Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. 15N -Labeled QconCAT Expression, Purification, and Characterization
2.2. Preparation of Microvesicles and Exosomes
2.3. AF4
2.4. LC-MS/MS Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Assignment of Protein Markers
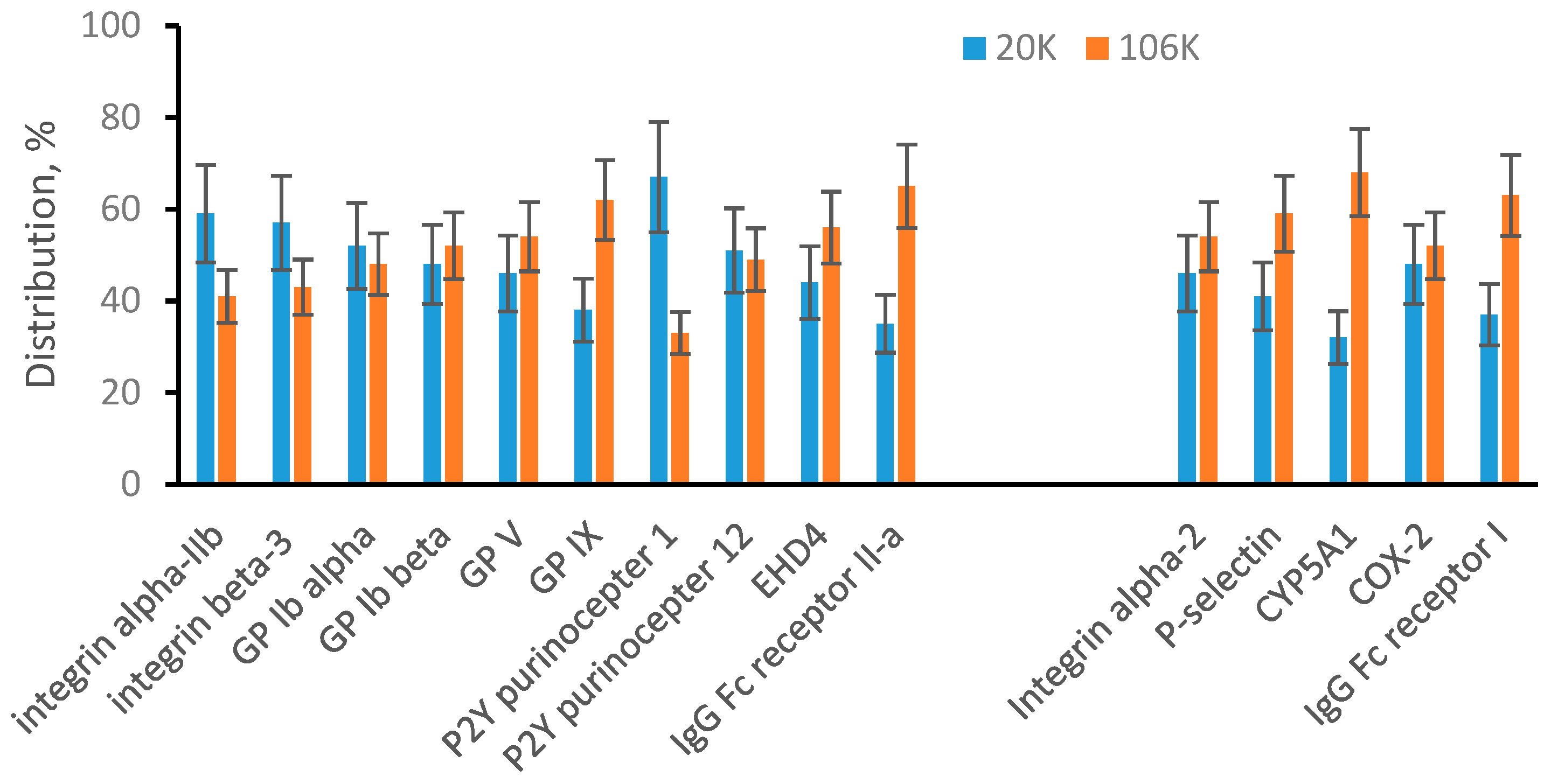
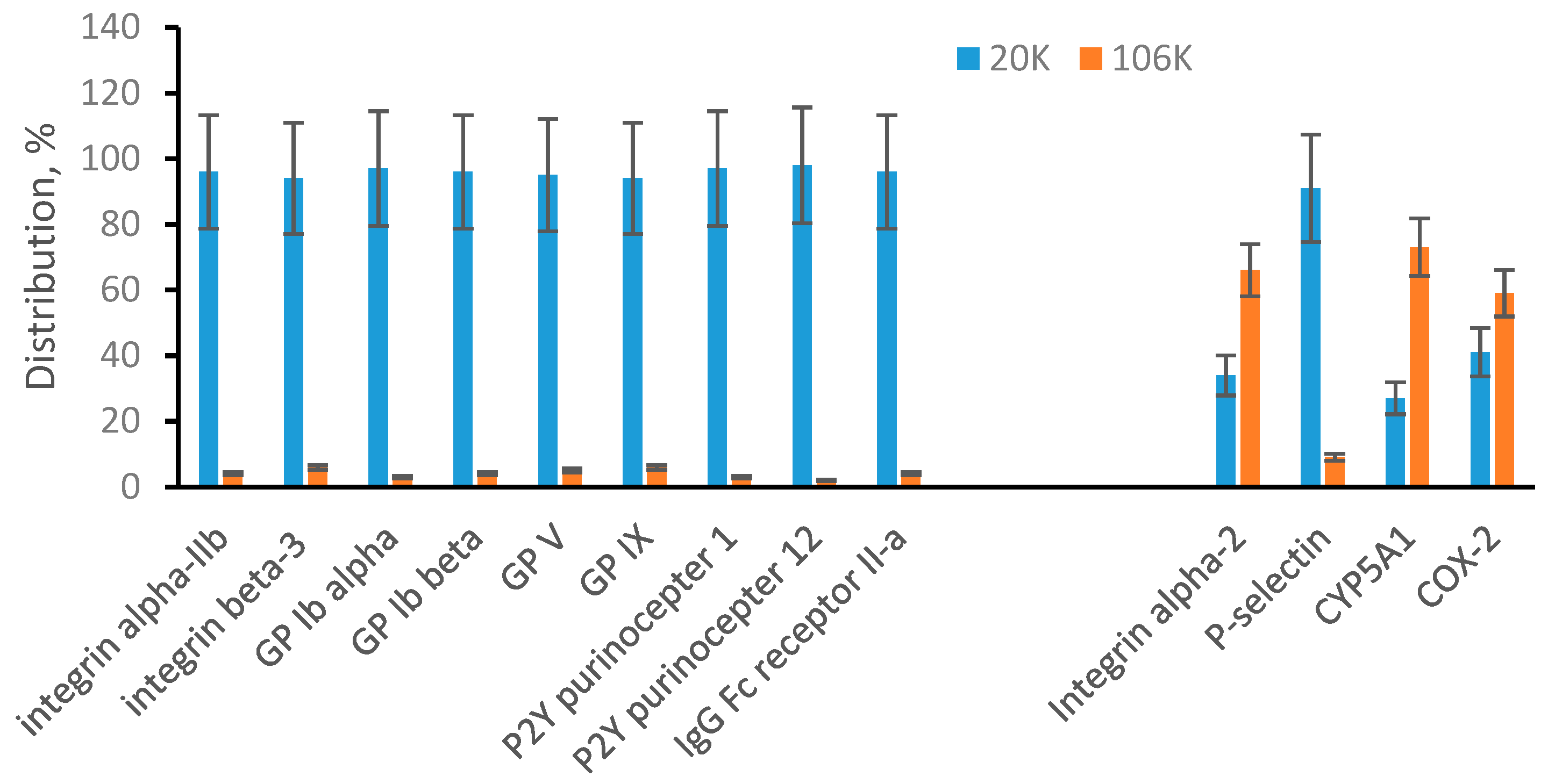
3.2. Sonicated Platelets
3.3. EVs from Human Plasma
3.4. EVs from ARPE-19 Cell Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF4 | asymmetrical-flow field-flow fractionation |
| EV | extracellular vesicle |
| GP | glycoprotein |
| MRM | multiple reaction monitoring |
| QconCATs | quantification concatamer |
References
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, Y.; Du, Y.; Liu, J. Exosomes: Diagnostic biomarkers and therapeutic delivery vehicles for can-cer. Mol. Pharm. 2019, 16, 3333–3349. [Google Scholar]
- Roy, S.; Hochberg, F.H.; Jones, P.S. Extracellular vesicles: The growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 2018, 7, 1438720. [Google Scholar] [CrossRef] [PubMed]
- Gudbergsson, J.M.; Jonsson, K.; Simonsen, J.B.; Johnsen, K.B. Systematic review of targeted extracellular vesicles for drug delivery—Consideration of methodological and biological heregeneity. J. Cont. Rel. 2019, 306, 108–120. [Google Scholar]
- Reiner, A.T.; Witwer, K.W.; Van Balkom, B.W.; De Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; De Kleijn, D.; et al. Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. STEM CELLS Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, iso-lation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef]
- Busatto, S.; Zendrini, A.; Radeghieri, A.; Paolini, L.; Romano, M.; Presta, M.; Bergese, P. The nanostruc-tured secretome. Biomater. Sci. 2019, 8, 39–63. [Google Scholar]
- Gudbergsson, J.M.; Johnsen, K.B. Exosomes and autophagy: Rekindling the vesicular waste hypothesis. J. Cell Commun. Signal. 2019, 13, 443–450. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Appoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar]
- Jang, S.C.; Crescitelli, R.; Cvjetkovic, A.; Belgrano, V.; Bagge, R.O.; Sundfeldt, K.; Ochiya, T.; Kalluri, R.; Lötvall, J. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 2019, 8, 1635420. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Jang, S.C.; Cvjetkovic, A.; Malmhäll, C.; Karimi, N.; Höög, J.L.; Johansson, I.; Fuchs, J.; Thorsell, A.; et al. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous popula-tions of extracellular vesicle subtypes. Proc. Nat. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanick, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by assymetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [PubMed]
- Anderson, L.; Hunter, C.L. Quantitative mass spectrometry multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteom. 2006, 5, 573–588. [Google Scholar]
- Liebler, D.C.; Zimmerman, L.J. Targeted quantification of proteins by mass spectrometry. Biochemistry 2013, 52, 3797–3806. [Google Scholar]
- Williams, S.K.R.; Runyon, J.R.; Murtaza, G. Field-flow fractionation: Addressing the nano challenge. Anal. Chem. 2011, 83, 634–642. [Google Scholar] [CrossRef]
- Qureshi, R.N.; Kok, W.T. Application of flow field-flow fractionation for the characterization of macro-molecules of biological interest: A review. Anal. Bioanal. Chem. 2011, 399, 1401–1411. [Google Scholar]
- Sitar, S.; Kejzar, A.; Pahovnik, D.; Kogej, K.; Tusek-Znidaric, M.; Lenassi, M.; Zagar, E. Size characteriza-tion and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar]
- Yang, J.S.; Lee, J.C.; Byeon, S.K.; Rha, K.H.; Moon, M.H. Size dependent lipidomic analysis of urinary exosomes from patients with prostate cancer by flow field-flow fractionation and nanoflow liquid chro-matography-tandem mass spectrometry. Anal. Chem. 2017, 89, 2488–2496. [Google Scholar]
- Oeyen, E.; Mol, K.V.; Baggerman, G.; Willems, H.; Boonen, K.; Rolfo, C.; Pauwels, P.; Jacobs, A.; Schil-dermans, K.; Cho, W.C.; et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterization of extracellular vesicles from urine. J. Extracell. Vesicles 2018, 7, 1490143. [Google Scholar]
- Pratt, J.M.; Simpson, D.M.; Doherty, M.K.; Rivers, J.; Gaskell, S.J.; Beynon, R.J. Multiplexed absolute quan-tification for proteomics using concatenated signature peptides. Nat. Protoc. 2006, 1, 1029–1043. [Google Scholar]
- Chen, J.; Turko, I.V. Trends in QconCATs for targeted proteomics. TrAC Trends Anal. Chem. 2014, 57, 1–5. [Google Scholar] [CrossRef]
- Wang, T.; Anderson, K.W.; Turko, I.V. Assessment of extracellular vesicles purity using proteomic stand-ards. Anal. Chem. 2017, 89, 11070–11075. [Google Scholar] [PubMed]
- Wang, T.; Turko, I.V. Proteomic toolbox to standardize the separation of extracellular vesicles and lipo-protein particles. J. Proteome Res. 2018, 17, 3104–3113. [Google Scholar]
- Au, A.E.; Josefsson, E.C. Regulation of platelet membrane protein shedding in health and disease. Platelets 2016, 28, 342–353. [Google Scholar] [CrossRef]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Nielsen, T.; Kristensen, A.F.; Pedersen, S.; Christiansen, G.; Kristensen, S.R. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J. Extracell. Vesicles 2018, 7, 1454777. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Assymmetric-flow field-flow fractionation technology for exomere and small extra-cellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar]
- Simonsen, J.B. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ. Res. 2017, 121, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-L.; Turko, I.V. Accumulation of large protein fragments in prematurely senescent ARPE-19 Cells. Investig. Opthalmol. Vis. Sci. 2009, 50, 4992–4997. [Google Scholar] [CrossRef]
- Witwer, K.W.; Thery, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influ-encing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [PubMed]

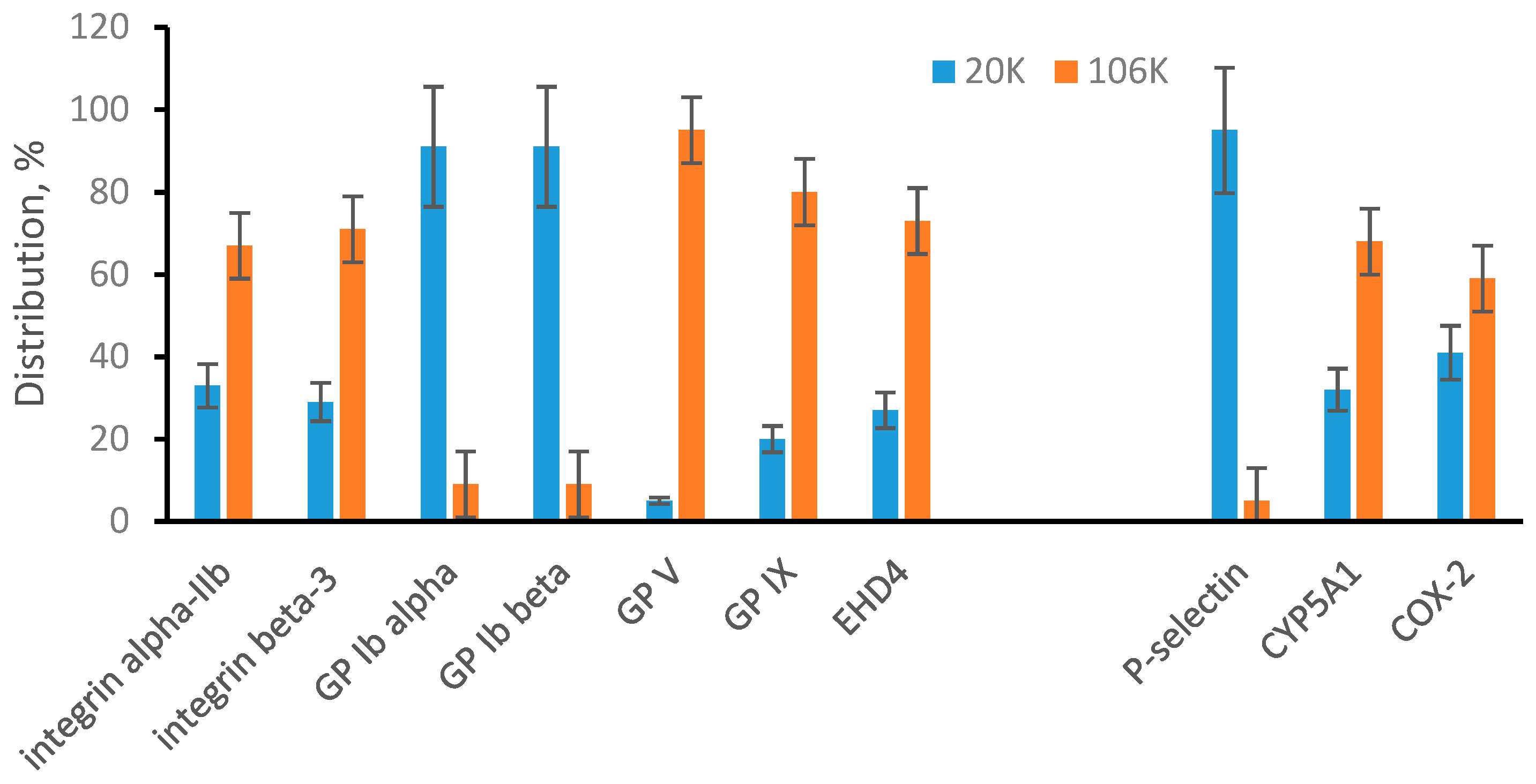
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Parot, J.; Hackley, V.A.; Turko, I.V. Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles. Proteomes 2020, 8, 33. https://doi.org/10.3390/proteomes8040033
Zhang L, Parot J, Hackley VA, Turko IV. Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles. Proteomes. 2020; 8(4):33. https://doi.org/10.3390/proteomes8040033
Chicago/Turabian StyleZhang, Linwen, Jeremie Parot, Vincent A. Hackley, and Illarion V. Turko. 2020. "Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles" Proteomes 8, no. 4: 33. https://doi.org/10.3390/proteomes8040033
APA StyleZhang, L., Parot, J., Hackley, V. A., & Turko, I. V. (2020). Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles. Proteomes, 8(4), 33. https://doi.org/10.3390/proteomes8040033




