and cardiovascular disease. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Foss, Y.J. Vitamin D deficiency is the cause of common obesity. Med. Hypotheses 2009, 72, 314–321. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Aleksova, A.; Beltrami, A.P.; Belfiore, R.; Barbati, G.; Di Nucci, M.; Scapol, S.; De Paris, V.; Carriere, C.; Sinagra, G. U-shaped relationship between vitamin D levels and long-term outcome in large cohort of survivors of acute myocardial infarction. Int. J. Cardiol. 2016, 223, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Montemayor, L.; Castillo, E.; Rodríguez-López, C.; Villarreal-Calderón, J.; Gómez-Carmona, M.; Tenorio-Martínez, S.; Nieblas, B.; García-Rivas, G. Seasonal Variation in Vitamin D in Association with Age, Inflammatory Cytokines, Anthropometric Parameters, and Lifestyle Factors in Older Adults. Mediators Inflamm. 2017, 2017, 5719461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, R.; Carbonell, J.; Mitchell, T. Machine Learning: An Artificial Intelligence Approach; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Kotsiantis, S.; Zaharakis, I.; Pintelas, P. Supervised machine learning: A review of classification techniques. Emerg. Artif. Intell. Appl. Comput. Eng. 2007, 160, 3–24. [Google Scholar]
- Dey, D.; Diaz Zamudio, M.; Schuhbaeck, A.; Juarez Orozco, L.E.; Otaki, Y.; Gransar, H.; Li, D.; Germano, G.; Achenbach, S.; Berman, D.S.; et al. Relationship between Quantitative Adverse Plaque Features from Coronary Computed Tomography Angiography and Downstream Impaired Myocardial Flow Reserve by 13N-Ammonia Positron Emission Tomography: A Pilot Study. Circ. Cardiovasc. Imaging 2015, 8, e003255. [Google Scholar] [CrossRef] [Green Version]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine Learning and Data Mining Methods in Diabetes Research. Comput. Struct. Biotechnol. J. 2017, 15, 104–116. [Google Scholar] [CrossRef]
- Zou, Q.; Qu, K.; Luo, Y.; Yin, D.; Ju, Y.; Tang, H. Predicting Diabetes Mellitus With Machine Learning Techniques. Front. Genet. 2018, 9, 515. [Google Scholar] [CrossRef]
- Krittanawong, C.; Bomback, A.S.; Baber, U.; Bangalore, S.; Messerli, F.H.; Wilson Tang, W.H. Future Direction for Using Artificial Intelligence to Predict and Manage Hypertension. Curr. Hypertens. Rep. 2018, 20, 75. [Google Scholar] [CrossRef]
- Qawqzeh, Y.K.; Bajahzar, A.S.; Jemmali, M.; Otoom, M.M.; Thaljaoui, A. Classification of Diabetes Using Photoplethysmogram (PPG) Waveform Analysis: Logistic Regression Modeling. Biomed Res. Int. 2020, 2020, 3764653. [Google Scholar] [CrossRef]
- Tiwari, P.; Colborn, K.; Smith, D.; Xing, F.; Ghosh, D.; Rosenberg, M. Assessment of a machine learning model applied to harmonized electronic health record data for the prediction of incident atrial fibrillation. JAMA Netw. Open 2020, 3, e1919396. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef]
- Saravanan, R.; Sujatha, P. A State of Art Techniques on Machine learning algorithms: A perspective of supervised learning approaches in data classification. In Proceedings of the 2018 Second International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 14–15 June 2018; pp. 945–949. [Google Scholar]
- Zhu, X.; Goldberg, A. Introduction to semi-supervised learning. Synth. Lect. Artif. Intell. Mach. Learn. 2009, 31, 1–130. [Google Scholar] [CrossRef] [Green Version]
- Narang, R.K.; Gamble, G.G.; Khaw, K.T.; Camargo, C.A.; Sluyter, J.D.; Scragg, R.K.R.; Reid, I.R. A prediction tool for vitamin D deficiency in New Zealand adults. Arch. Osteoporos. 2020, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-C.; Kim, D.; An, H.; Son, C.-S.; Cho, S.; Lee, J.-H. A Novel Biosensor and Algorithm to Predict Vitamin D Status by Measuring Skin Impedance. Sensors 2021, 21, 8118. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Garcia Carretero, R.; Vigil-Medina, L.; Barquero-Perez, O.; Mora-Jimenez, I.; Soguero-Ruiz, C.; Ramos-Lopez, J. Machine learning approaches to constructing predictive models of vitamin D deficiency in a hypertensive population: A comparative study. Informatics Heal. Soc. Care 2021, 46, 355–369. [Google Scholar] [CrossRef]
- Guo, S.; Lucas, R.M.; Ponsonby, A.L.; Chapman, C.; Coulthard, A.; Dear, K.; Dwyer, T.; Kilpatrick, T.; McMichael, T.; Pender, M.P.; et al. A novel approach for prediction of vitamin D status using support vector regression. PLoS ONE 2013, 8, e79970. [Google Scholar] [CrossRef]
- Ricciardi, C.; Cantoni, V.; Improta, G.; Iuppariello, L.; Latessa, I.; Cesarelli, M.; Triassi, M.; Cuocolo, A. Application of data mining in a cohort of Italian subjects undergoing myocardial perfusion imaging at an academic medical center. Comput. Methods Programs Biomed. 2020, 189, 105343. [Google Scholar] [CrossRef]
- Gomez-Marcos, M.A.; Martinez-Salgado, C.; Gonzalez-Sarmiento, R.; Hernandez-Rivas, J.M.; Sanchez-Fernandez, P.L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; Garca-Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross-sectional, descriptive observational study. BMJ Open 2016, 6, e011031. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Rubio Hererra, M.A.; Barbany, M.; Moreno, B. Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. (Barc). 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 05 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.; Kupper, L.; Nizam, A.; Muller, K. Applied Regression Analysis and Multivariable Methods, 4th ed.; Duxbury Press: Pacific Grove, CA, USA, 2007. [Google Scholar]
- Hilbe, J. Logistic Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kleinbaum, D. Logistic Regression: A Self-Learning Text; Springer: New York, NY, USA, 1994. [Google Scholar]
- Maalouf, M. Logistic regression in data analysis: An overview. Int. J. Data Anal. Tech. Strateg. 2011, 3, 281–299. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009. [Google Scholar]
- Berrar, D. Bayes’ Theorem and Naive Bayes Classifier. Encycl. Bioinform. Comput. Biol. 2018, 1, 403–412. [Google Scholar] [CrossRef]
- Hand, D.; Chan, Y. Idiot’s Bayes—Not so stupid after all? Int. Stat. Rev. 2001, 69, 385–398. [Google Scholar]
- Jahan, R. Applying Naive Bayes Classification Technique for Classification of Improved Agricultural Land soils. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 189–193. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Bernard, S.; Adam, S.; Heutte, L. Dynamic Random Forests. Pattern Recognit. Lett. 2012, 33, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Classification and Regression Trees; Chapman & Hall: New York, NY, USA, 1984. [Google Scholar]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A Systematic Review of Vitamin D Status in Southern European Countries; Springer: Berlin/Heidelberg, Germany, 2018; Volume 57, ISBN 0039401715. [Google Scholar]
- Díaz-López, A.; Paz-Graniel, I.; Alonso-Sanz, R.; Marqués-Baldero, C.; Mateos-Gil, C.; Arija-Val, V. Vitamin D deficiency in primary health care users at risk in Spain. Nutr. Hosp. 2021, 38, 1058–1067. [Google Scholar]
- Mansouri, M.; Miri, A.; Varmaghani, M.; Abbasi, R.; Taha, P.; Ramezani, S.; Rahmani, E.; Armaghan, R.; Sadeghi, O. Vitamin D deficiency in relation to general and abdominal obesity among high educated adults. Eat. Weight Disord. 2019, 24, 83–90. [Google Scholar] [CrossRef]
- Vanlint, S. Vitamin D and obesity. Nutrients 2013, 5, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jääskeläinen, T.; Männistö, S.; Härkänen, T.; Sääksjärvi, K.; Koskinen, S.; Lundqvist, A. Does Vitamin D status predict weight gain or increase in waist circumference? Results from the longitudinal Health 2000/2011 Survey. Public Health Nutr. 2020, 23, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Cătoi, A.F.; Iancu, M.; Pârvu, A.E.; Cecan, A.D.; Bidian, C.; Chera, E.I.; Pop, I.D.; Macri, A.M. Relationship between 25 hydroxyvitamin d, overweight/obesity status, pro-inflammatory and oxidative stress markers in patients with type 2 diabetes: A simplified empirical path model. Nutrients 2021, 13, 2889. [Google Scholar] [CrossRef] [PubMed]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.C. Obesity is associated with Vitamin D deficiency in Danish children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef]
- Viprey, M.; Merle, B.; Riche, B.; Freyssenge, J.; Rippert, P.; Chakir, M.A.; Thomas, T.; Malochet-guinamand, S.; Cortet, B.; Breuil, V.; et al. Development and validation of a predictive model of hypovitaminosis d in general adult population: SCOPYD study. Nutrients 2021, 13, 2526. [Google Scholar] [CrossRef]
- Izadi, A.; Aliasghari, F.; Gargari, B.P.; Ebrahimi, S. Strong association between serum vitamin D and vaspin levels, AIP, VAI and liver enzymes in NAFLD patients. Int. J. Vitam. Nutr. Res. 2020, 90, 59–66. [Google Scholar] [CrossRef]
- Toro, L.Z.; Polo, J.R.T.; Díez-Tabernilla, M.; Bernal, L.G.; Sebastián, A.A.; Rico, R.C. Fórmula CUN-BAE y factores bioquímicos como marcadores predictivos de obesidad y enfermedad cardiovascular en pacientes pre y post gastrectomía vertical. Nutr. Hosp. 2014, 30, 281–286. [Google Scholar]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef]
- Deschasaux, M.; Souberbielle, J.C.; Andreeva, V.A.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Latino-Martel, P.; Druesne-Pecollo, N.; De Edelenyi, F.S.; Galan, P.; et al. Quick and easy screening for Vitamin D insufficiency in adults a scoring system to be implemented in daily clinical practice. Medicine 2016, 95, e2783. [Google Scholar] [CrossRef]
- Lopes, J.B.; Fernandes, G.H.; Takayama, L.; Figueiredo, C.P.; Pereira, R.M.R. A predictive model of vitamin D insufficiency in older community people: From the São Paulo Aging & Health Study (SPAH). Maturitas 2014, 78, 335–340. [Google Scholar]
- Sohl, E.; Heymans, M.W.; De Jongh, R.T.; Den Heijer, M.; Visser, M.; Merlijn, T.; Lips, P.; Van Schoor, N.M. Prediction of vitamin D deficiency by simple patient characteristics. Am. J. Clin. Nutr. 2014, 99, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Overall (n = 501) | Females (n = 252) | Males (n = 249) | p1 | Normal Levels of Vitamin D (n = 327) | Vitamin D Deficit (n = 174) | p2 |
|---|---|---|---|---|---|---|---|
| Cardiovascular risk factors | |||||||
| Age, years | 55.90 ± 14.24 | 55.85 ± 14.19 | 55.95 ± 14.30 | 0.934 | 55.77 ± 14.43 | 56.14 ± 13.90 | 0.782 |
| Smoker, n (%) | 90 (18.00) | 41 (16.30) | 49 (19.70) | 0.320 | 47 (14.4) | 43 (24.7) | 0.004 |
| SBP, mmHg | 120.69 ± 23.13 | 114.99 ± 24.96 | 126.47 ± 19.52 | <0.001 | 120.62 ± 25.78 | 120.83 ± 17.14 | 0.921 |
| DBP, mmHg | 75.53 ± 10.10 | 73.67 ± 10.46 | 77.40 ± 9.37 | <0.001 | 75.30 ± 10.39 | 75.95 ± 9.54 | 0.496 |
| Hypertension, n (%) | 147 (25.80) | 65 (29.30) | 82 (32.90) | 0.079 | 96 (29.4) | 51 (29.3) | 0.991 |
| Total cholesterol, (mg/dL) | 194.76 ± 32.50 | 196.88 ± 32.64 | 192.61 ± 32.26 | 0.142 | 193.96 ± 32.21 | 196.27 ± 33.07 | 0.450 |
| LDL-C, mg/dL | 115.51 ± 29.37 | 113.61 ± 28.54 | 117.43 ± 14.12 | 0.148 | 114.37 ± 28.68 | 117.65 ± 30.59 | 0.236 |
| HDL-C, mg/dL | 58.88 ± 16.15 | 64.27 ± 16.14 | 53.43 ± 14.23 | <0.001 | 60.27 ± 16.33 | 56.27 ± 15.51 | 0.008 |
| Triglycerides, mg/dL | 103.12 ± 53.11 | 94.07 ± 50.48 | 112.27 ± 54.23 | <0.001 | 97.90 ± 46.63 | 112.93 ± 62.51 | 0.002 |
| Dyslipidemia, n (%) | 191 (38.1) | 96 (38.2) | 95 (38.1) | 0.905 | 208 (64.0) | 118 (67.8) | 0.393 |
| Glycemia, mg/dL | 88.21 ± 17.37 | 86.30 ± 15.73 | 90.14 ± 18.71 | 0.013 | 87.05 ± 15.20 | 90.39 ± 20.72 | 0.040 |
| HbA1c, (%) | 5.49 ± 0.56 | 5.44 ± 0.47 | 5.54 ± 0.63 | 0.043 | 5.48 ± 0.50 | 5.51 ± 0.65 | 0.466 |
| Diabetes mellitus, n (%) | 38 (7.60) | 12 (4.8) | 26 (10.50) | 0.016 | 23 (7.0) | 15 (8.6) | 0.523 |
| CVR score (%) | 11.80 ± 13.00 | 6.48 ± 6.67 | 17.22 ± 15.43 | <0.001 | 10.99 ± 12.38 | 13.33 ± 14.02 | 0.056 |
| Vitamin D | 25.56 ± 19.30 | 26.55 ± 25.60 | 24.61 ± 10.11 | 0.276 | --- | --- | --- |
| Drugs | |||||||
| Antihypertensive drugs, n (%) | 96 (19.20) | 46 (18.30) | 50 (20.10) | 0.604 | 58 (17.7) | 38 (21.8) | 0.267 |
| Lipid-lowering drugs, n (%) | 102 (20.40) | 53 (21.00) | 49 (19.70) | 0.707 | 72 (22.0) | 30 (17.2) | 0.206 |
| Antidiabetic drugs, n (%) | 35 (7.00) | 12 (4.8) | 23 (9.20) | 0.049 | 22 (6.7) | 13 (7.5) | 0.756 |
| Anthropometric parameters | |||||||
| Height, cm | 165.11 ± 9.68 | 158.70 ± 6.98 | 171.60 ± 7.46 | <0.001 | 165.59 ± 9.67 | 164.21 ± 9.67 | 0.128 |
| Weight, kg | 72.41 ± 13.61 | 65.67 ± 11.87 | 79.22 ± 11.75 | <0.001 | 71.68 ± 12.99 | 73.76 ± 4.65 | 0.104 |
| WC, (cm) | 93.33 ± 11.99 | 87.95 ± 11.68 | 98.76 ± 9.65 | <0.001 | 92.32 ± 11.78 | 95.21 ± 12.20 | 0.010 |
| Hip circumference, (cm) | 103.13 ± 9.24 | 103.55 ± 9.34 | 102.71 ± 9.13 | 0.313 | 102.29 ± 9.38 | 104.72 ± 8.78 | 0.005 |
| BMI ≥ 30, n (%) | 94 (18.80) | 52 (20.6) | 42 (16.90) | 0.280 | 52 (15.9) | 42 (24.1) | 0.025 |
| BMI, (kg/m2) | 26.52 ± 4.23 | 26.14 ± 4.79 | 26.90 ± 3.54 | 0.044 | 26.11 ± 4.08 | 27.28 ± 4.40 | 0.003 |
| WHtR | 0.57 ± 0.07 | 0.56 ± 0.08 | 0.58 ± 0.06 | 0.001 | 0.56 ± 0.07 | 0.58 ± 0.07 | 0.001 |
| BRI | 4.79 ± 1.57 | 4.59 ± 1.73 | 4.98 ± 1.36 | 0.005 | 4.62 ± 1.55 | 5.09 ± 1.56 | 0.002 |
| VAI | 3.26 ± 2.42 | 3.22 ± 2.59 | 3.30 ± 2.25 | 0.728 | 3.02 ± 2.26 | 3.71 ± 2.65 | 0.002 |
| CUN-BAE | 33.20 ± 7.86 | 38.50 ± 6.37 | 27.82 ± 5.07 | <0.001 | 32.73 ± 7.74 | 34.07 ± 8.02 | 0.068 |
| Overall | Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | IC 95% | p | OR | IC 95% | p | OR | IC 95% | p |
| WC | |||||||||
| Model 1 | 1.021 | 1.005–1.037 | 0.011 | 1.016 | 0.993–1.038 | 0.177 | 1.038 | 1.009–1.067 | 0.009 |
| Model 2 | 1.021 | 1.005–1.038 | 0.010 | 1.017 | 0.994–1.041 | 0.149 | 1.039 | 1.009–1.070 | 0.010 |
| Model 3 | 1.022 | 1.005–1.039 | 0.011 | 1.014 | 0.990–1.040 | 0.252 | 1.040 | 1.010–1.071 | 0.010 |
| BMI | |||||||||
| Model 1 | 1.068 | 1.022–1.116 | 0.003 | 1.061 | 1.005–1.121 | 0.034 | 1.080 | 1.003–1.163 | 0.042 |
| Model 2 | 1.069 | 1.022–1.118 | 0.004 | 1.065 | 1.007–1.127 | 0.027 | 1.078 | 1.001–1.162 | 0.048 |
| Model 3 | 1.068 | 1.021–1.118 | 0.004 | 1.059 | 0.999–1.123 | 0.056 | 1.078 | 1.000–1.163 | 0.050 |
| WHtR*1000 | |||||||||
| Model 1 | 1.004 | 1.002–1.007 | 0.001 | 1.003 | 1.000–1.006 | 0.089 | 1.007 | 1.002–1.011 | 0.003 |
| Model 2 | 1.005 | 1.002–1.008 | 0.001 | 1.003 | 1.000–1.007 | 0.055 | 1.008 | 1.003–1.013 | 0.002 |
| Model 3 | 1.005 | 1.002–1.008 | 0.001 | 1.003 | 0.999–1.007 | 0.118 | 1.008 | 1.003–1.014 | 0.001 |
| VAI | |||||||||
| Model 1 | 1.119 | 1.038–1.207 | 0.003 | 1.073 | 0.973–1.183 | 0.157 | 1.188 | 1.054–1.339 | 0.005 |
| Model 2 | 1.120 | 1.038–1.208 | 0.003 | 1.080 | 0.976–1.194 | 0.135 | 1.189 | 1.055–1.340 | 0.005 |
| Model 3 | 1.122 | 1.039–1.212 | 0.003 | 1.064 | 0.959–1.181 | 0.242 | 1.203 | 1.064–1.360 | 0.003 |
| BRI | |||||||||
| Model 1 | 1.209 | 1.073–1.361 | 0.002 | 1.126 | 0.969–1.308 | 0.122 | 1.362 | 1.118–1.660 | 0.002 |
| Model 2 | 1.250 | 1.095–1.426 | 0.001 | 1.157 | 0.982–1.363 | 0.080 | 1.447 | 1.152–1.818 | 0.002 |
| Model 3 | 1.249 | 1.092–1.430 | 0.001 | 1.126 | 0.945–1.340 | 0.184 | 1.467 | 1.163–1.851 | 0.001 |
| CUN-BAE | |||||||||
| Model 1 | 1.022 | 0.998–1.046 | 0.068 | 1.044 | 1.001–1.089 | 0.044 | 1.052 | 0.998–1.108 | 0.061 |
| Model 2 | 1.024 | 0.999–1.050 | 0.065 | 1.060 | 1.011–1.113 | 0.016 | 1.057 | 0.996–1.122 | 0.067 |
| Model 3 | 1.025 | 0.999–1.052 | 0.062 | 1.056 | 1.005–1.110 | 0.030 | 0.070 | 0.995–1.122 | 0.070 |
| Variable | Accuracy | Error | Precision | Specificity | Sensitivity | AUC-ROC (95% CI) |
|---|---|---|---|---|---|---|
| Algorithms | ||||||
| Logistic Regression | ||||||
| WC | 0.635 | 0.365 | 0.924 | 0.500 | 0.650 | 0.528 (0.494–0.563) |
| BMI | 0.641 | 0.359 | 0.919 | 0.526 | 0.655 | 0.538 (0.502–0.574) |
| WHtR | 0.638 | 0.362 | 0.910 | 0.512 | 0.654 | 0.538 (0.499–0.575) |
| BRI | 0.635 | 0.365 | 0.910 | 0.500 | 0.653 | 0.533 (0.497–0.570) |
| VAI | 0.633 | 0.367 | 0.906 | 0.488 | 0.652 | 0.531 (0.494–0.568) |
| CUN-BAE | 0.641 | 0.359 | 0.924 | 0.528 | 0.654 | 0.536 (0.501–0.572) |
| Naïve Bayes | ||||||
| WC | 0.607 | 0.393 | 0.856 | 0.118 | 0.669 | 0.546 (0.487–0.604) |
| BMI | 0.653 | 0.347 | 0.885 | 0.333 | 0.697 | 0.555 (0.495–0.616) |
| WHtR | 0.620 | 0.380 | 0.875 | 0.133 | 0.674 | 0.556 (0.499–0.613) |
| BRI | 0.620 | 0.380 | 0.875 | 0.133 | 0.674 | 0.556 (0.499–0.613) |
| VAI | 0.687 | 0.313 | 0.942 | 0.455 | 0.705 | 0.503 (0.458–0.547) |
| CUN-BAE | 0.640 | 0.360 | 0.923 | 0.000 | 0.676 | 0.503 (0.465–0.542) |
| Random Forest | ||||||
| WC | 0.580 | 0.420 | 0.786 | 0.185 | 0.667 | 0.449 (0.388–0.509) |
| BMI | 0.607 | 0.393 | 0.817 | 0.240 | 0.680 | 0.474 (0.412–0.536) |
| WHtR | 0.640 | 0.360 | 0.885 | 0.250 | 0.687 | 0.486 (0.434–0.537) |
| BRI | 0.653 | 0.347 | 0.894 | 0.313 | 0.694 | 0.501 (0.447–0.556) |
| VAI | 0.633 | 0.367 | 0.846 | 0.304 | 0.693 | 0.499 (0.436–0.562) |
| CUN-BAE | 0.613 | 0.387 | 0.827 | 0.250 | 0.683 | 0.479 (0.417–0.540) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patino-Alonso, C.; Gómez-Sánchez, M.; Gómez-Sánchez, L.; Sánchez Salgado, B.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A. Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters. Mathematics 2022, 10, 616. https://doi.org/10.3390/math10040616
Patino-Alonso C, Gómez-Sánchez M, Gómez-Sánchez L, Sánchez Salgado B, Rodríguez-Sánchez E, García-Ortiz L, Gómez-Marcos MA. Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters. Mathematics. 2022; 10(4):616. https://doi.org/10.3390/math10040616
Chicago/Turabian StylePatino-Alonso, Carmen, Marta Gómez-Sánchez, Leticia Gómez-Sánchez, Benigna Sánchez Salgado, Emiliano Rodríguez-Sánchez, Luis García-Ortiz, and Manuel A. Gómez-Marcos. 2022. "Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters" Mathematics 10, no. 4: 616. https://doi.org/10.3390/math10040616
APA StylePatino-Alonso, C., Gómez-Sánchez, M., Gómez-Sánchez, L., Sánchez Salgado, B., Rodríguez-Sánchez, E., García-Ortiz, L., & Gómez-Marcos, M. A. (2022). Predictive Ability of Machine-Learning Methods for Vitamin D Deficiency Prediction by Anthropometric Parameters. Mathematics, 10(4), 616. https://doi.org/10.3390/math10040616







