Phosphoproteomic Analysis of the Amygdala Response to Adolescent Glucocorticoid Exposure Reveals G-Protein Coupled Receptor Kinase 2 as a Target for Reducing Motivation for Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Drugs
2.3. Chronic Corticosterone Exposure
2.4. Operant Self-Administration
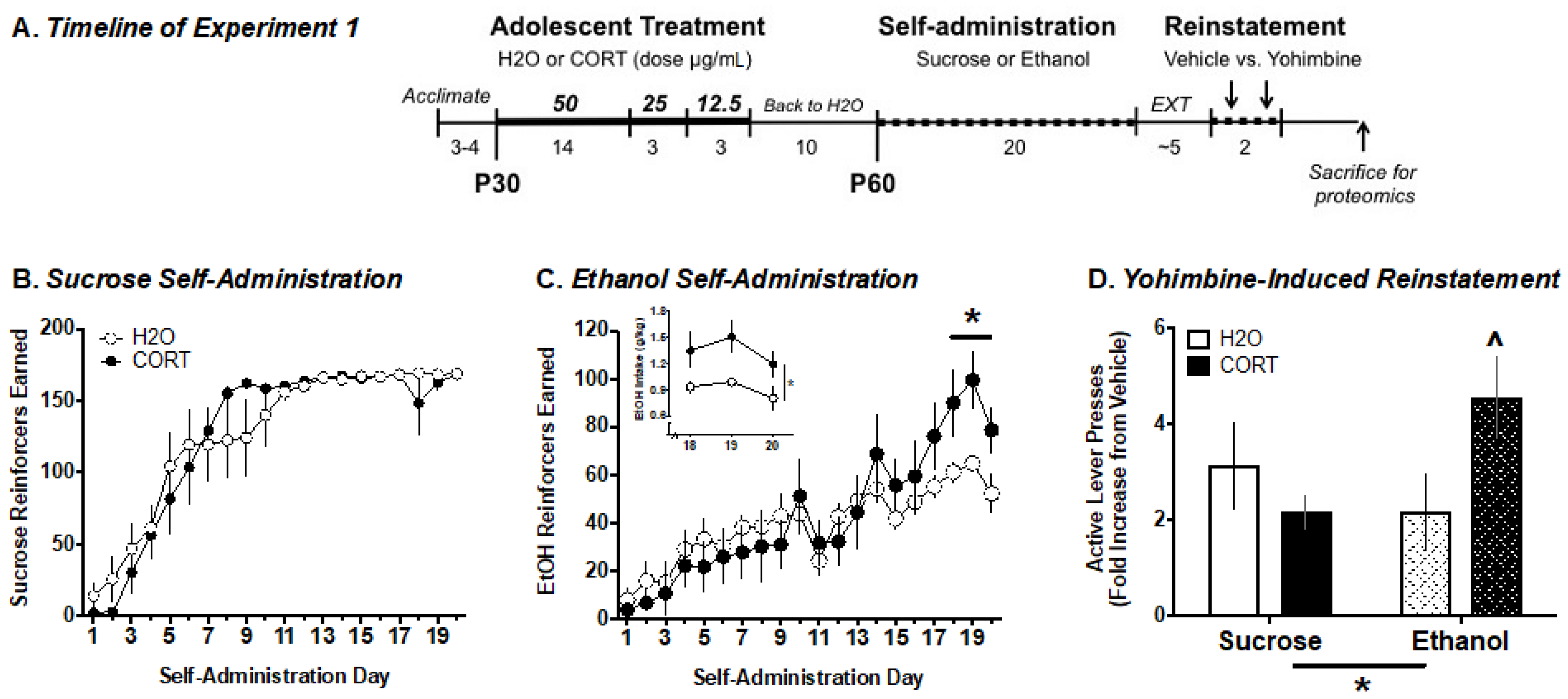
2.5. Experiment 1. Analysis of Adolescent CORT Effects on Adult Ethanol Self-Administration and the Amygdala Phosphoproteome
Quantitative Label-Free Phosphoproteomics
2.6. Experiment 2. Determining the Role of GRK2 in Regulating Ethanol-Motivated Behaviors
2.6.1. Surgery
2.6.2. Progressive Ratio (PR)
2.6.3. Extinction/Yohimbine-Induced Reinstatement of EtOH Seeking
2.7. Statistical Analysis
2.7.1. Behavioral Data
2.7.2. Proteomics Data
3. Results
3.1. Experiment 1. Analysis of Adolescent CORT Effects on Adult Ethanol Self-Administration and the Amygdala Phosphoproteome
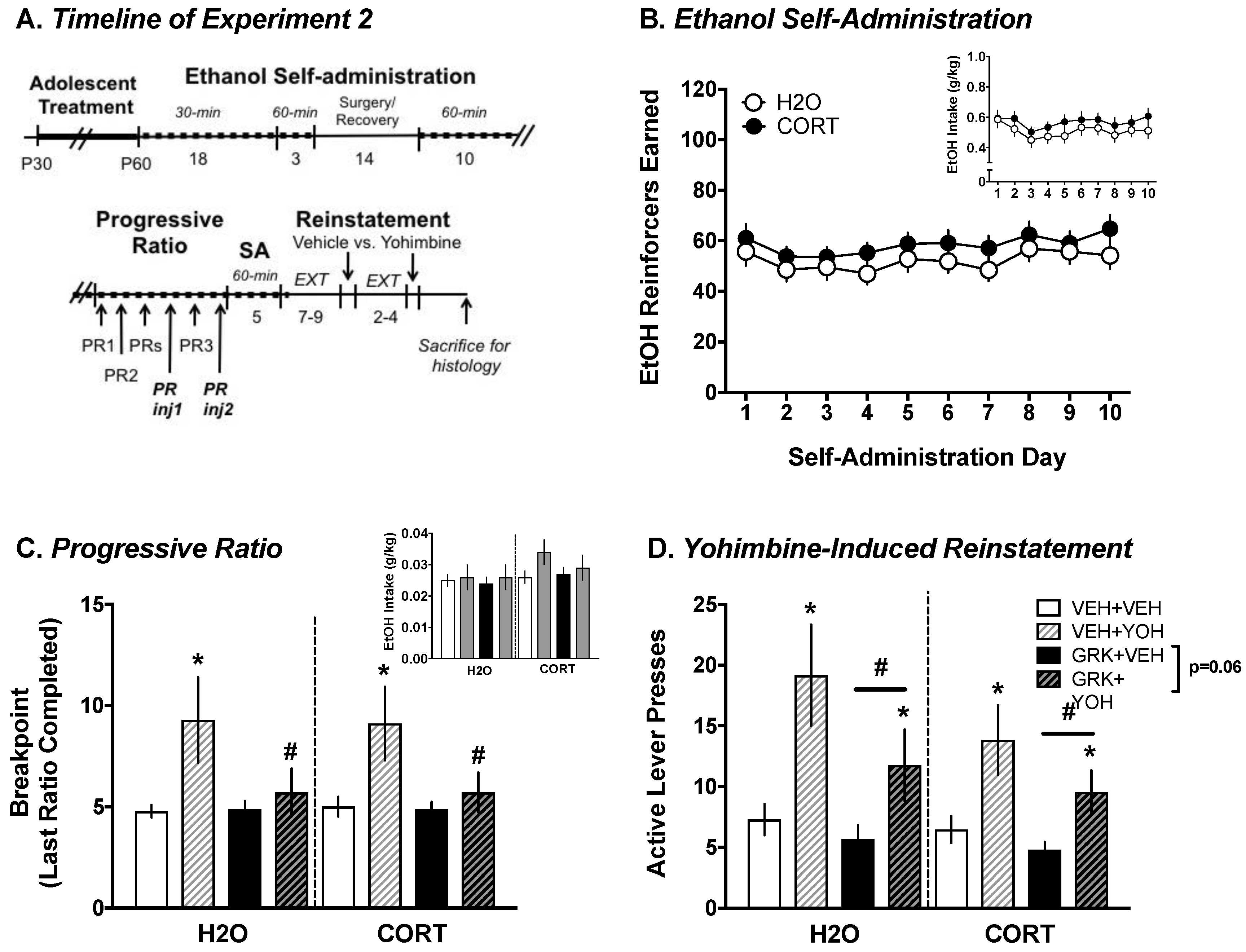
3.1.1. Ethanol Self-Administration
3.1.2. Yohimbine-Induced Reinstatement of EtOH vs. Sucrose Seeking
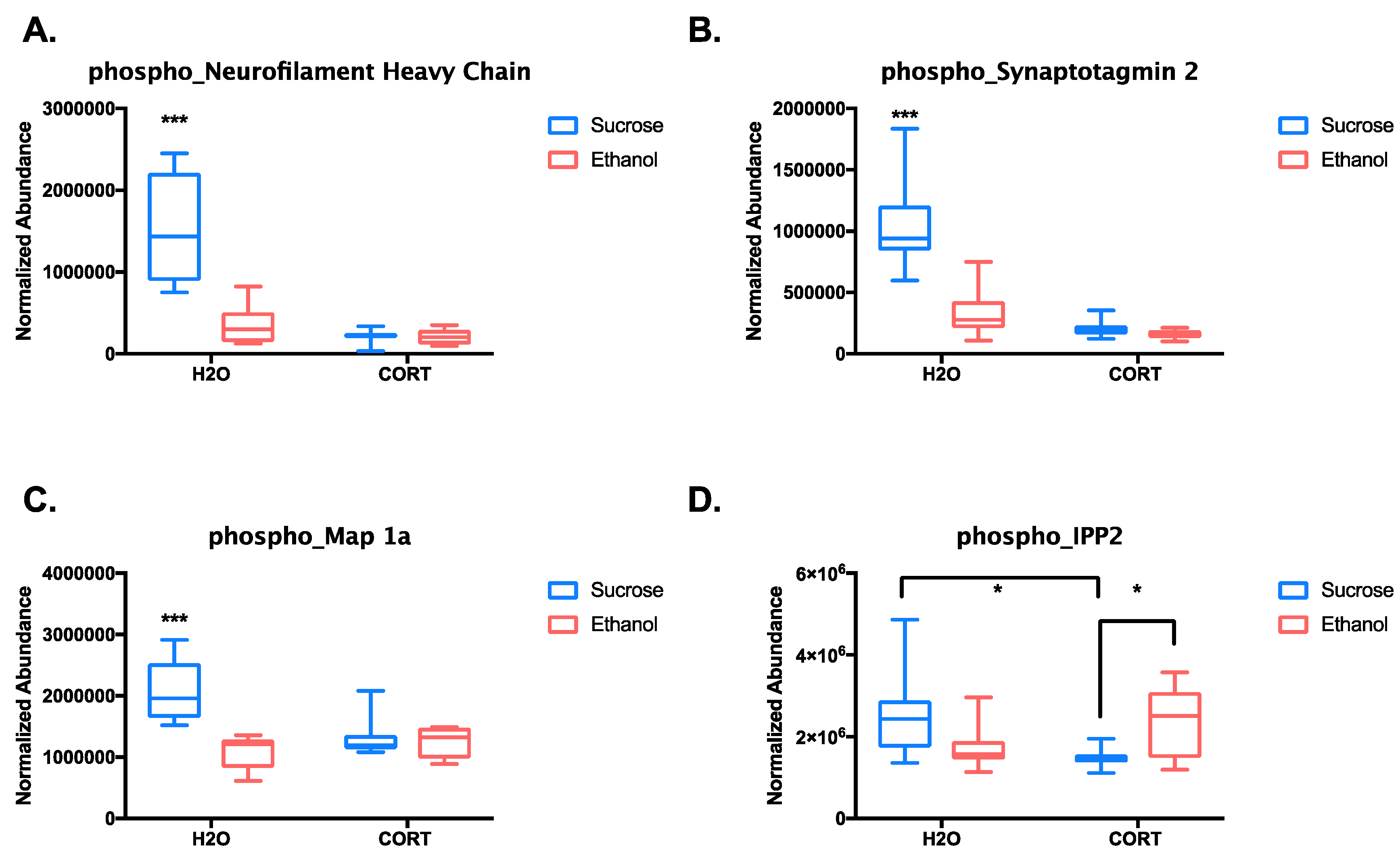
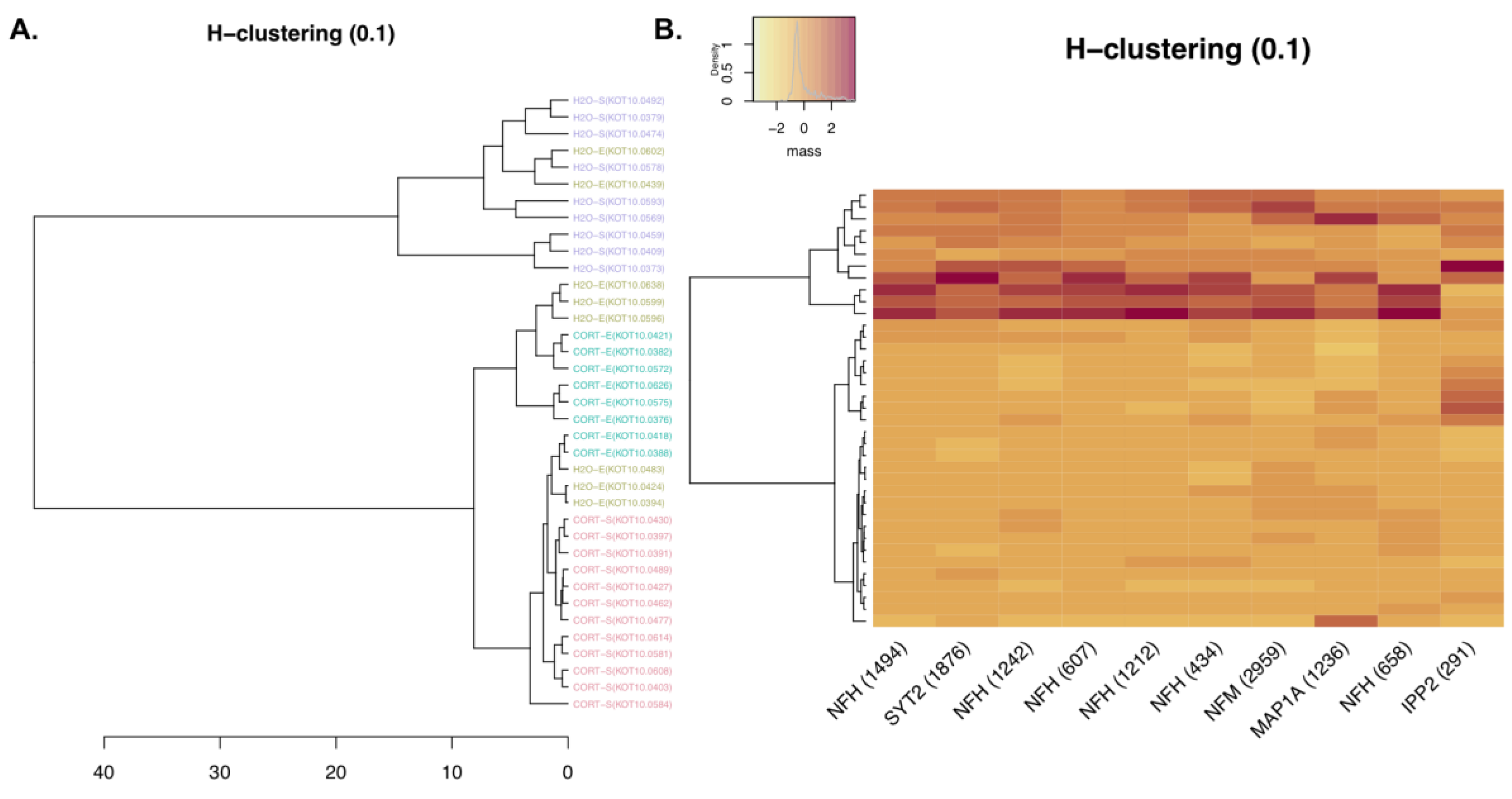
3.1.3. Phosphoproteomic Analysis
3.2. Experiment 2. Determining the Role of GRK2 in Regulating Ethanol Motivated Behaviors
3.2.1. Ethanol Self-Administration
3.2.2. Progressive Ratio Testing: Yohimbine vs. Vehicle
3.2.3. Yohimbine-Induced Reinstatement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Penza, K.M.; Heim, C.; Nemeroff, C.B. Neurobiological effects of childhood abuse: Implications for the pathophysiology of depression and anxiety. Arch. Womens. Ment. Health 2003, 6, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.-K.; Laucht, M.; Ridinger, M.; Wodarz, N.; Rietschel, M.; Maier, W.; Lathrop, M.; Lourdusamy, A.; Zimmermann, U.S.; Desrivieres, S.; et al. KCNJ6 is associated with adult alcohol dependence and involved in gene × early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology 2011, 36, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.; He, J.; Hodge, C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.R.; Miczek, K.A. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology 2014, 231, 1557–1580. [Google Scholar] [CrossRef] [PubMed]
- Eiland, L.; Romeo, R.D. Stress and the developing adolescent brain. Neuroscience 2013, 249, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pilowsky, D.J.; Keyes, K.M.; Hasin, D.S. Adverse childhood events and lifetime alcohol dependence. Am. J. Public Health 2009, 99, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.A.; Turner, R.J. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug Alcohol Depend. 2008, 93, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Wu, F.J.; Kiraly, D.D.; Ploski, J.E.; Kedves, A.T.; Duman, R.S.; Taylor, J.R. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry 2008, 63, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Taylor, J.R. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr. Protoc. Neurosci. 2009. Chapter 9. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.M.; Xie, M.; Taylor, J.R. Chronic Corticosterone Exposure during Adolescence Reduces Impulsive Action but Increases Impulsive Choice and Sensitivity to Yohimbine in Male Sprague-Dawley Rats. Neuropsychopharmacology 2012, 37, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.H. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav. Process. 2011, 87, 10–17. [Google Scholar] [CrossRef] [PubMed]
- McCool, B.A.; Chappell, A.M. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol. Clin. Exp. Res. 2009, 33, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Skelly, M.J.; Chappell, A.E.; Carter, E.; Weiner, J.L. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology 2015, 97, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Deehan, G.A.; Cain, M.E.; Kiefer, S.W. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol. Clin. Exp. Res. 2007, 31, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Bertholomey, M.L.; Nagarajan, V.; Torregrossa, M.M. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology 2016, 233, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Kedves, A.T.; Olausson, P.; Taylor, J.R. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 2009, 34, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hunsucker, S.W.; Solomon, B.; Gawryluk, J.; Geiger, J.D.; Vacano, G.N.; Duncan, M.W.; Patterson, D. Assessment of post-mortem-induced changes to the mouse brain proteome. J. Neurochem. 2008, 105, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.T.; Abbott, T.B.; Chung, L.; Gulcicek, E.E.; Stone, K.L.; Colangelo, C.M.; Lam, T.T.; Nairn, A.C.; Taylor, J.R.; Torregrossa, M.M. Phosphoproteomic analysis reveals a novel mechanism of CaMKIIα regulation inversely induced by cocaine memory extinction versus reconsolidation. J. Neurosci. 2016, 36, 7613–7627. [Google Scholar] [CrossRef] [PubMed]
- Eason, M.G.; Moreira, S.P.; Liggett, S.B. Four consecutive serines in the third intracellular loop are the sites for beta-adrenergic receptor kinase-mediated phosphorylation and desensitization of the α2A-adrenergic receptor. J. Biol. Chem. 1995, 270, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Bertholomey, M.L.; Verplaetse, T.L.; Czachowski, C.L. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behav. Brain Res. 2013, 238, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Feltenstein, M.W.; Henderson, A.R.; See, R.E. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: Sex differences and the role of the estrous cycle. Psychopharmacology 2011, 216, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Taus, T.; Kocher, T.; Pichler, P.; Paschke, C.; Schmidt, A.; Henrich, C.; Mechtler, K. Universal and confident phosphorylation site localization using PhosphoRS. J. Proteome Res. 2011, 10, 5354–5362. [Google Scholar] [CrossRef] [PubMed]
- Hirosawa, M.; Hoshida, M.; Ishikawa, M.; Toya, T. MASCOT: Multiple alignment system for protein sequences based on three-way dynamic programming. Comput. Appl. Biosci. 1993, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Akkus, F.; Mihov, Y.; Treyer, V.; Ametamey, S.M.; Johayem, A.; Senn, S.; Rösner, S.; Buck, A.; Hasler, G. Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl. Psychiatry 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr. Drug Abuse Rev. 2009, 2, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, D.K.; Courson, J.; Wroten, M.G.; Greentree, D.I.; Lum, E.N.; Campbell, R.R.; Thompson, A.B.; Maliniak, D.; Worley, P.F.; Jonquieres, G.; et al. Binge Alcohol Drinking by Mice Requires Intact Group1 Metabotropic Glutamate Receptor Signaling Within the Central Nucleus of the Amygdale. Neuropsychopharmacology 2014, 39, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Besheer, J.; Grondin, J.J.M.; Cannady, R.; Sharko, A.C.; Faccidomo, S.; Hodge, C.W. Metabotropic Glutamate Receptor 5 Activity in the Nucleus Accumbens Is Required for the Maintenance of Ethanol Self-Administration in a Rat Genetic Model of High Alcohol Intake. Biol. Psychiatry 2010, 67, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.M.; Cleva, R.M.; Hood, L.E.; Olive, M.F.; Gass, J.T. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol. Biochem. Behav. 2012, 101, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rosado, L.; Solan, J.L.; Dunn, C.A.; Norris, R.P.; Lampe, P.D. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim. Biophys. Acta 2012, 1818, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Reuss, L.; Altenberg, G.A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004, 279, 20058–20066. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Limbird, L.E. Regulation of alpha2AR trafficking and signaling by interacting proteins. Biochem. Pharmacol. 2007, 73, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, I.L.; Ralevski, E.; Desai, N.; Trevisan, L.; Gueorguieva, R.; Rounsaville, B.; Krystal, J.H. Noradrenergic vs. Serotonergic Antidepressant with or without Naltrexone for Veterans with PTSD and Comorbid Alcohol Dependence. Neuropsychopharmacology 2012, 37, 996–1004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haass-Koffler, C.L.; Swift, R.M.; Leggio, L. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology 2018, 235, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, D.D.; Alexander, L.; Malone, J.; Federoff, D.; Froehlich, J.C. The α2-adrenergic receptor agonist, clonidine, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol 2014, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Roeckner, A.R.; Bowling, A.; Butler, T.R. Chronic social instability increases anxiety-like behavior and ethanol preference in male Long Evans rats. Physiol. Behav. 2017, 173, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C.; Wahlsten, D.; Dudek, B.C. Genetics of mouse behavior: Interactions with laboratory environment. Science 1999, 284, 1670–1672. [Google Scholar] [CrossRef] [PubMed]
- Wahlsten, D.; Metten, P.; Phillips, T.J.; Boehm, S.L.; Burkhart-Kasch, S.; Dorow, J.; Doerksen, S.; Downing, C.; Fogarty, J.; Rodd-Henricks, K.; et al. Different data from different labs: Lessons from studies of gene-environment interaction. J. Neurobiol. 2003, 54, 283–311. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, M.L.; Bergdall, V.K.; Davis, I.C.; Hickman-Davis, J.M. Effect of Ventilated Caging on Water Intake and Loss in 4 Strains of Laboratory Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 525–533. [Google Scholar] [PubMed]
- Shan, L.; Schipper, P.; Nonkes, L.J.P.; Homberg, J.R. Impaired fear extinction as displayed by serotonin transporter knockout rats housed in open cages is disrupted by IVC cage housing. PLoS ONE 2014, 9, e91472. [Google Scholar] [CrossRef] [PubMed]
- Verplaetse, T.L.; Rasmussen, D.D.; Froehlich, J.C.; Czachowski, C.L. Effects of Prazosin, an α1-Adrenergic Receptor Antagonist, on the Seeking and Intake of Alcohol and Sucrose in Alcohol-Preferring (P) Rats. Alcohol. Clin. Exp. Res. 2012, 36, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Koob, G.F. Effects of β-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology 2010, 212, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sofuoglu, M.; Rosenheck, R.; Petrakis, I. Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addict. Behav. 2014, 39, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Weiner, J.L. Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes Brain Behav. 2017, 16, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-M.; Yuan, S.-N.; Guan, X.-T.; Xie, X.; Shao, F.; Wang, W.-W. Juvenile stress affects anxiety-like behavior and limbic monoamines in adult rats. Physiol. Behav. 2014, 135, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Comasco, E.; Todkar, A.; Granholm, L.; Nilsson, K.; Nylander, I. α2a-Adrenoceptor Gene Expression and Early Life Stress-Mediated Propensity to Alcohol Drinking in Outbred Rats. Int. J. Environ. Res. Public Health 2015, 12, 7154–7171. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, A.N.; Alexander, N.J.; McCool, B.A.; Weiner, J.L.; Jones, S.R. Chronic social isolation during adolescence augments catecholamine response to acute ethanol in the basolateral amygdala. Synapse 2015, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.M.; Carrasco, G.A. Cocaine Potentiates Multiple 5-HT2A Receptor Signaling Pathways and Is Associated with Decreased Phosphorylation of 5-HT2A Receptors In Vivo. J. Mol. Neurosci. 2015, 55, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Nimitvilai, S.; McElvain, M.A.; Brodie, M.S. Reversal of Dopamine D2 Agonist-Induced Inhibition of Ventral Tegmental Area Neurons by Gq-Linked Neurotransmitters Is Dependent on Protein Kinase C, G Protein-Coupled Receptor Kinase, and Dynamin. J. Pharmacol. Exp. Ther. 2013, 344, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Hasbi, A.; O’Dowd, B.F.; George, S.R. Dopamine D1-D2 Receptor Heteromer-mediated Calcium Release Is Desensitized by D1 Receptor Occupancy with or without Signal Activation. J. Biol. Chem. 2010, 285, 35092–35103. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Bartolomé, M.; García-Sevilla, J.A. Dysregulation of cannabinoid CB1 receptor and associated signaling networks in brains of cocaine addicts and cocaine-treated rodents. Neuroscience 2013, 247, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ferguson, S.S.; Barak, L.S.; Bodduluri, S.R.; Laporte, S.A.; Law, P.Y.; Caron, M.G. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc. Natl. Acad. Sci. USA 1998, 95, 7157–7162. [Google Scholar] [CrossRef] [PubMed]
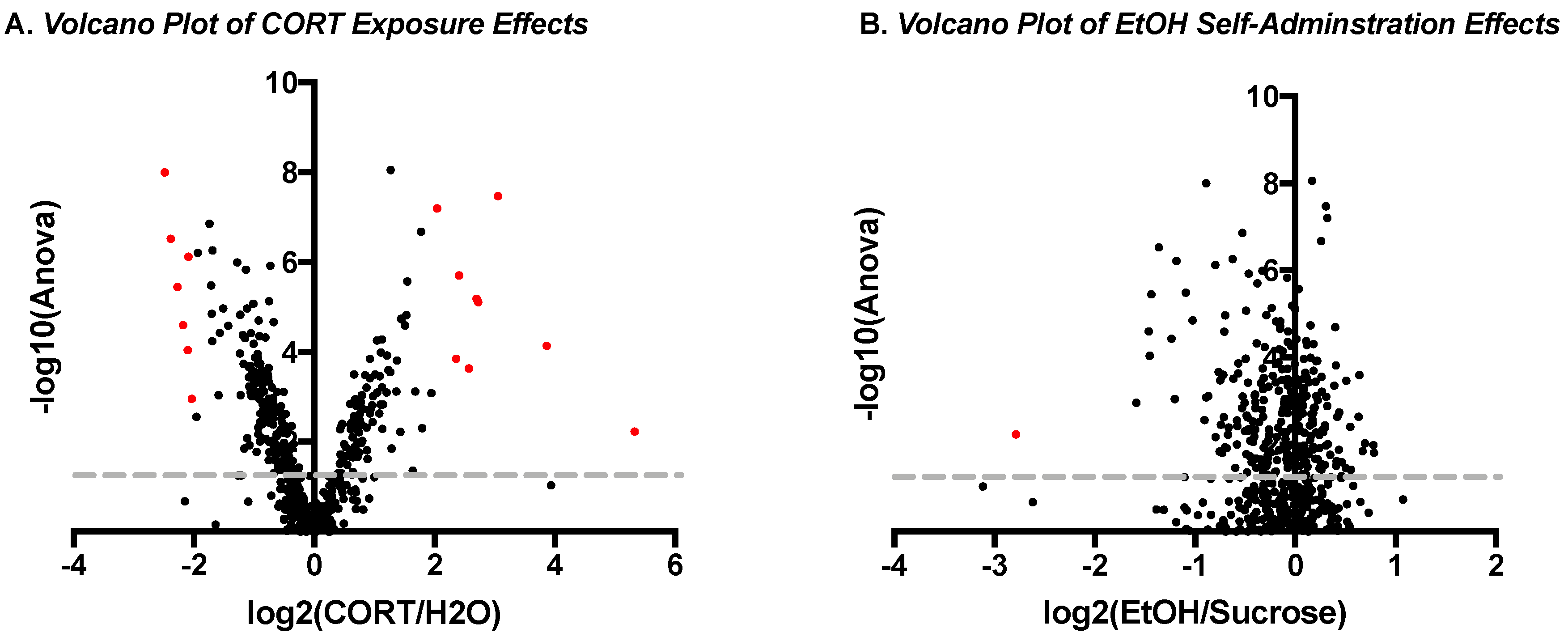

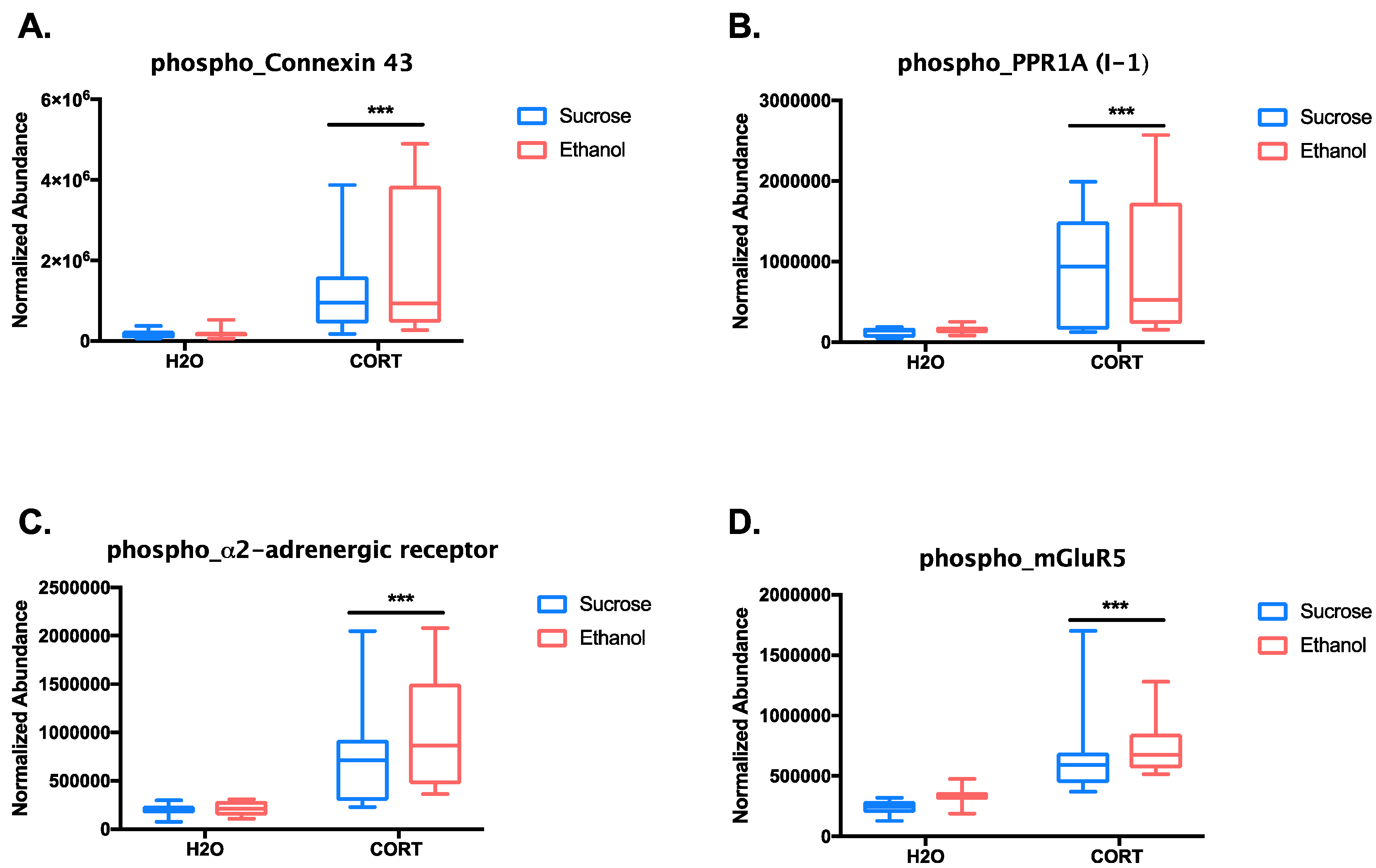
| Top Up-Regulated Phosphopeptides | |||
|---|---|---|---|
| Protein | Peptide Sequence + Phosphorylation Sites (in Red) | Log2(CORT/H2O)-Magnitude | p-Value (ANOVA) |
| Microtubule-associated protein 2 | RLSNVSSSGSINLLESPQLATLAEDVTAALAK | 5.325915864 | 0.005821436 |
| Microtubule-associated protein 2 | RLSNVSSSGSINLLESPQLATLAEDVTAALAK | 3.863577679 | 7.21 × 10−5 |
| Gap junction alpha-1 protein | VAAGHELQPLAIVDQRPSSRASSR | 3.054725626 | 3.34 × 10−8 |
| Microtubule-associated protein 2 | RLSNVSSSGSINLLESPQLATLAEDVTAALAK | 2.726525438 | 7.69 × 10−6 |
| Protein phosphatase 1 regulatory subunit 1A | RRPTPATLVLTSDQSSPEVDEDRIPNPLLK | 2.69773257 | 6.46 × 10−6 |
| Canalicular multispecific organic anion transporter 2 | IPLNLLPQLISGMTQTSVSLK | 2.568107399 | 0.000230896 |
| Microtubule-associated protein tau | HLSNVSSTGSIDMVDSPQLATLADEVSASLAK | 2.409694538 | 1.97 × 10−6 |
| Microtubule-associated protein 2 | RLSNVSSSGSINLLESPQLATLAEDVTAALAK | 2.359792109 | 0.000139602 |
| Alpha-2A adrenergic receptor | DGDALDLEESSSSEHAERPQGPGKPER | 2.043347801 | 6.23 × 10−8 |
| Top Down-Regulated Phosphopeptides | |||
| Neurofilament light polypeptide | AEEAKDEPPSEGEAEEEEK | −2.48762892 | 9.91 × 10−9 |
| Neurofilament heavy polypeptide | TLDVKSPEAK | −2.38663396 | 2.96 × 10−7 |
| Neurofilament heavy polypeptide | SLAEAKSPEK | −2.276582671 | 3.53 × 10−6 |
| Neurofilament heavy polypeptide | SPAEAKSPAEAKPPAEAK | −2.178346111 | 2.49 × 10−5 |
| Neurofilament heavy polypeptide | SPVEVKSPEK | −2.100576776 | 9.08 × 10−5 |
| Neurofilament heavy polypeptide | SPAEAKSPAEVK | −2.093897637 | 7.44 × 10−7 |
| Neurofilament medium polypeptide | AEEEGGSEEEVGDKSPQESK | −2.035085213 | 0.001096364 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertholomey, M.L.; Stone, K.; Lam, T.T.; Bang, S.; Wu, W.; Nairn, A.C.; Taylor, J.R.; Torregrossa, M.M. Phosphoproteomic Analysis of the Amygdala Response to Adolescent Glucocorticoid Exposure Reveals G-Protein Coupled Receptor Kinase 2 as a Target for Reducing Motivation for Alcohol. Proteomes 2018, 6, 41. https://doi.org/10.3390/proteomes6040041
Bertholomey ML, Stone K, Lam TT, Bang S, Wu W, Nairn AC, Taylor JR, Torregrossa MM. Phosphoproteomic Analysis of the Amygdala Response to Adolescent Glucocorticoid Exposure Reveals G-Protein Coupled Receptor Kinase 2 as a Target for Reducing Motivation for Alcohol. Proteomes. 2018; 6(4):41. https://doi.org/10.3390/proteomes6040041
Chicago/Turabian StyleBertholomey, Megan L., Kathryn Stone, TuKiet T. Lam, Seojin Bang, Wei Wu, Angus C. Nairn, Jane R. Taylor, and Mary M. Torregrossa. 2018. "Phosphoproteomic Analysis of the Amygdala Response to Adolescent Glucocorticoid Exposure Reveals G-Protein Coupled Receptor Kinase 2 as a Target for Reducing Motivation for Alcohol" Proteomes 6, no. 4: 41. https://doi.org/10.3390/proteomes6040041
APA StyleBertholomey, M. L., Stone, K., Lam, T. T., Bang, S., Wu, W., Nairn, A. C., Taylor, J. R., & Torregrossa, M. M. (2018). Phosphoproteomic Analysis of the Amygdala Response to Adolescent Glucocorticoid Exposure Reveals G-Protein Coupled Receptor Kinase 2 as a Target for Reducing Motivation for Alcohol. Proteomes, 6(4), 41. https://doi.org/10.3390/proteomes6040041





