Abstract
Objective: The purpose of this study was to assess the clinical effectiveness of using a combination of enamel matrix protein derivative and acellular dermal matrix in comparison to acellular dermal matrix alone for treating gingival recessions. Methods: The Cochrane Library (Wiley), PubMed by Medline (NLM), Medline (EBSCO), and Embase (Ovid) databases were searched for entries up to April 2020. Only clinical trials were included. Primary outcomes were root coverage (%), changes in keratinized tissue width and recession (mm). Meta-analysis was conducted for root coverage, changes in keratinized tissue width, recession, clinical attachment level and probing depth. Results: Four studies were selected for the analysis. In primary outcomes, root coverage, change in keratinized tissue width and recession analysis showed a mean difference of 4.99% (p = 0.11), 0.20 mm (p = 0.14) and 0.13 mm (p = 0.23) respectively between the two groups. Secondary outcomes analysis also exhibited a statistically insignificant difference between the test and control group with mean difference of 0.11 mm (p = 0.32) in clinical attachment level gain and -0.03 mm (p = 0.29) in probing depth reduction analysis. Conclusions: Within the limits of this study, enamel matrix protein derivative combined with acellular dermal matrix used for treating gingival recession defects resulted in no beneficial effect clinically than acellular dermal matrix only.
1. Introduction
Gingival recession and pathological loss of keratinized tissues are the most prevalent mucogingival deformities demanding surgical treatment to restore the lost supportive tissues [1]. Consequently, root coverage techniques and soft tissue augmentation are critical techniques of muco-gingival surgery protocols, including coronally advanced flaps (CAF), laterally advanced flaps (LAF), double papillae repositioned flaps, free gingival grafts (FGG), sub-epithelial connective tissue grafts (CTG), the tunnel technique as well as the pedicle flaps bilaminar technique and guided tissue regeneration or grafts [2,3,4].
Autogenous tissue grafts, particularly CTG, unquestionably remain the gold standard for root coverage treatments and soft tissue augmentation [5,6]. Considerable evidence has established that a CAF with CTG attains improved root coverage of recession [7]. However, some of the obvious drawbacks of harvesting autogenous tissue, includes post-operative bleeding and discomfort or pain at the donor site, a limited tissue supply, enhanced morbidity, much lengthier duration of surgery, and further proficiency of the surgeon [8].
To overcome such issues, various allograft substitutes (non-vital) have been investigated for plastic periodontal surgery. One of the examples is acellular dermal matrix (ADM), a human skin derivative [9]. The dermal allograft preparation includes removal of cell component and preservation of the ultrastructural integrity to prevent the inflammatory reaction [9,10,11]. Originally, ADM has been used in plastic surgery for the management of full-thickness burn wounds [10] and as an alternative to autogenous gingival grafts in root coverage procedures for last two decades [12,13,14,15,16,17]. The ADM has shown to enhance keratinized tissue, especially while treating challenging cases including thinner palatal tissues, multiple teeth recession, a restricted period of treatment, and individuals with a decreased pain threshold [12,18,19]. Moreover, ADM increased gingival thickness [14,18] and thickness of the keratinized tissue than the CAF only [16]. A histologic study suggested that the attachment of gingival tissue to the root surface was likewise for ADM and CTG, revealing epithelialization and connective tissue adherence, with the alveolar bone essentially not affected [20]. A histometric evaluation at 6-months post-operatively showed an enhanced thickness of marginal tissue, corresponding to a palatal tissue graft [20,21].
A biologic mediator, known as enamel matrix protein derivative (EMD) promotes and accelerates the regeneration of periodontal tissue (cementum, alveolar bone, and attachment tissues). Several studies including randomized controlled clinical trials (RCTs), case reports and systematic reviews (SRs) have established that EMD stimulates the new connective tissue, cementum, periodontal ligament (PDL) and bone formation for treating the periodontal osseous defects including intra-bony and furcation defects [22,23,24,25,26,27,28], periodontal reconstructive surgery [27] and root coverage [29]. For the root coverage techniques, EMD has been related to an effective root coverage, foreseeable and easy to perform with reduced patient morbidity [30,31]. In addition, EMD demonstrated positive results with regards to keratinized tissue width (KTW) and complete root coverage [32]. In contrast, few studies revealed no significant difference for the gingival recessions in the clinical results between EMD treated and non-EMD treated sites [33,34,35].
Till date, there are no definite evidence showing the extent of using the combination of ADM and different other biomolecules in terms of additional improvements of the clinical parameters compared to using the ADM alone. Furthermore, although a very few systematic reviews have been reported concerning ADM [36,37,38]; however, there are no published systematic reviews (including meta-analysis) analyzing the studies reporting combination of AMD and EMD. Hence, the extent of clinical improvements which can be achievable after the combination technique then the use of ADM alone is still debatable. Therefore, the present systematic review and meta-analysis was aimed to explore whether the addition of EMD to ADM improves the clinical outcomes with respect to root coverage (RC), KTW and gingival recession (REC) reduction compared to using the ADM alone.
2. Methodology
The present systematic review was conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) principles [39] and compared the clinical efficacy of EMD + ADM and ADM in treating gingival recession defects.
2.1. Focused Question
The focused question was devised based on the population intervention control outcome (PICO) principle. The focused question for this study was, “What are the clinical benefits of using a combination of enamel matrix derivative (EMD) and acellular dermal matrix (ADM) with coronally advanced flap (CAF) for the treatment of gingival recession lesions”.
The PICO was designed as:
Population (P): Gingival recession defects (Millers class I to III)
Intervention types (I): CAF + EMD + ADM
Comparison/control (C): CAF + ADM
Outcome measures (O): Primary outcomes → Percentage of RC, change in KTW and REC reduction. Secondary Outcomes → Clinical attachment level (CAL) gain, pocket depth (PD) reduction
2.2. Literature Search
The literature search was performed on electronic databases until April 2020 using Cochrane Library (Wiley), PubMed (NLM), Medline (Ovid) and Embase (Ovid) databases. Following keywords (italics) with Boolean operators were used for search process, P: “gingival recession” OR “recession defect” OR “root coverage” AND I: “enamel matrix derivative” OR “enamel matrix proteins” OR “EMD” OR “acellular dermal matrix allograft” OR “acellular dermal matrix” OR “ADM” OR “allograft” AND C: “coronally advanced flap” OR “CAF” OR “coronally positioned flap” OR “CPF” OR “surgical flaps” AND O: “keratinized tissue width” OR “complete root coverage”. In addition, the Open Grey (www.opengrey.eu, accessed on 3 March 2021) was used for searching the Grey literature. The reference list of related articles was hand searched for more studies. Furthermore, the Periodontology specialty journals were also searched. In the case of ambiguous or missing data, experts were contacted directly.
2.3. Literature Selection
Criteria for including a study was as follows:
- Controlled Clinical Trials (CCTs) or RCTs in which combination of EMD and ADM is compared to ADM only.
- Millers class I to III gingival recession of ≥2 mm (measured from cemento-enamel junction to the gingival margin).
- Mean follow-up period of ≥6-months.
The exclusion criteria included:
- Class IV gingival recession.
- Laboratory or animal-based (in vitro and histologic) studies
- Reviews or systematic reviews, case series and case reports studies.
- Studies other than in English Language.
2.4. Literature Screening
A systematic screening of the retrieved articles was carried out by two independent researchers (MSS and MAL) in three stages. In the first stage, researchers independently read the retrieved titles and keywords to assess the fulfillment of the inclusion criteria. In the second stage, the abstracts were carefully screened for their relevance to the research question. In the third stage, the full-text of the screened articles were retrieved and meticulously analyzed according to the eligibility criteria. All the included studies were analyzed for the primary (RC (%), change in KTW (mm) and REC reduction (mm)) and the secondary (CAL gain (mm), PD reduction (mm)) outcome measures. The kappa coefficient (κ) was used to analyze inter-rater agreement between the researchers [40]. In case of any discrepancy, consent was be obtained by discussion with a third reviewer (MSZ).
2.5. Assessment of Quality and Bias Risk
In regard to RCTs and CCTs, the Cochrane risk of bias tool [41] was applied to perform the quality assessment, analyzing different domains including:
- Selection bias
- Performance bias
- Detection bias
- Attrition bias
- Reporting bias and
- Other types of bias
2.6. Data Analysis
The homogeneity of outcomes between the studies allowed the quantitative synthesis by meta-analysis using the Review Manager 5.3. (Cochrane Collaboration, Oxford, UK) for MacOS software. For primary and secondary outcomes, mean differences along with 95% confidence intervals of differences (95% CI) were estimated. A p value of less than 0.05 was regarded to be of statistical significance. The forest plot to elucidate the weighted mean difference (WMD) of the outcome in every study as well as the final estimate was used. Based on the assumption of a population of studies with probable variations, a random-effects model (inverse-variance) was implemented. Heterogeneity resultant from the discrepant treatment effect of each paper was calculated via the Cochran’s test [42]. The outcomes of the I2 test (low: 25%; moderate: 50%; high heterogeneity: 75%) and Q statistic (significant at p < 0.10) were applied to measure the heterogeneity level [43]. Sensitivity analyses as well as subgroup analyses were conducted where indicated.
2.7. Publication Bias
The meta-analyses with fewer than 10 research papers were not assessed, contemplating the power to detect the publication bias [44]; else funnel plots were used to assess the publication bias [45].
3. Results
The screening and search results are shown in Figure 1. Out of 1423 titles, five studies were selected for the full-text evaluation. A further manual search added three more articles, making a total of eight papers assessed for the eligibility. Four studies were excluded for not fulfilling the selection criteria [animal and histologic studies (n = 2), case report (n = 1), full-text not available (n = 1)] (Table 1), and four RCTs were finally included for the quantitative as well as qualitative analysis (Figure 1).
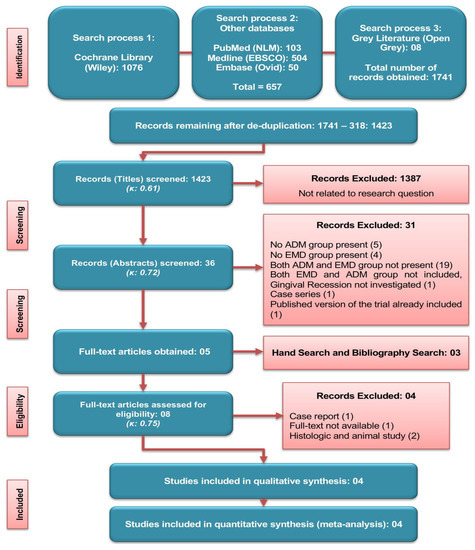
Figure 1.
Literature search strategy and screening criteria used.
From the 31 excluded abstracts, 22 papers used several different sources of soft tissue grafting (supplemental figure) such as autogenous [10 papers (45%)], xenografts [2 papers (9%)], synthetic graft [one paper (5%)], and combined sources [8 papers (36%)]. Only one paper (5%), used two allografts including ADM, but due to no EMD interventional group, this study was excluded.
3.1. Study Characteristics
The study characteristics of papers included are demonstrated in Table 2. All the four RCTs [46,47,48,49] were single-center, split-mouth randomized controlled clinical trials, used antibiotics and were conducted in the institutional setup. Furthermore, in each study, the intervention group used EMD combined with ADM, where the control group used only ADM [46,47,48,49]. The follow-up time period varied between the included RCTs (i.e., 3- and 6-months [46], 2-, 4- and 6-months [47], 6-months [48] and 3-, 6- and 12-months [49]). The comparatively shorter follow-up time periods, especially for Shin et al. [46] and Pourabbas et al. [47], are one of the limitations of these studies.
The extent of inter-rater reliability between the two reviewers was tested using the Cohen’s Kappa coefficient. The calculated scores of Cohen’s Kappa statistic κ were 0.61 0.72 and 0.75 indicating substantial to almost perfect agreement [40].

Table 1.
Excluded studies after full-text analysis.
Table 1.
Excluded studies after full-text analysis.
| Author/s | Reason for Exclusion |
|---|---|
| Saadoun [50] | Case report |
| Wallace [51] | No full-text available |
| de Oliveira et al. [52] Shirakata et al. [53] | Histologic and animal study |

Table 2.
Characteristics of the studies.
Table 2.
Characteristics of the studies.
| Authors | Study Design | Treatment | Antibiotics | Follow-Up (Months) | |
|---|---|---|---|---|---|
| Test Group | Control Group | ||||
| Shin et al. [46] | Single-center Split-mouth RCT Prospective | EMD + ADM + CAF | ADM + CAF | Y | 3, 6 |
| Pourabbas et al. [47] | Single-center Split-mouth RCT Prospective | EMD + ADM + CAF | ADM + CAF | Y | 2, 4, 6 |
| Alves et al. [48] | Single-center Split-mouth RCT Prospective | EMD + ADM + CAF | ADM + CAF | Y | 6 |
| Costa et al. [49] | Single-center Split-mouth RCT Prospective | EMD + ADM + CAF | ADM + CAF | Y | 3, 6, 12 |
ADM (Acellular dermal matrix), CAF (Coronally advanced flap), EMD (Enamel matrix protein derivative), RCT (Randomized controlled clinical trial), Y (Yes).
3.2. Participant and Defect Characteristics
A total of 58 patients with a range of age between 23 and 63 years were reported from the included studies. Only one study reported the mean age of 45.4 years [46]; however, three studies [47,48,49] did not report the mean age. Three studies reported the number of males and females [46,47,48], whereas one study did not mention the gender of the participants [49]. Three studies included the smokers [46,48,49] and one study did not include the smokers [47]. One dropout was reported in one study [48] with reason, while there were no drop-outs in other three included studies [46,47,49] (Table 3). The studies reported a total of 194 defects (ranging from 36 to 82). In three papers, class I and II gingival recession lesions (Millers) were cured [47,48,49], whereas in one publication, Miller class I to III gingival recession defects were selected [46]. Furthermore, in every study, anterior and premolars were the type of tooth treated (Table 3).

Table 3.
Characteristics of participants and defects.
3.3. Quality Assessment of Included Trials
Figure 2 shows percentages across every included paper for individual risk of bias item, whereas Figure 3 demonstrates risk of bias item for every included study. Out of four studies, one RCT each was categorized as low risk [48] and high risk [46] of bias, and two RCTs [47,49] were categorized as unclear risk of bias.
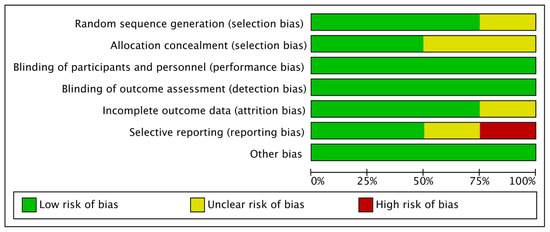
Figure 2.
Bias risk graph: Individual risk of bias item shown as percentages across each included paper.
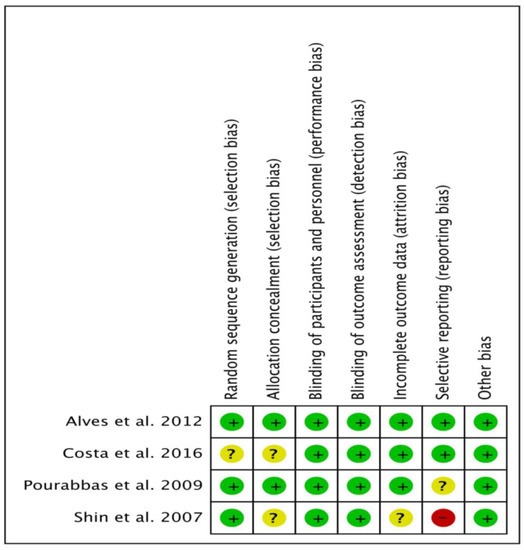
Figure 3.
Bias risk summary: Each risk of bias item for the included papers.
3.4. Effects of Primary and Secondary Outcomes
Clinical changes in terms of RC (%), KTW (mm), REC (mm) (primary outcomes), and CAL (mm) and PD (mm) (secondary outcomes) are summarized in Table 4. Quantitative analysis of three primary outcomes is shown in Figure 4 and two secondary outcomes is revealed in Figure 5. Four studies each were included in every analysis.

Table 4.
Effects of intervention and evaluation of various parameters.
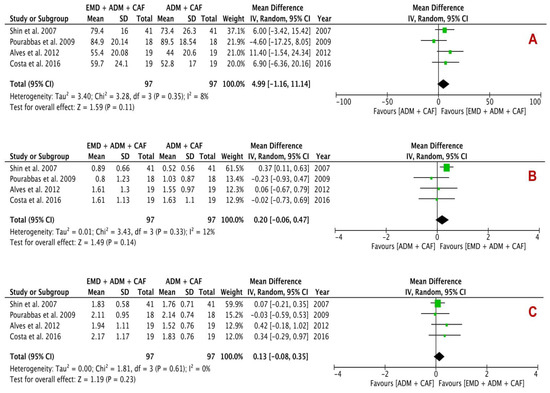
Figure 4.
Forest plots evaluating primary outcomes (A) RC, (B) Change in KTW and (C) REC reduction following surgical treatment using EMD + ADM and ADM alone (random effects; 95% CI; weighted mean difference).
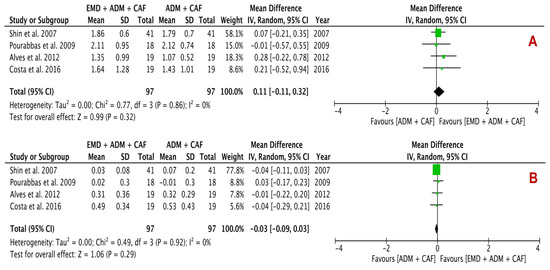
Figure 5.
Forest plots evaluating secondary outcomes (A) CAL gain and (B) PD reduction following surgical treatment using EMD + ADM and ADM alone (random effects; 95% CI; weighted mean difference).
In primary outcomes, RC, change in KTW and REC analysis showed a mean difference of 4.99% (p = 0.11; 95% CI −1.16–11.14), 0.20 mm (p = 0.14; 95% CI −0.06–0.47) and 0.13 mm (p = 0.23; 95% CI −0.08–0.35) respectively between the experimental group and control group, indicating no beneficial effect of EMD + ADM than ADM. The analysis of RC and change in KTW showed low percentage of heterogeneity with I2 value of 8% and 12% respectively; however, change in KTW analysis demonstrated no heterogeneity (I2: 0%).
The secondary outcomes analysis included CAL gain and PD reduction, presenting a statistically insignificant change between the EMD + ADM and ADM alone with difference in mean of 0.11 mm (p = 0.32; 95% CI −0.11–0.32) in CAL gain and −0.03 mm (p = 0.29; 95% CI −0.09–0.03) in PD reduction analysis. The I2 value was found to be 0% for both analyses, suggesting no heterogeneity.
4. Discussion
This systematic review estimated the clinical efficacy of using either ADM alone or EMD and ADM combination for the regenerative periodontal surgery of gingival recession defects. For this purpose, various primary [RC (%), change in KTW (mm) and REC reduction (mm)] and secondary [CAL (mm) and PD (mm)] parameters for gingival recession defects were evaluated. The quantitative analysis using forest plots (Figure 4 and Figure 5) demonstrated a statistically insignificant difference between the test group (EMD + ADM) and control group (ADM only) (p < 0.05). Only two forest plots (Figure 4) showed low heterogeneity, whereas other forest plots revealed no heterogeneity, suggesting little variability between the studies. All the included studies conducted qualitative and quantitative analysis using the split-mouth designs. A fundamental benefit of this design of study is that each individual act as his/her own control and hence the variability between the subjects is almost eliminated therefore enhancing the study power. In addition, the requirements of sample size for this study design is around half than that of a parallel study design [54].
In terms of smoking history, three studies included smokers [46,48,49], whereas one study include only non-smokers [47]. Further studies are needed to differentiate smokers and non-smokers to adequately evaluate the efficacy of these treatment modalities. All the four included studies used postoperative antibiotics; therefore, the beneficial effects of postoperative antibiotics have not been evaluated. It is plausible that the use of postoperative antibiotics may act as a confounding factor [55,56].
Histological studies on recession defects using EMD combined with CAF [57], CTG [58,59] barrier membranes [60,61] revealed tissue formation essential for periodontal regeneration including cementum, PDL fibers and alveolar bone. An RCT using CAF plus EMD showed that it is a predictable therapeutic modality for gingival recession defects, easy to implement, low morbidity and a significant increase in the RC percentage, change in KTW and CAL [62]. In a paper assessing the role of EMD in the surgical management of recession defects, Cheng et al. (2015) in a SR showed that CAF + CTG and CAF + EMD significantly improved KTW compared to the CAF alone. Using EMD reduced PD; however, the difference was insignificant [63]. Modica et al. (2000), Discepoli et al. (2019) and Górski et al. (2020) showed that there is no difference between EMD treated gingival recessions and non-EMD treated gingival recessions with respect to the clinical outcomes [64,65,66]. In another study, this biologically active regenerative material increased the percentage of RC and KTW than the control group [67]. The present review validated that both ADM + EMD and ADM only enhanced the clinical variables considerably; however, between the two treatments, there was no statistically significant difference. Besides, a histological study [52] found that EMD does not contribute to improved cementum and bone regeneration when associated with ADM. On the contrary, another histological study [53] demonstrated better cementum and bone regeneration for EMD + ADM group as compared to ADM only and CAF only groups.
The study hypothesis was that the EMD might increase the potential of wound healing due to its potential of stimulating angiogenesis [68,69] and fibroblast activity [70,71] when applied to the interface of ADM-soft tissue. Additionally, EMD may also play a role in the propagation of PDL cells, increase formation of proteins including collagen, and promote mineralization [30,70,71] when used at the ADM-root surface interface. Another study exhibited that EMD is found at the site of healing during majority of the essential phases occurring during wound healing of the gingival tissues [72,73].
Cummings et al. (2005), reported the management of teeth with severe gingival recessions (Miller Class III/IV) using a combination of CAF and ADM. Formation of cementum in the apical part of the recession defects was observed with no signs of novel osteogenesis. Additionally, ADM adherence to the surface of root was accomplished via the formation of both long junctional epithelium as well as connective tissue adherence however there were no signs of PDL fibers entrance to the surface of root [20]. Inductive effects of EMD on the periodontium components; predominantly fibroblasts have been reported by in vitro studies [74,75], while the in vivo studies suggest controversial findings [64,76]. This can be attributed to technique sensitiveness of EMD application leading to change in outcomes and disagreement. The application of ADM may lead to EMD dispersion and emission from the wound area as a result of its physical pressure and effects on thickness of flap as well as tension. In contrast, ADM permeability to EMD has not been confirmed and ADM presence may disrupt the interaction between fibroblasts and EMD. In addition, ADM on its own may stimulate proliferation of cells and ultrastructural cellular changes, accredited to the presence of extracellular materials [14] and leading to an improved inductive role of ADM on EMD.
A routine encounter for root coverage surgical techniques is the graft’s ability to persist at the surgical receptor site. Especially when dealing with a non-vital ADM graft, this must be contemplated as an even more critical factor [77]. Studies investigated variations in gingival as well as alveolar mucosa microcirculation after various surgical flaps or incisions in a fluorescein angiographic evaluation [78] and laser Doppler flowmetry [79,80] the flaps must be wide enough at their base to comprise the main vessels of gingival tissue. The vertical releasing incisions should never be in close proximity to tooth with gingival recession defect. Since the principle gingival vascular supply is directed caudocranially to the gingival margin from the vestibule [81], a wider flap enhances the amount of blood vessels accessible to contribute in the process of healing. The modified technique [82] was used in two studies [48,49] and based on the extension of surgical site to adjacent teeth with the intention to give more nutrients, vascular supply, and an enhanced source of cells to support the ADM absorption. A wider flap also permitted for much improved tissue manipulation, particularly for attaining a tension free CAF to completely cover the allograft [82,83]. Whereas, Shin et al. (2007) used the incision design by Zucchelli and Sanctis [84] for recession defects involving multiple teeth, eluding vertical releasing incisions. Therefore, healing of several root coverage procedures and the impact of some other variables including thickness of flap, the coronal flaps tension and vestibular depth on the outcomes imply a compulsion for more human, animal, or histological studies to explore their plausible effect as prognostic factors. Nevertheless, ADM is the most widely studied soft tissue allograft till date than any other allografts and it has already provided very favorable results in general plastic surgeries. Comparing ADM to autogenous grafts, it prevents the need for second surgical site and consequently post-operative pain and discomfort [8].
In the present review, two studies revealed unclear risk of allocation concealment, which can introduce selection bias. Furthermore, one study showed high risk of selective reporting, indicating presence of reporting bias. Overall, quality appraisal of the selected papers in the meta-analysis were mostly categorized as unclear risk. The choice to include each study in the meta-analysis was due to scarcity of data in the literature as only four RCTs could be identified according to the exhaustive literature search. Moreover, the results of this study should be interpreted cautiously due to the fact that only four RCTs were included after the exhaustive search, not providing a very strong evidence. Other limitations of this review were shorter follow-up time periods and small sample sizes of the studies included in the quantitative analysis. Therefore, for future research, more interventional studies particularly RCTs with much bigger sample sizes and longer-term follow-ups are required for further validation of the effect of combination therapy of EMD + ADM against ADM alone in gingival recession defects.
Furthermore, the availability of data from the clinical trials (mostly Class I/II gingival recession cases) could be a limitation to conclude for this application setting and therefore, future research should also emphasize on these limitations and directing the lack of evidence for severe recession cases.
5. Conclusions
Enamel matrix protein derivative and acellular dermal matrix are commonly explored for gingival recessions regenerative therapy. Using a combination of EMD and ADM for treating gingival recession defects resulted no remarkable beneficial effects compared to ADM only in terms of RC, change in KTW, REC, CAL, and PD statistically. Accordingly, both treatments can serve as an alternate to root coverage of Millers class I and II gingival recession lesions. However, the cost-benefit ratio associated with adding EMD to ADM procedure should be carefully evaluated.
Author Contributions
Conceptualization: M.S.S. and M.S.Z. Methodology: M.S.S., M.A.L. (Mohid Abrar Lone) and A.H.S. Software: M.S.S. Writing Original Draft: M.S.S., H.M. and M.S.Z. Writing Review and Editing: M.S.Z. and M.A.L. (Muneeb Ahmed Lone). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be found at Cochrane Library (Wiley), PubMed by Medline (NLM), Medline (EBSCO) and Embase (Ovid) databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89, S204–S213. [Google Scholar] [CrossRef]
- Bouchard, P.; Malet, J.; Borghetti, A. Decision-making in aesthetics: Root coverage revisited. Periodontology 2000 2001, 27, 97–120. [Google Scholar] [CrossRef]
- Zucchelli, G.; Amore, C.; Sforza, N.; Montebugnoli, L.; De Sanctis, M. Bilaminar techniques for the treatment of recession-type defects. A comparative clinical study. J. Clin. Periodontol. 2003, 30, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stathopoulou, P.G. Tunneling Techniques for Root Coverage. Curr. Oral Health Rep. 2019, 6, 237–243. [Google Scholar] [CrossRef]
- Chambrone, L.; Chambrone, D.; Pustiglioni, F.E.; Chambrone, L.A.; Lima, L.A. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J. Dent. 2008, 36, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, E.T.; Sanz, M.; Dibart, S.; Greenwell, H.; John, V.; Kim, D.M.; Langer, L.; Neiva, R.; Rasperini, G. Periodontal soft tissue non–root coverage procedures: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Pagliaro, U.; Nieri, M. Treatment of gingival recession with coronally advanced flap procedures: A systematic review. J. Clin. Periodontol. 2008, 35, 136–162. [Google Scholar] [CrossRef]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef]
- Rhee, P.H.; Friedman, C.D.; Ridge, J.A.; Kusiak, J. The use of processed allograft dermal matrix for intraoral resurfacing: An alternative to split-thickness skin grafts. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1201–1204. [Google Scholar] [CrossRef]
- Wainwright, D. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995, 21, 243–248. [Google Scholar] [CrossRef]
- Wainwright, D.; Madden, M.; Luterman, A.; Hunt, J.; Monafo, W.; Heimbach, D.; Kagan, R.; Sittig, K.; Dimick, A.; Herndon, D. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J. Burn Care Res. 1996, 17, 124–136. [Google Scholar] [CrossRef]
- Tal, H. Subgingival acellular dermal matrix allograft for the treatment of gingival recession: A case report. J Periodontol. 1999, 70, 1118–1124. [Google Scholar] [CrossRef]
- Harris, R.J. A comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft: Results of 107 recession defects in 50 consecutively treated patients. Int. J. Periodontics Restor. Dent. 2000, 20, 51–59. [Google Scholar]
- Aichelmann-Reidy, M.E.; Yukna, R.A.; Evans, G.H.; Nasr, H.F.; Mayer, E.T. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J. Periodontol. 2001, 72, 998–1005. [Google Scholar] [CrossRef]
- Harris, R.J. Acellular Dermal Matrix Used for Root Coverage: 18-Month Follow-up Observation. Int. J. Periodontics Restor. Dent. 2002, 22, 157–163. [Google Scholar]
- Woodyard, J.G.; Greenwell, H.; Hill, M.; Drisko, C.; Iasella, J.M.; Scheetz, J. The clinical effect of acellular dermal matrix on gingival thickness and root coverage compared to coronally positioned flap alone. J. Periodontol. 2004, 75, 44–56. [Google Scholar] [CrossRef]
- de Resende, D.R.B.; Greghi, S.L.A.; Siqueira, A.F.; Benfatti, C.A.M.; Damante, C.A.; Zangrando, M.S.R. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; Dolci, M.; Esposito, P.; D’Archivio, D.; Lisanti, L.; Di Luccio, A.; Perinetti, G. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: A comparative 1-year clinical study. J. Periodontol. 2002, 73, 1299–1307. [Google Scholar] [CrossRef]
- Tal, H.; Moses, O.; Zohar, R.; Meir, H.; Nemcovsky, C. Root coverage of advanced gingival recession: A comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J. Periodontol. 2002, 73, 1405–1411. [Google Scholar] [CrossRef]
- Cummings, L.C.; Kaldahl, W.B.; Allen, E.P. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J. Periodontol. 2005, 76, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. Gingival Augmentation with an Acellular Dermal Matrix: Human Histologic Evaluation of a Case-Placement of the Graft on Periosteum. Int. J. Periodontics Restor. Dent. 2004, 24, 379–385. [Google Scholar]
- Heijl, L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J. Clin. Periodontol. 1997, 24, 693–696. [Google Scholar] [PubMed]
- Sculean, A.; Chiantella, G.C.; Windisch, P.; Donos, N. Clinical and Histologic Evaluation of Human lntrabony Defects Treated with an Enamel Matrix Protein Derivative (Emdogain). Int. J. Periodontics Restor. Dent. 2000, 20, 375–381. [Google Scholar]
- Jepsen, S.; Heinz, B.; Jepsen, K.; Arjomand, M.; Hoffmann, T.; Richter, S.; Reich, E.; Sculean, A.; Gonzales, J.R.; Bödeker, R.H.; et al. A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal Class II furcation involvement in mandibular molars. Part I: Study design and results for primary outcomes. J. Periodontol. 2004, 75, 1150–1160. [Google Scholar] [CrossRef]
- Francetti, L.; Trombelli, L.; Lombardo, G.; Guida, L.; Cafiero, C.; Roccuzzo, M.; Carusi, G.; Del Fabbro, M. Evaluation of efficacy of enamel matrix derivative in the treatment of intrabony defects: A 24-month multicenter study. Int. J. Periodontics Restor. Dent. 2005, 25, 461–473. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 247–266. [Google Scholar]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: A systematic review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Pisani, F.; De Vito, D.; Lone, M.A.; Almasri, M. Long-term Clinical Performance of Regeneration versus Conservative Surgery in the Treatment of Infra-bony Defects: A Systematic Review. J. Int. Acad. Periodontol. 2021, 23, 31–56. [Google Scholar]
- Del Pizzo, M.; Zucchelli, G.; Modica, F.; Villa, R.; Debernardi, C. Coronally advanced flap with or without enamel matrix derivative for root coverage: A 2-year study. J. Clin. Periodontol. 2005, 32, 1181–1187. [Google Scholar] [CrossRef]
- Gestrelius, S.; Andersson, C.; Lidström, D.; Hammarström, L.; Somerman, M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 1997, 24, 685–692. [Google Scholar] [CrossRef]
- Nemcovsky, C.E.; Artzi, Z.; Tal, H.; Kozlovsky, A.; Moses, O. A multicenter comparative study of two root coverage procedures: Coronally advanced flap with addition of enamel matrix proteins and subpedicle connective tissue graft. J. Periodontol. 2004, 75, 600–607. [Google Scholar] [CrossRef]
- Mercado, F.; Hamlet, S.; Ivanovski, S. A 3-year prospective clinical and patient-centered trial on subepithelial connective tissue graft with or without enamel matrix derivative in Class I-II Miller recessions. J. Periodontal Res. 2019, 55, 296–306. [Google Scholar] [CrossRef]
- Hägewald, S.; Spahr, A.; Rompola, E.; Haller, B.; Heijl, L.; Bernimoulin, J.P. Comparative study of Emdogain® and coronally advanced flap technique in the treatment of human gingival recessions: A prospective controlled clinical study. J. Clin. Periodontol. 2002, 29, 35–41. [Google Scholar] [CrossRef]
- Trabulsi, M.; Oh, T.J.; Eber, R.; Weber, D.; Wang, H.L. Effect of enamel matrix derivative on collagen guided tissue regeneration-based root coverage procedure. J. Periodontol. 2004, 75, 1446–1457. [Google Scholar] [CrossRef]
- Alexiou, A.; Vouros, I.; Menexes, G.; Konstantinidis, A. Comparison of enamel matrix derivative (Emdogain) and subepithelial connective tissue graft for root coverage in patients with multiple gingival recession defects: A randomized controlled clinical study. Quintessence Int. 2017, 48, 381–389. [Google Scholar] [PubMed]
- Gapski, R.; Parks, C.A.; Wang, H.L. Acellular dermal matrix for mucogingival surgery: A meta-analysis. J. Periodontol. 2005, 76, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.I.; Matthews, D.C. Acellular dermal matrix and subepithelial connective tissue grafts for root coverage: A systematic review. J. Indian Soc. Periodontol. 2017, 21, 439–448. [Google Scholar]
- Lu, W.; Qi, G.; Ding, Z.; Li, X.; Qi, W.; He, F. Clinical efficacy of acellular dermal matrix for plastic periodontal and implant surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 49, 1057–1066. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochemia medica: Biochem Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. Cochrane Handb. Syst. Rev. Interv. 2019, 349–374. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Cueva, M.A.; Kerns, D.G.; Hallmon, W.W.; Rivera-Hidalgo, F.; Nunn, M.E. A comparative study of root coverage using acellular dermal matrix with and without enamel matrix derivative. J. Periodontol. 2007, 78, 411–421. [Google Scholar] [CrossRef]
- Pourabbas, R.; Chitsazi, M.T.; Kosarieh, E.; Olyaee, P. Coronally advanced flap in combination with acellular dermal matrix with or without enamel matrix derivatives for root coverage. Indian J. Dent. Sci. 2009, 20, 320–325. [Google Scholar] [CrossRef]
- Alves, L.B.; Costa, P.P.; Scombatti de Souza, S.L.; de Moraes Grisi, M.F.; Palioto, D.B.; Taba, M., Jr.; Novaes, A., Jr. Acellular dermal matrix graft with or without enamel matrix derivative for root coverage in smokers: A randomized clinical study. J. Clin. Periodontol. 2012, 39, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.P.; Alves, L.B.; Souza, S.L.; Grisi, M.F.; Palioto, D.B.; Taba, M., Jr.; Novaes, A.B. Root Coverage in Smokers with Acellular Dermal Matrix Graft and Enamel Matrix Derivative: A 12-Month Randomized Clinical Trial. Int. J. Periodontics Restor. Dent. 2016, 36, 525–531. [Google Scholar] [CrossRef]
- Saadoun, A.P. Root coverage with Emdogain/AlloDerm: A new way to treat gingival recessions. Eur. J. Esthet. Dent. 2008, 3, 46–65. [Google Scholar] [PubMed]
- Wallace, S. Treating human gingival recession defects with acellular dermis matrix and enamel matrix derivative using coronally advanced flaps. Gen. Dent. 2014, 62, e12–e15. [Google Scholar]
- de Oliveira, C.A.; Spolidório, L.C.; Cirelli, J.A.; Marcantonio, R.A.C. Acellular dermal matrix allograft used alone and in combination with enamel matrix protein in gingival recession: Histologic study in dogs. Int. J. Periodontics Restor. Dent. 2005, 25, 595–603. [Google Scholar]
- Shirakata, Y.; Sculean, A.; Shinohara, Y.; Sena, K.; Takeuchi, N.; Bosshardt, D.; Noguchi, K. Healing of localized gingival recessions treated with a coronally advanced flap alone or combined with an enamel matrix derivative and a porcine acellular dermal matrix: A preclinical study. Clin. Oral Investig. 2016, 20, 1791–1800. [Google Scholar] [CrossRef]
- Hujoel, P.; DeRouen, T. Validity issues in split-mouth trials. J. Clin. Periodontol. 1992, 19, 625–627. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L. Systemic antibiotics in periodontal therapy. Aust. Dent. J. 2009, 54, S96–S101. [Google Scholar] [CrossRef]
- Oswal, S.; Ravindra, S.; Sinha, A.; Manjunath, S. Antibiotics in periodontal surgeries: A prospective randomised cross over clinical trial. J. Indian Soc. Periodontol. 2014, 18, 570–574. [Google Scholar] [CrossRef]
- McGuire, M.K.; Cochran, D.L. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histological evaluation. J. Periodontol. 2003, 74, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Carnio, J.; Camargo, P.M.; Kenney, E.B.; Schenk, R.K. Histological evaluation of 4 cases of root coverage following a connective tissue graft combined with an enamel matrix derivative preparation. J. Periodontol. 2002, 73, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Nakamura, T.; Shinohara, Y.; Nakamura-Hasegawa, K.; Hashiguchi, C.; Takeuchi, N.; Imafuji, T.; Sculean, A.; Noguchi, K. Split-mouth evaluation of connective tissue graft with or without enamel matrix derivative for the treatment of isolated gingival recession defects in dogs. Clin. Oral Investig. 2019, 23, 3339–3349. [Google Scholar] [CrossRef]
- Sallum, E.A.; Pimentel, S.P.; Saldanha, J.B.; Nogueira-Filho, G.R.; Casati, M.Z.; Nociti, F.H., Jr.; Sallum, A.W. Enamel matrix derivative and guided tissue regeneration in the treatment of dehiscence-type defects: A histomorphometric study in dogs. J. Periodontol. 2004, 75, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yamamoto, S.; Ota, M.; Shibukawa, Y.; Yamada, S. Coverage of gingival recession defects using guided tissue regeneration with and without adjunctive enamel matrix derivative in a dog model. Int. J. Periodontics Restor. Dent. 2011, 31, 247–253. [Google Scholar]
- Pilloni, A.; Paolantonio, M.; Camargo, P.M. Root coverage with a coronally positioned flap used in combination with enamel matrix derivative: 18-month clinical evaluation. J. Periodontol. 2006, 77, 2031–2039. [Google Scholar] [CrossRef]
- Cheng, G.L.; Fu, E.; Tu, Y.K.; Shen, E.C.; Chiu, H.C.; Huang, R.Y.; Yuh, D.Y.; Chiang, C.Y. Root coverage by coronally advanced flap with connective tissue graft and/or enamel matrix derivative: A meta-analysis. J. Periodontal Res. 2015, 50, 220–230. [Google Scholar] [CrossRef]
- Modica, F.; Pizzo, M.D.; Roccuzzo, M.; Romagnoli, R. Coronally advanced flap for the treatment of buccal gingival recessions with and without enamel matrix derivative. A split-mouth study. J. Periodontol. 2000, 71, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Discepoli, N.; Mirra, R.; Ferrari, M. Efficacy of Enamel Derivatives to Improve Keratinized Tissue as Adjunct to Coverage of Gingival Recessions: A Systematic Review and Meta-Analysis. Materials 2019, 12, 2790. [Google Scholar] [CrossRef] [PubMed]
- Górski, B.; Górska, R.; Wysokińska-Miszczuk, R.; Kaczyński, T. Tunnel technique with enamel matrix derivative in addition to subepithelial connective tissue graft compared with connective tissue graft alone for the treatment of multiple gingival recessions: A randomized clinical trial. Clin. Oral Investig. 2020, 24, 4475–4486. [Google Scholar] [CrossRef]
- Cueva, M.A.; Boltchi, F.E.; Hallmon, W.W.; Nunn, M.E.; Rivera-Hidalgo, F.; Rees, T. A comparative study of coronally advanced flaps with and without the addition of enamel matrix derivative in the treatment of marginal tissue recession. J. Periodontol. 2004, 75, 949–956. [Google Scholar] [CrossRef]
- Thoma, D.S.; Villar, C.C.; Carnes, D.L.; Dard, M.; Patricia Chun, Y.H.; Cochran, D.L. Angiogenic activity of an enamel matrix derivative (EMD) and EMD-derived proteins: An experimental study in mice. J. Clin. Periodontol. 2011, 38, 253–260. [Google Scholar] [CrossRef]
- Kasaj, A.; Meister, J.; Lehmann, K.; Stratul, S.; Schlee, M.; Stein, J.; Willershausen, B.; Schmidt, M. The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J. Periodontal Res. 2012, 47, 479–487. [Google Scholar] [CrossRef]
- Rodrigues, T.L.; Marchesan, J.T.; Coletta, R.D.; Novaes, A.B., Jr.; Grisi, M.F.D.M.; Souza, S.L.; Taba, M., Jr.; Palioto, D.B. Effects of enamel matrix derivative and transforming growth factor-β1 on human periodontal ligament fibroblasts. J. Clin. Periodontol. 2007, 34, 514–522. [Google Scholar] [CrossRef]
- Heng, N.H.; Zahlten, J.; Cordes, V.; Ong, M.M.A.; Goh, B.T.; N’Guessan, P.D.; Pischon, N. Effects of enamel matrix derivative and transforming growth factor-β1 on connective tissue growth factor in human periodontal ligament fibroblasts. J. Periodontol. 2015, 86, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Roccuzzo, M.; Francetti, L.; Acunzo, R.; Consonni, D.; Silvestri, M. Subepithelial connective tissue graft for treatment of gingival recessions with and without enamel matrix derivative: A multicenter, randomized controlled clinical trial. Int. J. Periodontics Restor. Dent. 2011, 31, 132–139. [Google Scholar]
- Miron, R.; Dard, M.; Weinreb, M. Enamel matrix derivative, inflammation and soft tissue wound healing. J. Periodontal Res. 2015, 50, 555–569. [Google Scholar] [CrossRef]
- Lindskog, S. Formation of intermediate cementum. I: Early mineralization of aprismatic enamel and intermediate cementum in monkey. J. Craniofac. Genet. Dev. Biol. 1982, 2, 147–160. [Google Scholar]
- Lindskog, S. Formation of intermediate cementum. II: A scanning electron microscopic study of the epithelial root sheath of Hertwig in monkey. J. Craniofac. Genet. Dev. Biol. 1982, 2, 161–169. [Google Scholar] [PubMed]
- Berlucchi, I.; Francetti, L.; Del Fabbro, M.; Testori, T.; Weinstein, R.L. Enamel matrix proteins (Emdogain) in combination with coronally advanced flap or subepithelial connective tissue graft in the treatment of shallow gingival recessions. Int. J. Periodontics Restor. Dent. 2002, 22, 583–594. [Google Scholar]
- Batista, E.L., Jr.; Batista, F.C.; Novaes, A.B., Jr. Management of soft tissue ridge deformities with acellular dermal matrix. Clinical approach and outcome after 6 months of treatment. J. Periodontol. 2001, 72, 265–273. [Google Scholar] [CrossRef]
- Mörmann, W.; Ciancio, S.G. Blood supply of human gingiva following periodontal surgery: A fluorescein angiographic study. J. Periodontol. 1977, 48, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Tonetti, M.; Donos, N. Comparison of gingival blood flow during healing of simplified papilla preservation and modified Widman flap surgery: A clinical trial using laser Doppler flowmetry. J. Clin. Periodontol. 2007, 34, 903–911. [Google Scholar] [CrossRef]
- Retzepi, M.; Tonetti, M.; Donos, N. Gingival blood flow changes following periodontal access flap surgery using laser Doppler flowmetry. J. Clin. Periodontol. 2007, 34, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Baldi, C.; Pini-Prato, G.; Pagliaro, U.; Nieri, M.; Saletta, D.; Muzzi, L.; Cortellini, P. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J. Periodontol. 1999, 70, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.R.; Novaes, A.B., Jr.; Grisi, M.F.; Souza, S.L.; Taba, M., Jr.; Palioto, D.B. A 6-month comparative clinical study of a conventional and a new surgical approach for root coverage with acellular dermal matrix. J. Periodontol. 2004, 75, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.R.; Novaes, A.B., Jr.; Grisi, M.F.; Souza, S.L.; Taba, M., Jr.; Palioto, D.B. New surgical approach for root coverage of localized gingival recession with acellular dermal matrix: A 12-month comparative clinical study. J. Esthet. Restor. Dent. 2005, 17, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; De Sanctis, M. Treatment of multiple recession-type defects in patients with esthetic demands. J. Periodontol. 2000, 71, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).