Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
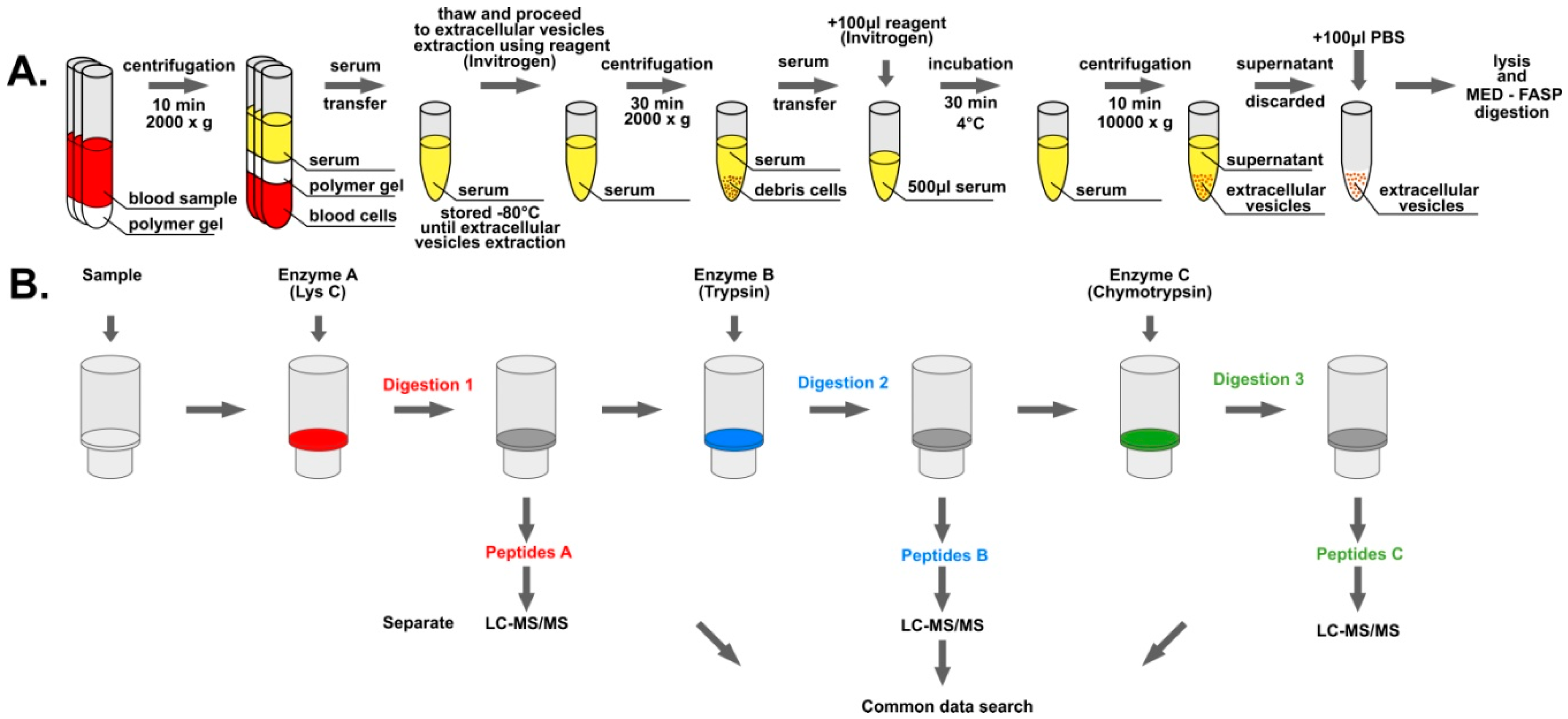
2.1. Blood Collection and Serum Isolation
2.2. Extracellular Vesicle Isolation
2.3. Protein Digestion
2.4. Mass spectra Acquisition and Data Analysis
3. Results
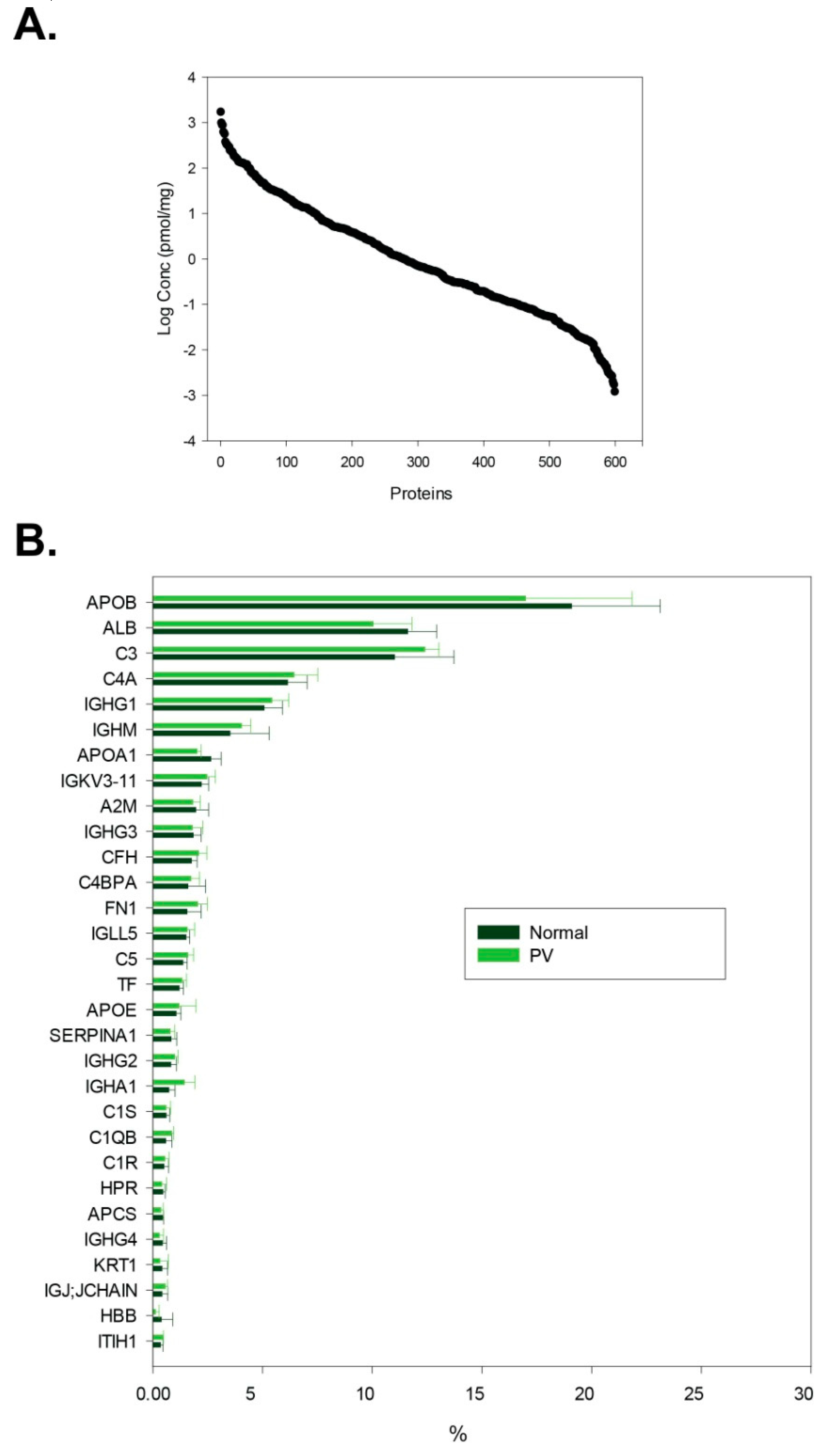
3.1. General Description of Isolated Extracellular Vesicle Material
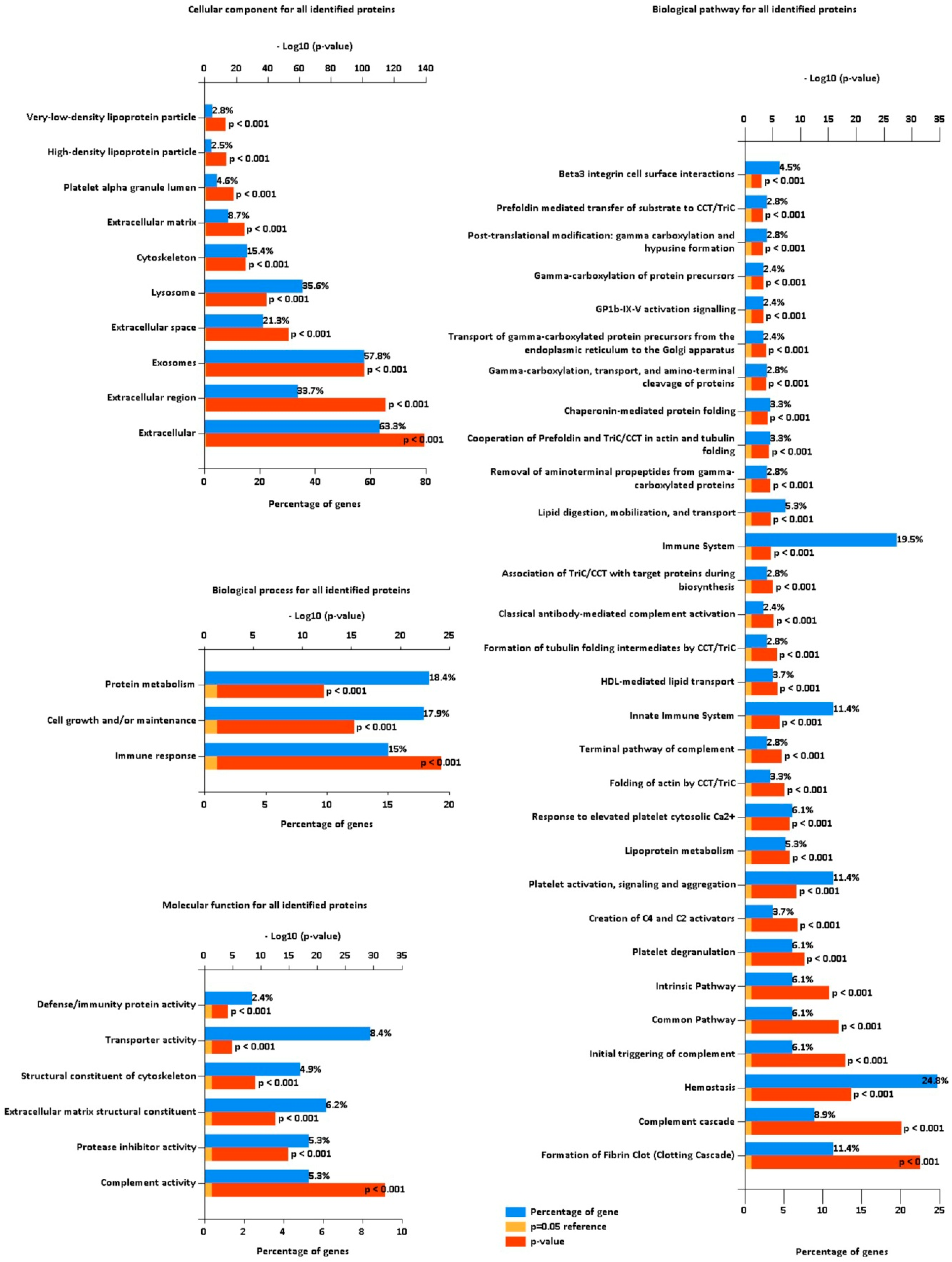
3.2. Functional Categorization of Identified Proteins
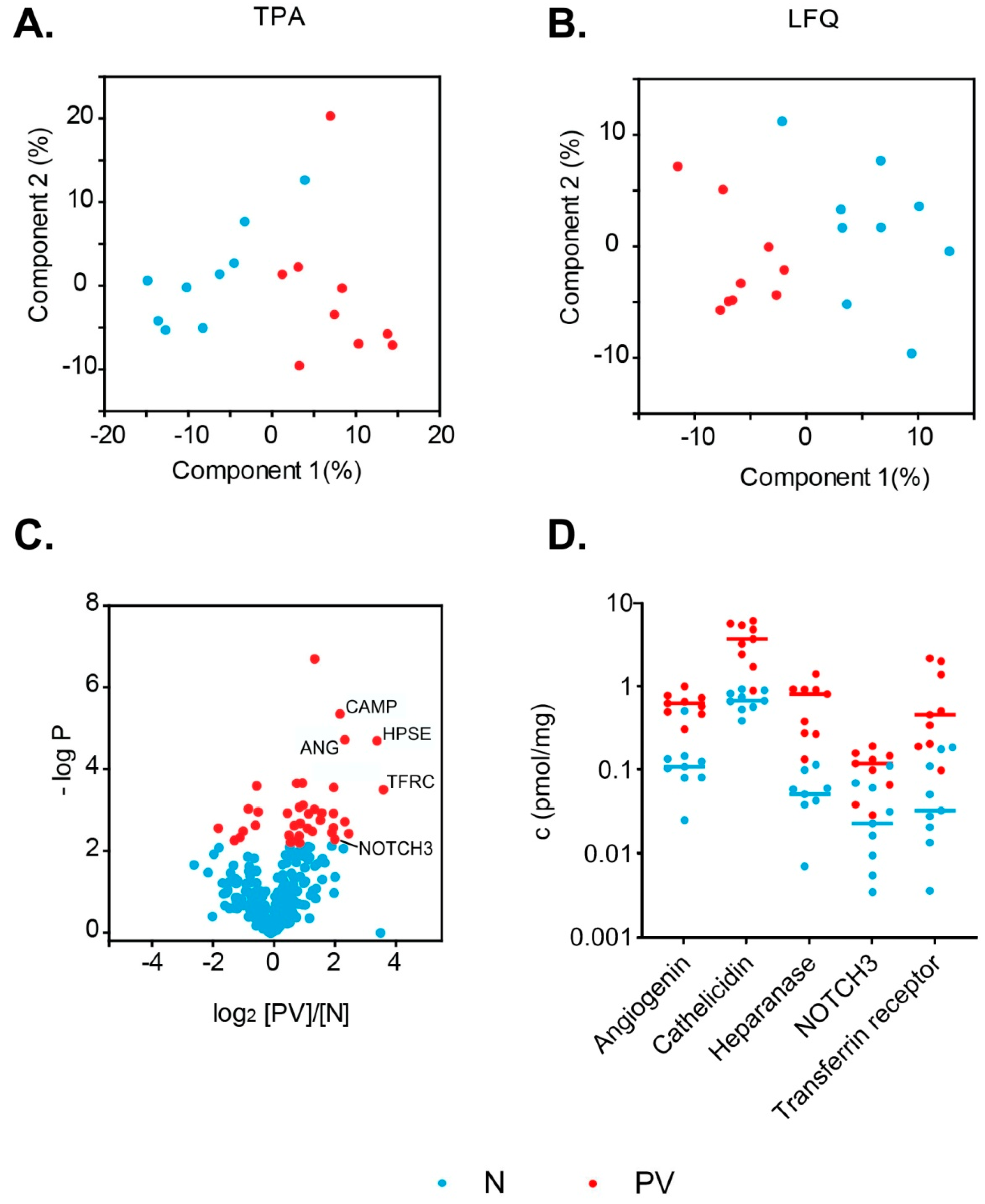
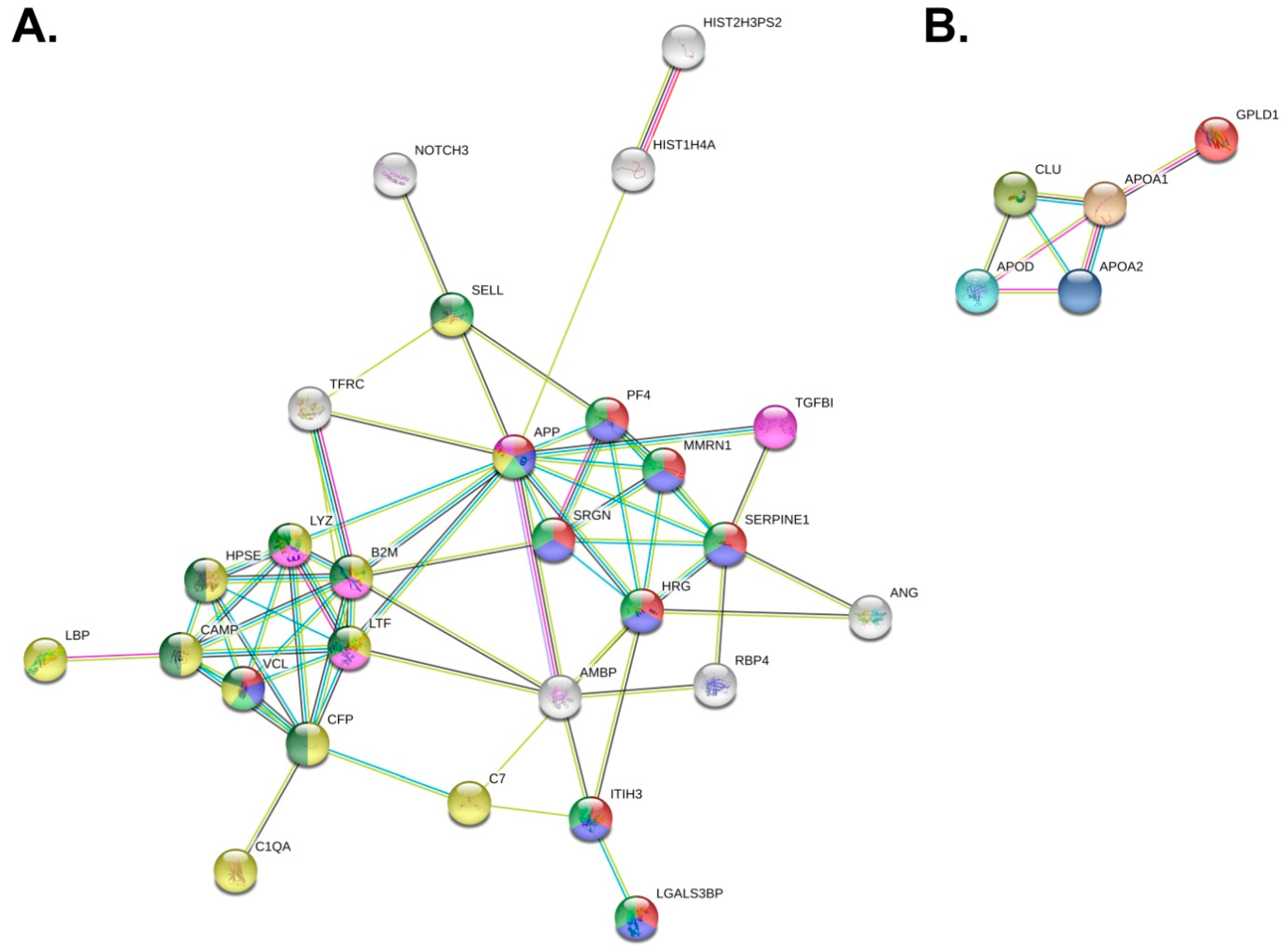
3.3. Differences in PV Patients’ Exosomal Proteomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Sirois, I.; Bell, C.; Hanafi, L.-A.; Hamelin, K.; Dieudé, M.; Rondeau, C.; Thibault, P.; Desjardins, M.; Hebert, M.-J. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 2013, 13, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Yu, H.; McKenzie, A.J.; Franklin, J.L.; Patton, J.G.; Liu, Q.; Weaver, A.M. Quantitative Proteomic Analysis of Small and Large Extracellular Vesicles (EVs) Reveals Enrichment of Adhesion Proteins in Small EVs. J. Proteome Res. 2019, 18, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gao, Y.; Zhang, L.; Chen, Y.; Ge, W.; Tang, P. Involvement of serum-derived exosomes of elderly patients with bone loss in failure of bone remodeling via alteration of exosomal bone-related proteins. Aging Cell 2018, 17, e12758. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015, 29, 281–290. [Google Scholar] [CrossRef]
- Turay, D.; Khan, S.; Diaz Osterman, C.J.; Curtis, M.P.; Khaira, B.; Neidigh, J.W.; Mirshahidi, S.; Casiano, C.A.; Wall, N.R. Proteomic Profiling of Serum-Derived Exosomes from Ethnically Diverse Prostate Cancer Patients. Cancer Invest. 2016, 34, 1–11. [Google Scholar] [CrossRef]
- Palazzolo, G.; Albanese, N.N.; DI Cara, G.; Gygax, D.; Vittorelli, M.L.; Pucci-Minafra, I. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res. 2012, 32, 847–860. [Google Scholar]
- An, M.; Lohse, I.; Tan, Z.; Zhu, J.; Wu, J.; Kurapati, H.; Morgan, M.A.; Lawrence, T.S.; Cuneo, K.C.; Lubman, D.M. Quantitative Proteomic Analysis of Serum Exosomes from Patients with Locally Advanced Pancreatic Cancer Undergoing Chemoradiotherapy. J. Proteome Res. 2017, 16, 1763–1772. [Google Scholar] [CrossRef]
- Wang, N.; Song, X.; Liu, L.; Niu, L.; Wang, X.; Song, X.; Xie, L. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. 2018, 109, 1701–1709. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, S.; Sun, K.; Chng, W.-J. The emerging roles of exosomes in leukemogeneis. Oncotarget 2016, 7, 50698–50707. [Google Scholar] [CrossRef] [PubMed]
- Ahadon, M.; Abdul Aziz, S.; Wong, C.L.; Leong, C.F. Plasma-derived microparticles in polycythaemia vera. Malays. J. Pathol. 2018, 40, 41–48. [Google Scholar] [PubMed]
- Taniguchi, Y.; Tanaka, H.; Luis, E.J.; Sakai, K.; Kumode, T.; Sano, K.; Serizawa, K.; Rai, S.; Morita, Y.; Hanamoto, H.; et al. Elevated plasma levels of procoagulant microparticles are a novel risk factor for thrombosis in patients with myeloproliferative neoplasms. Int. J. Hematol. 2017, 106, 691–703. [Google Scholar] [CrossRef]
- Tan, X.; Shi, J.; Fu, Y.; Gao, C.; Yang, X.; Li, J.; Wang, W.; Hou, J.; Li, H.; Zhou, J. Role of erythrocytes and platelets in the hypercoagulable status in polycythemia vera through phosphatidylserine exposure and microparticle generation. Thromb. Haemost. 2013, 109, 1025–1032. [Google Scholar] [PubMed]
- Siegel, F.P.; Petrides, P.E. Congenital and Acquired Polycythemias. Dtsch. Ärztebl. Int. 2008, 105, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Pardanani, A.; Tefferi, A.; Gilliland, D.G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat. Rev. Cancer 2007, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef]
- Socoro-Yuste, N.; Čokić, V.P.; Mondet, J.; Plo, I.; Mossuz, P. Quantitative Proteome Heterogeneity in Myeloproliferative Neoplasm Subtypes and Association with JAK2 Mutation Status. Mol. Cancer Res. 2017, 15, 852–861. [Google Scholar] [CrossRef]
- Mambet, C.; Necula, L.; Mihai, S.; Matei, L.; Bleotu, C.; Chivu-Economescu, M.; Stanca, O.; Tatic, A.; Berbec, N.; Tanase, C.; et al. Increased Dkk-1 plasma levels may discriminate disease subtypes in myeloproliferative neoplasms. J. Cell. Mol. Med. 2018, 22, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Michaelis, L.C.; Verstovsek, S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015, 29, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R. Quantitative Evaluation of Filter Aided Sample Preparation (FASP) and Multienzyme Digestion FASP Protocols. Anal. Chem. 2016, 88, 5438–5443. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Rakus, D. Multi-enzyme digestion FASP and the ’Total Protein Approach’-based absolute quantification of the Escherichia coli proteome. J. Proteomics 2014, 109, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Gaugaz, F.Z. Fast and sensitive total protein and Peptide assays for proteomic analysis. Anal. Chem. 2015, 87, 4110–4116. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. MCP 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.-S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Lucas, D.A.; Hise, D.; Schaefer, C.F.; Xiao, Z.; Janini, G.M.; Buetow, K.H.; Issaq, H.J.; Veenstra, T.D.; Conrads, T.P. Analysis of the human serum proteome. Clin. Proteomics 2004, 1, 101–225. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Spivak, J.L.; Considine, M.; Williams, D.M.; Talbot, C.C.; Rogers, O.; Moliterno, A.R.; Jie, C.; Ochs, M.F. Two Clinical Phenotypes in Polycythemia Vera. N. Engl. J. Med. 2014, 371, 808–817. [Google Scholar] [CrossRef]
- Niemann, C.U.; Kjeldsen, L.; Ralfkiaer, E.; Jensen, M.K.; Borregaard, N. Serglycin proteoglycan in hematologic malignancies: A marker of acute myeloid leukemia. Leukemia 2007, 21, 2406–2410. [Google Scholar] [CrossRef]
- Wickenhauser, C.; Thiele, J.; Schmitz, B.; Frimpong, S.; Neumann, I.; Schramm, K.; Zankovich, R.; Fischer, R. Polycythemia vera megakaryocytes store and release lysozyme to a higher extent than megakaryocytes in secondary polycythemia (polyglobuly). Leuk. Res. 1999, 23, 299–306. [Google Scholar] [CrossRef]
- Malmquist, J. Serum Lactoferrin in Leukaemia and Polycythaemia vera. Scand. J. Haematol. 1972, 9, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Norfolk, D.R.; Child, J.A.; Roberts, B.E.; Forbes, M.A.; Cooper, E.H. Serum beta-2-microglobulin in disorders of myeloid proliferation. Acta Haematol. 1983, 69, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kogan, I.; Chap, D.; Hoffman, R.; Axelman, E.; Brenner, B.; Nadir, Y. JAK-2 V617F mutation increases heparanase procoagulant activity. Thromb. Haemost. 2016, 115, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, K.A.; Hu, G.-F. Mechanism and Function of Angiogenin in Hematopoietic Malignancy. Zhongguo Sheng Wu Hua Xue Yu Fen Zi Sheng Wu Xue Bao Chin. J. Biochem. Mol. Biol. 2015, 31, 1267–1275. [Google Scholar]
- Purow, B. NOTCH inhibition as a promising new approach to cancer therapy. Adv. Exp. Med. Biol. 2012, 727, 305–319. [Google Scholar]
- Joutel, A.; Andreux, F.; Gaulis, S.; Domenga, V.; Cecillon, M.; Battail, N.; Piga, N.; Chapon, F.; Godfrain, C.; Tournier-Lasserve, E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J. Clin. Investig. 2000, 105, 597–605. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; A B da Cruz E Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Fujita, H.; Hamaki, T.; Handa, N.; Ohwada, A.; Tomiyama, J.; Nishimura, S. Hypocholesterolemia in patients with polycythemia vera. J. Clin. Exp. Hematop. JCEH 2012, 52, 85–89. [Google Scholar] [CrossRef][Green Version]
| Uniprot Accession | Gene Name | Protein Name | Median Concentration [pmol/mg] | |
|---|---|---|---|---|
| Controls (N) | Patients (PV) | |||
| Transmembrane or GPI-anchored proteins associated to plasma membrane and/or endosomes 1 | ||||
| a)Non-tissue specific | ||||
| P08514 | ITGA2B | Integrin alpha-IIb (CD41) | 1.03 × 10−1 | 7.57 × 10−2 |
| P05106 | ITGB3 | Integrin beta-3 (CD61) | 5.46 × 10−2 | 8.54 × 10−2 |
| b)Tissue-specific | ||||
| P05067 | APP | Amyloid-beta precursor protein | 5.29 × 10−1 | 1.35 |
| P08571 | CD14 | Monocyte differentiation antigen CD14 | 3.14 × 10−1 | 4.96 × 10−1 |
| P14770 | GP9 | Platelet glycoprotein IX (CD42a) | 8.09 × 10−2 | 5.77 × 10−2 |
| P08514 | ITGA2B | Integrin alpha-IIb (CD41) | 1.03 × 10−1 | 7.57 × 10−2 |
| Cytosolic proteins recovered in EVs 1 | ||||
| a)With lipid or membrane protein-binding ability | ||||
| P04083 | ANXA1 | Annexin A1 | Not quantifiable | Not quantifiable |
| P61586 | RHOA | Transforming protein RhoA | Not quantifiable | Not quantifiable |
| b)Promiscous incorporation in EVs | ||||
| P60709 | ACTB | Actin, cytoplasmic 1 | 3.38 | 3.84 |
| P63267 | ACTG2 | Actin, gamma-enteric smooth muscle | 2.68 × 10−1 | 5.59 × 10−1 |
| P04406 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 1.84 × 10−1 | 2.83 × 10−1 |
| P68366 | TUBA4A | Tubulin alpha-4A chain | 9.78 × 10−2 | 5.54 × 10−2 |
| P07437 | TUBB | Tubulin beta chain | 1.93 × 10−2 | Not quantifiable |
| Q9H4B7 | TUBB1 | Tubulin beta-1 chain | 2.75 × 10−2 | 3.77 × 10−3 |
| Major components of non-EV co-isolated structures (abundant in plasma, serum) 1 | ||||
| P02768 | ALB | Serum albumin | 1.67 × 103 | 1.45 × 103 |
| P02647 | APOA1 | Apolipoprotein A-I | 8.57 × 102 | 6.53 × 102 |
| P02652 | APOA2 | Apolipoprotein A-II | 1.77 × 102 | 1.26 × 102 |
| P04114 | APOB | Apolipoprotein B-100 | 3.70 × 102 | 3.29 × 102 |
| Transmembrane, lipid-bound and soluble proteins associated to other intracellular compartments than PM/endosomes 1 | ||||
| a)Nucleus | ||||
| P33778 | HIST1H2BB | Histone H2B type 1-B | 8.83 × 10−2 | 2.73 × 10−1 |
| P62805 | HIST1H4A | Histone H4 | 2.98 × 10−1 | 2.83 |
| b)Secretory pathway (endoplasmic reticulum, Golgi apparatus) | ||||
| P11021 | HSPA5 | Endoplasmic reticulum chaperone BiP | 4.86 × 10−1 | 7.16 × 10−1 |
| c)Others (autophagosomes, cytoskeleton, ...) | ||||
| P12814 | ACTN1 | Alpha-actinin-1 | 1.89 × 10−1 | 2.97 × 10−1 |
| Secreted proteins recovered with EVs 1 | ||||
| a)Cytokines and growth factors | ||||
| Q9GZP0 | PDGFD | Platelet-derived growth factor D | Not quantifiable | 8.34 × 10−3 |
| P01137 | TGFB1 | Transforming growth factor beta-1 proprotein | 3.47 × 10−1 | 1.01 |
| b)Adhesion and extracellular matrix proteins | ||||
| P02765 | AHSG | Alpha-2-HS-glycoprotein | 1.39 × 101 | 1.07 × 101 |
| O43866 | CD5L | CD5 antigen-like | 7.59 × 101 | 6.59 × 101 |
| Q99715 | COL12A1 | Collagen alpha-1(XII) chain | 9.68 × 10−3 | 1.03 × 10−2 |
| P39060 | COL18A1 | Collagen alpha-1(XVIII) chain | 1.33 × 10−1 | 1.04 × 10−1 |
| P12109 | COL6A1 | Collagen alpha-1(VI) chain | 4.09 × 10−2 | 2.25 × 10−2 |
| P12111 | COL6A3 | Collagen alpha-3(VI) chain | 8.48 × 10−2 | 1.37 × 10−1 |
| P02751 | FN1 | Fibronectin | 5.91 × 101 | 7.78 × 101 |
| Q08380 | LGALS3BP | Galectin-3-binding protein | 5.23 | 1.07 × 101 |
| Protein | Gene | p Value | [PV]/[N] 1 | Peptides | Conc. (pmol/mg) | Fraction of Total Protein% | ||
|---|---|---|---|---|---|---|---|---|
| N | PV | N | PV | |||||
| Transferrin receptor protein 1 | TFRC | 3.30 × 10−4 | 13 | 19 | 0.03 | 0.46 | 2.80 × 10−4 | 3.91 × 10−3 |
| Heparanase | HPSE | 2.10 × 10−5 | 11 | 11 | 0.05 | 0.82 | 3.18 × 10−4 | 5.00 × 10−3 |
| Plasminogen activator inhibitor 1 | SERPINE1 | 3.90 × 10−3 | 5.9 | 6 | 0.05 | 0.37 | 2.39 × 10−4 | 1.66 × 10−3 |
| Angiogenin | ANG | 2.00 × 10−5 | 5.4 | 3 | 0.11 | 0.63 | 1.83 × 10−4 | 1.04 × 10−3 |
| Histone H4 | HIST1H4A | 2.00 × 10−3 | 5.4 | 5 | 0.3 | 2.83 | 3.38 × 10−4 | 3.21 × 10−3 |
| Cathelicidin antimicrobial peptide | CAMP | 4.60 × 10−6 | 4.9 | 5 | 0.68 | 3.73 | 1.31 × 10−3 | 7.20 × 10−3 |
| Neurogenic locus notch homolog protein 3 | NOTCH3 | 5.30 × 10−3 | 4.3 | 8 | 0.02 | 0.12 | 5.62 × 10−4 | 2.93 × 10−3 |
| Lysozyme C | LYZ | 2.90 × 10−4 | 4.2 | 8 | 0.82 | 2.44 | 1.35 × 10−3 | 4.03 × 10−3 |
| Histone H3 | HIST2H3PS2 | 1.20 × 10−3 | 4.2 | 4 | 0.28 | 1.27 | 4.38 × 10−4 | 1.96 × 10−3 |
| L-selectin | SELL | 2.80 × 10−3 | 4.2 | 4 | 0.05 | 0.21 | 2.31 × 10−4 | 8.66 × 10−4 |
| Lactotransferrin | LTF | 3.70 × 10−3 | 4 | 23 | 0.27 | 1.09 | 2.10 × 10−3 | 8.54 × 10−3 |
| Vinculin | VCL | 1.20 × 10−3 | 3.2 | 13 | 0.08 | 0.2 | 9.44 × 10−4 | 2.43 × 10−3 |
| Multimerin-1 | MMRN1 | 1.80 × 10−3 | 3.1 | 32 | 0.39 | 1 | 5.35 × 10−3 | 1.38 × 10−2 |
| Beta-2-microglobulin | B2M | 2.10 × 10−7 | 2.7 | 6 | 2.52 | 6.83 | 3.46 × 10−3 | 9.37 × 10−3 |
| Nidogen-2 | NID2 | 9.80 × 10−3 | 2.7 | 6 | 0.02 | 0.04 | 2.57 × 10−4 | 6.52 × 10−4 |
| Amyloid beta A4 protein | APP | 3.40 × 10−4 | 2.6 | 12 | 0.53 | 1.35 | 4.60 × 10−3 | 1.18 × 10−2 |
| Serglycin | SRGN | 1.30 × 10−3 | 2.4 | 5 | 1.51 | 3.25 | 2.66 × 10−3 | 5.73 × 10−3 |
| Platelet glycoprotein V | GP5 | 2.90 × 10−3 | 2.3 | 11 | 0.18 | 0.57 | 1.11 × 10−3 | 3.49 × 10−3 |
| Retinol-binding protein 4 | RBP4 | 2.30 × 10−4 | 2.1 | 13 | 4.65 | 9.59 | 1.07 × 10−2 | 2.21 × 10−2 |
| Lipopolysaccharide-binding protein | LBP | 7.80 × 10−4 | 2.1 | 12 | 1.44 | 3.32 | 7.69 × 10−3 | 1.77 × 10−2 |
| TGFβ-induced protein ig-h3 | TGFBI | 2.20 × 10−3 | 2 | 12 | 0.19 | 0.41 | 1.41 × 10−3 | 3.07 × 10−3 |
| Properdin | CFP | 8.80 × 10−4 | 1.9 | 19 | 6.74 | 12.83 | 3.45 × 10−2 | 6.58 × 10−2 |
| Galectin-3-binding protein | LGALS3BP | 4.10 × 10−3 | 1.9 | 27 | 5.23 | 10.74 | 3.42 × 10−2 | 7.02 × 10−2 |
| Inter-alpha-trypsin inhibitor heavy chain H3 | ITIH3 | 6.10 × 10−3 | 1.9 | 29 | 1.53 | 3.15 | 1.53 × 10−2 | 3.15 × 10−2 |
| Alpha-1-microglobulin | AMBP | 2.2 × 10−4 | 1.8 | 17 | 7.75 | 14.69 | 3.02 × 10−2 | 5.73 × 10−2 |
| Platelet factor 4 | PF4 | 5.60 × 10−3 | 1.8 | 7 | 124.41 | 288.88 | 1.35 × 10−1 | 3.13 × 10−1 |
| Complement component C7 | C7 | 2.50 × 10−3 | 1.7 | 47 | 11.11 | 21.7 | 1.04 × 10−1 | 2.03 × 10−1 |
| Complement C1q subcomponent subunit A | C1QA | 1.20 × 10−3 | 1.5 | 18 | 72.33 | 114.86 | 1.88 × 10−1 | 2.99 × 10−1 |
| Histidine-rich glycoprotein | HRG | 4.00 × 10−3 | 1.5 | 22 | 18.28 | 31.2 | 1.09 × 10−1 | 1.86 × 10−1 |
| N-acetylmuramoyl-L-alanine amidase | PGLYRP2 | 5.90 × 10−3 | 1.5 | 17 | 2.24 | 3.37 | 1.39 × 10−2 | 2.10 × 10−2 |
| Clusterin | CLU | 1.10 × 10−3 | 0.75 | 19 | 31.13 | 23.19 | 1.63 × 10−1 | 1.22 × 10−1 |
| Apolipoprotein A-I | APOA1 | 2.70 × 10−3 | 0.73 | 41 | 857.47 | 652.78 | 2.64 | 2.01 |
| Apolipoprotein A-II | APOA2 | 2.40 × 10−3 | 0.71 | 11 | 177.37 | 126.08 | 1.98 × 10−1 | 1.41 × 10−1 |
| Apolipoprotein D | APOD | 9.60 × 10−4 | 0.6 | 13 | 61.85 | 37.16 | 1.49 × 10−1 | 8.98 × 10−2 |
| Fibulin-1 | FBLN1 | 3.40 × 10−3 | 0.54 | 20 | 3.01 | 1.63 | 2.24 × 10−2 | 1.21 × 10−2 |
| Phosphatidylinositol-glycan-specific phospholipase D | GPLD1 | 4.60 × 10−3 | 0.5 | 9 | 0.19 | 0.11 | 1.74 × 10−3 | 1.04 × 10−3 |
| Peroxiredoxin-6 | PRDX6 | 5.50 × 10−3 | 0.44 | 4 | 4.45 | 2.93 | 1.11 × 10−2 | 7.35 × 10−3 |
| Salivary acidic proline-rich phosphoprotein 1/2 | PRH1 | 2.90 × 10−3 | 0.3 | 3 | 0.19 | 0.06 | 3.23 × 10−4 | 1.01 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fel, A.; Lewandowska, A.E.; Petrides, P.E.; Wiśniewski, J.R. Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls. Proteomes 2019, 7, 20. https://doi.org/10.3390/proteomes7020020
Fel A, Lewandowska AE, Petrides PE, Wiśniewski JR. Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls. Proteomes. 2019; 7(2):20. https://doi.org/10.3390/proteomes7020020
Chicago/Turabian StyleFel, Anna, Aleksandra E. Lewandowska, Petro E. Petrides, and Jacek R. Wiśniewski. 2019. "Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls" Proteomes 7, no. 2: 20. https://doi.org/10.3390/proteomes7020020
APA StyleFel, A., Lewandowska, A. E., Petrides, P. E., & Wiśniewski, J. R. (2019). Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls. Proteomes, 7(2), 20. https://doi.org/10.3390/proteomes7020020





