Ovarian Cancer: Tumor-Specific Urinary Micro-Peptides Profiling as Potential Biomarkers for Early Diagnosis
Abstract
1. Introduction
2. Experimental Section
2.1. Ethical Considerations
2.2. Study Design, Sites, and Duration
2.3. Study Population
2.4. Samples
2.5. Determination of Tumor Biomarker CA125 Using ELISA Technique [GenAsia Biotech Co. Ltd, Shanghai, China]
2.6. Polyacrylamide Gel -SDS Gel Electrophoresis (PAGE-SDS Electrophoresis)
Urine Protein Precipitation
2.7. Sequencing and Identification of Selected Urine Peptides
2.8. Statistical Analysis
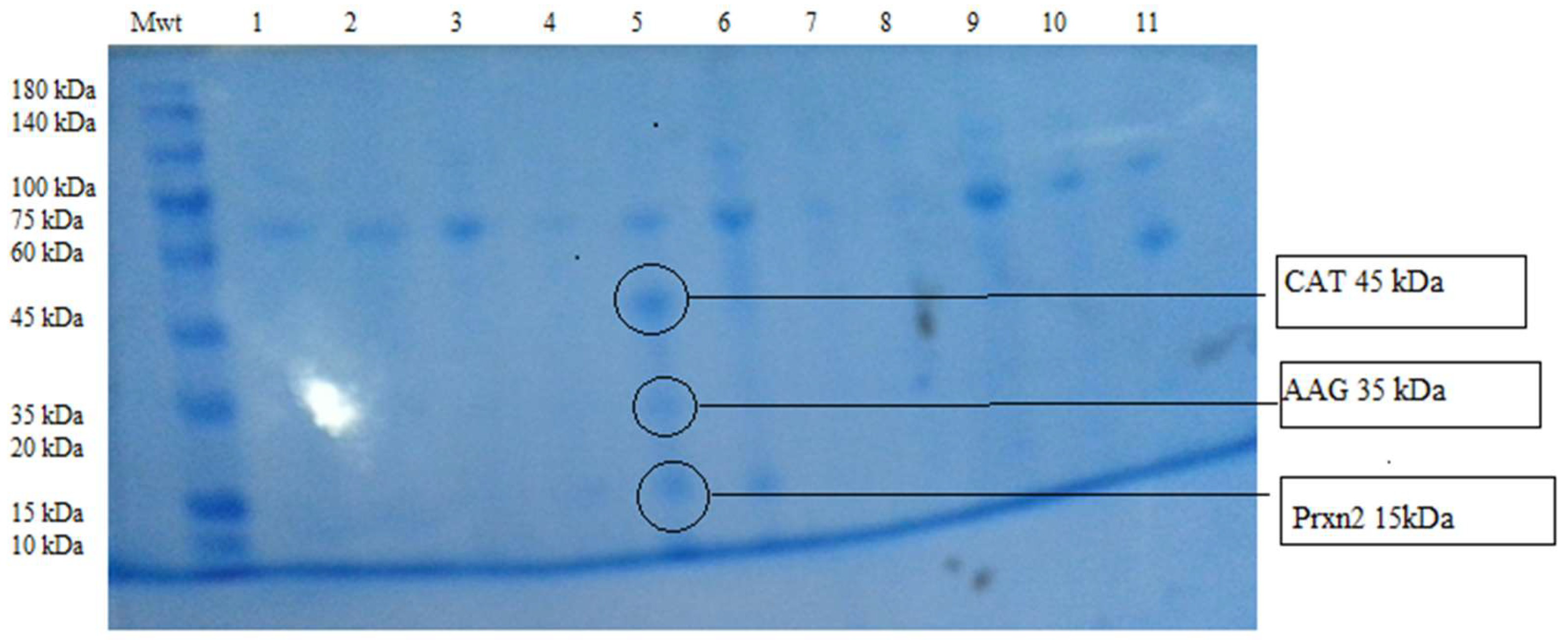
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 9 July 2020).
- Abuidris, D.O.; Weng, H.Y.; Elhaj, A.M.; Eltayeb, E.A.; Elsanousi, M.; Ibnoof, R.S.; Mohammed, S.I. Incidence and survival rates of ovarian cancer in low-income women in Sudan. Mol. Clin. Oncol. 2016, 5, 823–828. [Google Scholar] [CrossRef]
- Badgwell, D.; Bast, R.C. Early detection of ovarian cancer. Dis. Markers 2007, 23, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Holalkere, N.S.; Katur, A.M.; Lee, S.I. Issues in imaging malignant neoplasms of the female reproductive system. Curr. Probl. Diagn. Radiol. 2009, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Clarke-Pearson, D.L. Screening for Ovarian Cancer. N. Engl. J. Med. 2009, 361, 170–177. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for ovarian cancer: U.S. Preventive services task force reaffirmation recommendation statement. Ann. Intern. Med. 2012, 157, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.A.A.; Wicklund, K.G.; Cushing-Haugen, K.L.; Weiss, N.S. Predictive value of symptoms for early detection of ovarian cancer. J. Natl. Cancer Inst. 2010, 102, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liu, H.; Dong, Z.; Feng, Y.; Zhang, X.; Tian, Y. Searching for Potential Ovarian Cancer Biomarkers with Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Am. J. Biomed. Sci. 2009, 1, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S.; Taguchi, A. Application of proteomics to cancer early detection. Cancer J. 2011, 17, 423–428. [Google Scholar] [CrossRef]
- Sarojini, S.; Tamir, A.; Lim, H.; Li, S.; Zhang, S.; Goy, A.; Suh, K.S. Early detection biomarkers for ovarian cancer. J. Oncol. 2012. [Google Scholar] [CrossRef]
- Belczacka, I.; Latosinska, A.; Metzger, J.; Marx, D.; Vlahou, A.; Mischak, H.; Frantzi, M. Proteomics biomarkers for solid tumors: Current status and future prospects. Mass Spectrom. Rev. 2018, 38, 49–78. [Google Scholar] [CrossRef]
- Lee, C.; Im, E.; Moon, P.; Baek, M. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jing, Y.; Xu, H. Mining for missed sORF-encoded peptides. Expert Rev. Proteom. 2018, 16, 257–266. [Google Scholar] [CrossRef]
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 9 July 2020).
- The European Bioinformatics Institute (EMBL-EBI). Available online: https://www.ebi.ac.uk/ (accessed on 9 July 2020).
- Libbing, C.L.; Versagli, C.A. Catalase mediates the survival of anchorage-independent ovarian cancer cells [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Rodríguez, R.B.; Martínez-Cordero, E.; Santiago, J. The Relevance of Alpha-1-Acid Glycoprotein in Human Cancer: A Mini-review. Adv. Can. Res. Clin. Imaging 2019, 2. [Google Scholar] [CrossRef]
- Nicolussi, A.; D’Inzeo, S.; Capalbo, C.; Giannini, G.; Coppa, A. The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 2017, 6, 139–153. [Google Scholar] [CrossRef]
- McLemore, M.R.; Miaskowski, C.; Aouizerat, B.E.; Chen, L.M.; Dodd, M.J. Epidemiological and genetic factors associated with ovarian cancer. Cancer Nurs. 2009, 32, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, C.R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S.T.; Austoker, J. Identifying symptoms of ovarian cancer: A qualitative and quantitative study. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.G.; Yang, G.; Liu, G.; Mercado-Uribe, I.; Chang, B.; Xiao, X.S. Ovarian cancer: pathology, biology, and disease models. Front. Biosci. 2009, 14, 2089–2102. [Google Scholar] [CrossRef]
- Tanvir, I.; Riaz, S.; Hussain, A.; Mehboob, R.; Shams, M.; Khan, H. Hospital-based study of epithelial malignancies of endometrial cancer frequency in Lahore, Pakistan, and common diagnostic pitfalls. Pathol. Res. Int. 2014, 2014, 5. [Google Scholar] [CrossRef]
- Whitwell, H.J.; Worthington, J.; Blyuss, O.; Gentry-Maharaj, A.; Ryan, A.; Gunu, R.; Timms, J.F. Improved early detection of ovarian cancer using longitudinal multimarker models. Br. J. Cancer 2020, 122, 847–856. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Bast, R.C., Jr. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef]
- Smith, C.R.; Batruch, J.; Bauça, J.M.; Kosanam, H.; Ridley, J.; Bernardini, M.Q.; Leung, F.; Diamandis, E.P.; Kulasingam, V. Deciphering the peptidome of urine from ovarian cancer patients and healthy controls. Clin. Proteomics 2014, 11, 1–10. [Google Scholar] [CrossRef]
| Study Groups: | Age (yrs ± SD) | Presentation | Disease Stage | |||||
|---|---|---|---|---|---|---|---|---|
| Abdominal | Pelvic | Vaginal | Stage I/II | Stage III/IV | ||||
| Discomfort | pain | bleeding | ||||||
| Apparently healthy | 46.5 ± 28 | Nil | Nil | Nil | ||||
| (n=200) | ||||||||
| Study patients | 42.5 ± 23 | >90% | >90% | 1% | 34/112 | 78/112 | ||
| (n = 112) | −30.40% | −69.40% | ||||||
| Urinary micro-peptides: | +ve | +ve | ||||||
| (Patients with urinary peptides = 70) | 10%/25.7% | 24.3%/40% | ||||||
| Patients with Urinary peptides and C125 reactivity: | 6/26 (23.2%) | |||||||
| Histological Types: | Sero adenocarcinoma | mucinous adenocarcinoma | other types | |||||
| 91/112(81.2%) | 11/112(9.8%) | 10/122(9.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgan, S.S.; Abd Elaziz, F.J.; Nasr, A.M.A.; Elfaki, M.E.E.; Khalil, E.A.G. Ovarian Cancer: Tumor-Specific Urinary Micro-Peptides Profiling as Potential Biomarkers for Early Diagnosis. Proteomes 2020, 8, 32. https://doi.org/10.3390/proteomes8040032
Murgan SS, Abd Elaziz FJ, Nasr AMA, Elfaki MEE, Khalil EAG. Ovarian Cancer: Tumor-Specific Urinary Micro-Peptides Profiling as Potential Biomarkers for Early Diagnosis. Proteomes. 2020; 8(4):32. https://doi.org/10.3390/proteomes8040032
Chicago/Turabian StyleMurgan, Sulafa S., Faisal J. Abd Elaziz, Abubakr M. A. Nasr, Mona E. E. Elfaki, and Eltahir A. G. Khalil. 2020. "Ovarian Cancer: Tumor-Specific Urinary Micro-Peptides Profiling as Potential Biomarkers for Early Diagnosis" Proteomes 8, no. 4: 32. https://doi.org/10.3390/proteomes8040032
APA StyleMurgan, S. S., Abd Elaziz, F. J., Nasr, A. M. A., Elfaki, M. E. E., & Khalil, E. A. G. (2020). Ovarian Cancer: Tumor-Specific Urinary Micro-Peptides Profiling as Potential Biomarkers for Early Diagnosis. Proteomes, 8(4), 32. https://doi.org/10.3390/proteomes8040032





