Thermodynamic Interpretation of a Machine-Learning-Based Response Surface Model and Its Application to Pharmacodynamic Synergy between Propofol and Opioids
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Preprocessing
2.1.1. Clinical Trials
2.1.2. Data Preprocessing and Pharmacokinetic Simulation
2.2. Model Derivation
2.2.1. Thermodynamic Interpretation
2.2.2. Machine-Learning-Based Response Surface Model
2.2.3. Multi-Drug MLRSM
3. Results
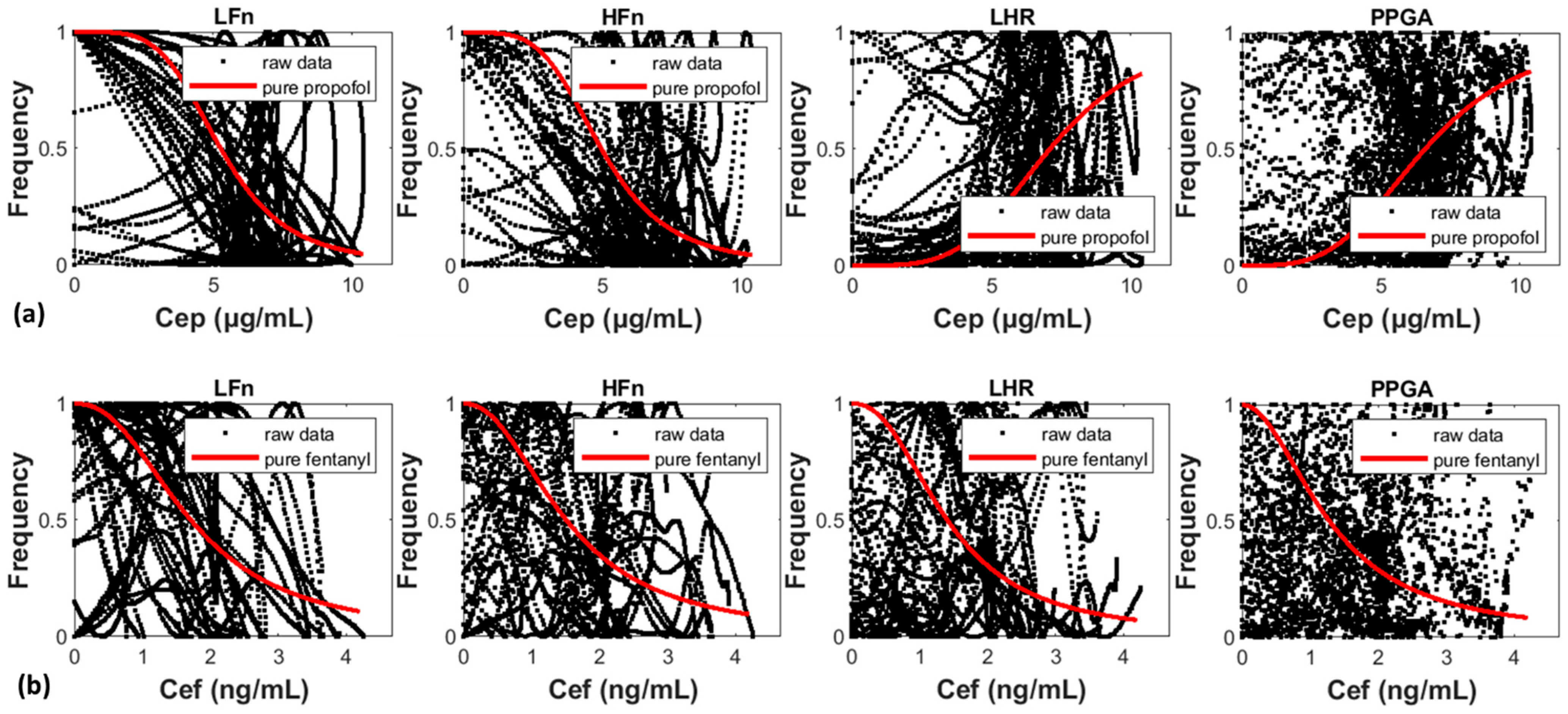
3.1. Validation and Visualization of the Single-Drug MLRSM
3.2. Two-Drug MLRSM vs. Conventional RSMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LaPierre, C.D.; Johnson, K.B.; Randall, B.R.; White, J.L.; Egan, T.D. An exploration of remifentanil-propofol combinations that lead to a loss of response to esophageal instrumentation, a loss of responsiveness, and/or onset of intolerable ventilatory depression. Anesth. Analg. 2011, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, J.F.A.; Eger, E.; Sonner, J.M.; Shafer, S.L. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth. Analg. 2008, 107, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Estafanous, F.G.; Brum, J.M.; Ribeiro, M.P.; Estafanous, M.; Starr, N.; Ferrario, C. Analysis of heart rate variability to assess hemodynamic alterations following induction of anesthesia. J. Cardiothorac. Vasc. Anesth. 1992, 6, 651–657. [Google Scholar] [CrossRef]
- Galletly, D.C.; Corfiatis, T.; Westenberg, A.M.; Robinson, B.J. Heart rate periodicities during induction of propofol-nitrous oxide-isoflurane anaesthesia. Br. J. Anaesth. 1992, 68, 360–364. [Google Scholar] [CrossRef][Green Version]
- Win, N.N.; Fukayama, H.; Kohase, H.; Umino, M. The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth. Analg. 2005, 101, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zickmann, B.; Hofmann, H.C.; Pottkämper, C.; Knothe, C.; Boldt, J.; Hempelmann, G. Changes in heart rate variability during induction of anesthesia with fentanyl and midazolam. J. Cardiothorac. Vasc. Anesth. 1996, 10, 609–613. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Gans, F.; Schumann, A.Y.; Kantelhardt, J.W.; Penzel, T.; Fietze, I. Cross-modulated amplitudes and frequencies characterize interacting components in complex systems. Phys. Rev. Lett. 2009, 102, 098701. [Google Scholar] [CrossRef]
- Diz, J.; Río, R.D.; Lamas, A.; Mendoza, M.; Durán, M.; Ferreira, L. Analysis of pharmacodynamic interaction of sevoflurane and propofol on Bispectral Index during general anaesthesia using a response surface model. Br. J. Anaesth. 2010, 104, 733–739. [Google Scholar] [CrossRef][Green Version]
- Johnson, K.B.; Syroid, N.D.; Gupta, D.K.; Manyam, S.C.; Egan, T.D.; Huntington, J.; White, J.L.; Tyler, D.; Westenskow, D.R. An evaluation of remifentanil propofol response surfaces for loss of responsiveness, loss of response to surrogates of painful stimuli and laryngoscopy in patients undergoing elective surgery. Anesth. Analg. 2008, 106, 471–479. [Google Scholar] [CrossRef]
- Kim, W.H.; Ahn, H.J.; Kim, J.A. Interactions of propofol and remifentanil on bispectral index under 66% N(2)O: Analysis by dose-effect curve, isobologram, and combination index. Korean J. Anesthesiol. 2010, 59, 371–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kern, S.E.; Xie, G.; White, J.L.; Egan, T.D. A Response Surface Analysis of Propofol–Remifentanil Pharmacodynamic Interaction in Volunteers. Anesthesiology 2004, 100, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.J.; Olofsen, E.; Engbers, F.H.M.; Burm, A.G.L.; Bovill, J.G.; Vuyk, J. Propofol reduces perioperative remifentanil requirements in a synergistic manner response surface modeling of perioperative remifentanil-propofol interactions. Anesthesiology 2003, 99, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Heyse, B.; Proost, J.H.; Hannivoort, L.N.; Eleveld, D.J.; Luginbühl, M.; Struys, M.M.R.F.; Vereecke, H.E.M. A response surface model approach for continuous measures of hypnotic and analgesic effect during sevoflurane-remifentanil interaction: Quantifying the pharmacodynamic shift evoked by stimulation. Anesthesiology 2014, 120, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar]
- Bol, C.J.; Vogelaar, J.P.; Tang, J.P.; Mandema, J.W. Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat. J. Pharmacol. Exp. Ther. 2000, 294, 347–355. [Google Scholar]
- Miller, R.D.; Eriksson, L.I.; Fleisher, L.A.; Wiener-Kronish, J.P.; Cohen, N.H.; Young, W.L. Miller’s Anesthesia E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Schnider, T.W.; Minto, C.F.; Shafer, S.L.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Youngs, E.J. The influence of age on propofol pharmacodynamics. Anesthesiology 1999, 90, 1502–1516. [Google Scholar] [CrossRef]
- Vuyk, J.; Lim, T.; Engbers, F.H.; Burm, A.G.; Vletter, A.A.; Bovill, J.G. The pharmacodynamic interaction of propofol and alfentanil during lower abdominal surgery in women. Anesthesiology 1995, 83, 8–22. [Google Scholar] [CrossRef]
- Bouillon, T.; Bruhn, J.; Radu-Radulescu, L.; Bertaccini, E.; Park, S.; Shafer, S. Non-steady state analysis of the pharmacokinetic interaction between propofol and remifentanil. Anesthesiology 2002, 97, 1350–1362. [Google Scholar] [CrossRef]
- Koitabashi, T.; Johansen, J.W.; Sebel, P.S. Remifentanil dose/electroencephalogram bispectral response during combined propofol/regional anesthesia. Anesth. Analg. 2002, 94, 1530–1533. [Google Scholar] [CrossRef]
- Vinik, H.R.; Bradley, E.L.; Kissin, I., Jr. Isobolographic analysis of propofol-thiopental hypnotic interaction in surgical patients. Anesth. Analg. 1999, 88, 667–670. [Google Scholar] [PubMed]
- Nieuwenhuijs, D.J.; Olofsen, E.; Romberg, R.R.; Sarton, E.; Ward, D.; Engbers, F.; Vuyk, J.; Mooren, R.; Teppema, L.J.; Dahan, A. Response surface modeling of remifentanil-propofol interaction on cardiorespiratory control and bispectral index. Anesthesiology 2003, 98, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Lo, M.-T.; Chen, K.-H.; Mandell, S.; Chang, W.-K.; Lin, C.; Ting, C.-K. Strong Early Phase Parasympathetic Inhibition Followed by Sympathetic Withdrawal During Propofol Induction: Temporal Response Assessed by Wavelet-Based Spectral Analysis and Photoplethysmography. Front. Physiol. 2021, 12, 705153. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.A.V.I.D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 1985, 248, H151–H153. [Google Scholar] [CrossRef]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef]
- Colombo, R.; Marchi, A.; Borghi, B.; Fossali, T.; Rech, R.; Castelli, A.; Corona, A.; Guzzetti, S.; Raimondi, F. Pulse Photoplethysmographic Analysis Estimates the Sympathetic Activity Directed to Heart and Vessels. Anesthesiology 2015, 123, 336–345. [Google Scholar] [CrossRef]
- Babchenko, A.; Davidson, E.; Ginosar, Y.; Kurz, V.; Faib, I.; Adler, D.; Nitzan, M. Photoplethysmographic measurement of changes in total and pulsatile tissue blood volume, following sympathetic blockade. Physiol. Meas. 2001, 22, 389–396. [Google Scholar] [CrossRef]
- Shafer, S.L.; Varvel, J.R.; Aziz, N.; Scott, J.C. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology 1990, 73, 1091–1102. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef]
- Chen, C.-C.; Juan, H.-H.; Tsai, M.-Y.; Lu, H.H.-S. Unsupervised Learning and Pattern Recognition of Biological Data Structures with Density Functional Theory and Machine Learning. Sci. Rep. 2018, 8, 557. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Huang, S.-J.; Chen, C.-C.; Lu, H.H.-S. Computational Complexity Reduction of Neural Networks of Brain Tumor Image Segmentation by Introducing Fermi–Dirac Correction Functions. Entropy 2021, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Lee, S.-I. Drug interaction: Focusing on response surface models. Korean J. Anesthesiol. 2010, 58, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camblor, P.; Corral, N. A general bootstrap algorithm for hypothesis testing. J. Stat. Plan. Inference 2012, 142, 589–600. [Google Scholar] [CrossRef]
- Watso, J.C.; Huang, M.; Belval, L.N.; Cimino, F.A.; Jarrard, C.P.; Hendrix, J.M.; Hinojosa-Laborde, C.; Crandall, C.G. Low-dose fentanyl reduces pain perception, muscle sympathetic nerve activity responses, and blood pressure responses during the cold pressor test. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R64–R76. [Google Scholar] [CrossRef]
- Hasegawa, G.; Hirata, N.; Yoshikawa, Y.; Yamakage, M. Differential effects of remimazolam and propofol on heart rate variability during anesthesia induction. J. Anesth. 2022, 36, 239–245. [Google Scholar] [CrossRef]
- Vettorello, M.; Colombo, R.; Grandis, C.E.D.; Costantini, E.; Raimondi, F. Effect of fentanyl on heart rate variability during spontaneous and paced breathing in healthy volunteers. Acta. Anaesthesiol. Scand. 2008, 52, 1064–1070. [Google Scholar] [CrossRef]


| Activity | Parameters | Propofol | Fentanyl | ||
|---|---|---|---|---|---|
| Modeling | Sampling | Modeling | Sampling | ||
| LFn | Steepness | −4.4084 | −4.4105 (0.0570) * | −2.3600 | −2.3624 (0.0275) |
| Normalization factor | 5.2611 | 5.2613 (0.0307) | 1.7159 | 1.7161 (0.0184) | |
| HFn | Steepness | −4.1503 | −4.1510 (0.0592) | −2.2044 | −2.2052 (0.0281) |
| Normalization factor | 4.9724 | 4.9740 (0.0322) | 1.5134 | 1.5129 (0.0184) | |
| LHR | Steepness | 4.0242 | 4.0220 (0.0521) | −2.3588 | −2.3409 (0.0285) |
| Normalization factor | 7.0896 | 7.0899 (0.0379) | 1.4091 | 1.4075 (0.0199) | |
| PPGA | Steepness | 3.5442 | 3.5432 (0.0429) | −2.0138 | −2.0371 (0.0291) |
| Normalization factor | 6.5799 | 6.5787 (0.0360) | 1.2836 | 1.2795 (0.0186) | |
| Activity | Para- Meters | Cp | Ce (Mixture) | Ce (Group 1) | Ce (Group 2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Modeling | Sampling | Modeling | Sampling | Modeling | Sampling | Modeling | Sampling | ||
| LFn | 1.2797 1.7051 | 1.28 (0.01) 1.70 (0.01) | −3.2024 −2.8392 | −3.20 (0.03) −2.84 (0.02) | -- * -- | −3.20 (0.03) −2.84 (0.02) | -- -- | −3.20 (0.03) −2.84 (0.02) | |
| 13.1206 10.3580 | 13.11 (0.15) 10.36 (0.09) | 3.9268 2.0578 | 3.93 (0.02) 2.06 (0.01) | -- -- | 3.93 (0.02) 2.06 (0.01) | -- -- | 3.93 (0.02) 2.06 (0.01) | ||
| 5.3293 6.4379 | 5.33 (0.06) 6.44 (0.06) | 4.2351 2.3693 | 4.23 (0.02) 2.37 (0.01) | 4.4430 2.8881 | 4.44 (0.03) 2.89 (0.01) | 4.0676 1.9515 | 4.07 (0.03) 1.95 (0.01) | ||
| 10.0290 | 12.19 (9.95) | −0.4678 | −0.47 (0.02) | −0.0579 | −0.06 (0.02) | −1.0517 | −1.05 (0.04) | ||
| 0.0693 | 0.07 (0.03) | 1.6778 | 1.68 (0.07) | 67.5643 | Inf | 0.9598 | 0.96 (0.02) | ||
| HFn | 1.2892 1.5988 | 1.29 (0.01) 1.60 (0.01) | −3.2263 −2.6622 | −3.23 (0.03) −2.66 (0.03) | -- -- | −3.23 (0.03) −2.66 (0.03) | -- -- | −3.23 (0.03) −2.66 (0.02) | |
| 15.0960 11.8426 | 15.10 (0.19) 11.85 (0.11) | 3.7128 1.8988 | 3.71 (0.02) 1.90 (0.01) | -- -- | 3.71 (0.02) 1.90 (0.01) | -- -- | 3.71 (0.02) 1.90 (0.01) | ||
| 5.3293 6.4379 | 5.42 (0.06) 6.44 (0.06) | 4.2351 2.3693 | 4.22 (0.02) 2.36 (0.01) | 4.4430 2.8881 | 4.44 (0.03) 2.89 (0.01) | 4.0676 1.9515 | 4.07 (0.03) 1.95 (0.01) | ||
| 4.1107 | 4.13 (0.26) | −0.2176 | −0.22 (0.02) | −0.1191 | −0.12 (0.02) | −0.6847 | −0.68 (0.03) | ||
| 0.1660 | 0.17 (0.01) | 3.9263 | 4.02 (0.60) | 5.7335 | 6.42 (3.38) | 1.2405 | 1.24 (0.04) | ||
| LHR | 1.2451 1.5897 | 1.25 (0.01) 1.47 (0.59) | 3.1158 −2.6172 | 3.12 (0.03) −2.03 (1.65) | -- -- | 3.12 (0.03) −2.02 (1.67) | -- -- | 3.12 (0.03) −2.06 (1.61) | |
| 14.8138 12.4434 | 14.80 (0.20) 12.11 (1.48) | 7.1478 1.8258 | 7.14 (0.04) 1.95 (0.34) | -- -- | 7.15 (0.04) 1.91 (0.30) | -- -- | 7.15 (0.04) 1.96 (0.35) | ||
| 5.4163 6.4236 | 5.42 (0.06) 6.42 (0.06) | 4.2173 2.3599 | 4.22 (0.02) 2.36 (0.01) | 4.3982 2.8534 | 4.40 (0.03) 2.85 (0.01) | 4.0676 1.9515 | 4.07 (0.03) 1.95 (0.01) | ||
| 3.6084 | 3.02 (2.47) | −0.5546 | −0.50 (0.71) | −0.8130 | −0.53 (0.73) | −0.0255 | −0.45 (1.61) | ||
| 0.1929 | 0.22 (0.15) | 0.6589 | 0.59 (0.15) | 0.3642 | 0.42 (0.23) | 508.7802 | Inf | ||
| PPGA | −1.0789 −1.4841 | −1.08 (0.01) −1.49 (0.01) | −2.7005 2.3635 | −2.70 (0.02) 2.36 (0.02) | -- -- | −2.70 (0.02) 2.36 (0.02) | -- -- | −2.70 (0.02) 2.36 (0.02) | |
| 4.9809 6.5615 | 4.98 (0.07) 6.56 (0.07) | 4.6255 2.5710 | 4.62 (0.03) 2.57 (0.02) | -- -- | 4.63 (0.03) 2.57 (0.02) | -- -- | 4.63 (0.03) 2.57 (0.02) | ||
| 5.2263 6.5400 | 5.22 (0.06) 6.54 (0.06) | 4.1871 2.2863 | 4.19 (0.02) 2.29 (0.01) | 4.3317 2.7539 | 4.33 (0.03) 2.75 (0.01) | 4.0815 1.9444 | 4.08 (0.03) 1.94 (0.01) | ||
| −0.9360 | −0.94 (0.03) | −1.2411 | −1.24 (0.03) | −1.2383 | −1.24 (0.03) | −1.3195 | −1.32 (0.05) | ||
| 1.0353 | 1.04 (0.02) | 0.8278 | 0.83 (0.02) | 0.9129 | 0.91 (0.01) | 0.6747 | 0.68 (0.02) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Y.; Liou, J.-Y.; Lin, C.; Ting, C.-K.; Chang, W.-K.; Lo, M.-T.; Chen, C.-C. Thermodynamic Interpretation of a Machine-Learning-Based Response Surface Model and Its Application to Pharmacodynamic Synergy between Propofol and Opioids. Mathematics 2022, 10, 1651. https://doi.org/10.3390/math10101651
Wang H-Y, Liou J-Y, Lin C, Ting C-K, Chang W-K, Lo M-T, Chen C-C. Thermodynamic Interpretation of a Machine-Learning-Based Response Surface Model and Its Application to Pharmacodynamic Synergy between Propofol and Opioids. Mathematics. 2022; 10(10):1651. https://doi.org/10.3390/math10101651
Chicago/Turabian StyleWang, Hsin-Yi, Jing-Yang Liou, Chen Lin, Chien-Kun Ting, Wen-Kuei Chang, Men-Tzung Lo, and Chien-Chang Chen. 2022. "Thermodynamic Interpretation of a Machine-Learning-Based Response Surface Model and Its Application to Pharmacodynamic Synergy between Propofol and Opioids" Mathematics 10, no. 10: 1651. https://doi.org/10.3390/math10101651
APA StyleWang, H.-Y., Liou, J.-Y., Lin, C., Ting, C.-K., Chang, W.-K., Lo, M.-T., & Chen, C.-C. (2022). Thermodynamic Interpretation of a Machine-Learning-Based Response Surface Model and Its Application to Pharmacodynamic Synergy between Propofol and Opioids. Mathematics, 10(10), 1651. https://doi.org/10.3390/math10101651








