Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers
Abstract
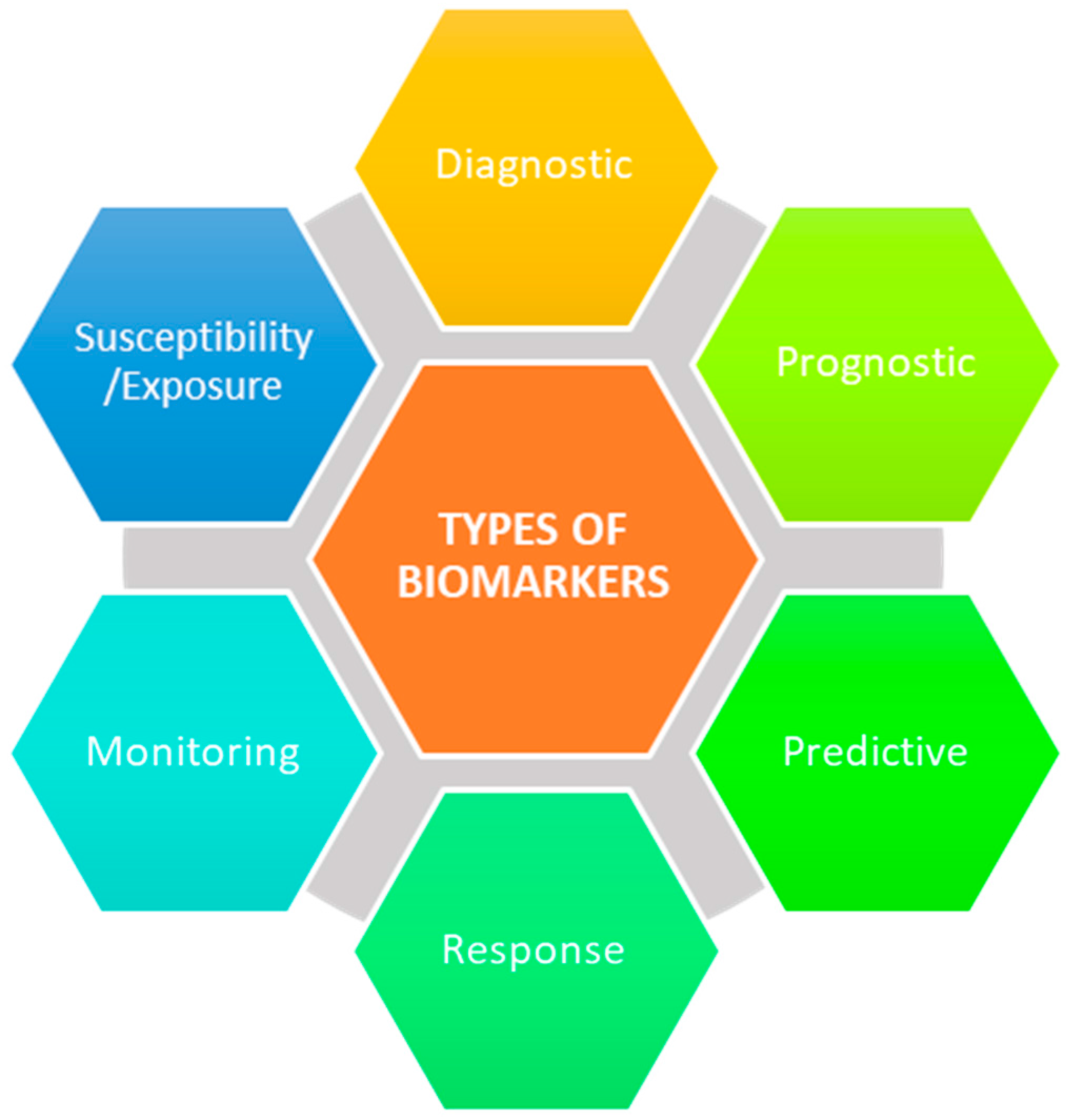
:1. Introduction
2. Serological Biomarkers
3. Synovial Biomarkers
4. Alternative Biomarker Sources
5. Future Perspectives
Author Contributions
Conflicts of Interest
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Schentag, C.T.; Brockbank, J.E.; Pellett, F.J.; Shanmugarajah, S.; Toloza, S.M.A.; Rahman, P.; Gladman, D.D. Familial aggregation of psoriatic arthritis. Ann. Rheum. Dis. 2009, 68, 664–667. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Anhorn, K.A.; Schachter, R.K.; Mervart, H. Hla antigens in psoriatic arthritis. J. Rheumatol. 1986, 13, 586–592. [Google Scholar] [PubMed]
- Eder, L.; Chandran, V.; Pellet, F.; Shanmugarajah, S.; Rosen, C.F.; Bull, S.B.; Gladman, D.D. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 2012, 71, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Sally, B.; Ciszewski, C.; Abadie, V.; Curran, S.A.; Groh, V.; FitzGerald, O.; Winchester, R.J.; Jabri, B. Interleukin 15 primes natural killer cells to kill via nkg2d and cpla2 and this pathway is active in psoriatic arthritis. PLoS ONE 2013, 8, e76292. [Google Scholar] [CrossRef] [PubMed]
- Mameli, A.; Cauli, A.; Taccari, E.; Scarpa, R.; Punzi, L.; Lapadula, G.; Peluso, R.; Ramonda, R.; Spadaro, A.; Iannone, F.; et al. Association of mica alleles with psoriatic arthritis and its clinical forms. A multicenter italian study. Clin. Exp. Rheumatol. 2008, 26, 649–652. [Google Scholar] [PubMed]
- Tillett, W.; Costa, L.; Jadon, D.; Wallis, D.; Cavill, C.; McHugh, J.; Korendowych, E.; McHugh, N. The classification for psoriatic arthritis (caspar) criteria—A retrospective feasibility, sensitivity, and specificity study. J. Rheumatol. 2012, 39, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Mc Ardle, A.; Flatley, B.; Pennington, S.R.; FitzGerald, O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res. Ther. 2015, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, K.; Qian, P.-Y. Proteomics: Challenges, techniques and possibilities to overcome biological sample complexity. Hum. Genom. Proteom. HGP 2009, 2009, 239204. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C.; Eriksson, C.; Alenius, G.-M. S-calprotectin (s100a8/s100a9): A potential marker of inflammation in patients with psoriatic arthritis. J. Immunol. Res. 2014, 2014, 696415. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Conigliaro, P.; Riccieri, V.; Di Franco, M.; Alessandri, C.; Spadaro, A.; Perricone, R.; Valesini, G. Distribution of interleukin-10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin. Exp. Immunol. 2015, 179, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Alenius, G.M.; Eriksson, C.; Rantapaa Dahlqvist, S. Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin. Exp. Rheumatol. 2009, 27, 120–123. [Google Scholar] [PubMed]
- Abji, F.; Pollock, R.A.; Liang, K.; Chandran, V.; Gladman, D.D. Brief report: Cxcl10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol. 2016, 68, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Cook, R.J.; Edwin, J.; Shen, H.; Pellett, F.J.; Shanmugarajah, S.; Rosen, C.F.; Gladman, D.D. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology 2010, 49, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Cretu, D.; Gao, L.; Liang, K.; Soosaipillai, A.; Diamandis, E.P.; Chandran, V. Novel serum biomarkers differentiate psoriatic arthritis from psoriasis without psoriatic arthritis. Arthritis Care Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dalmady, S.; Kiss, M.; Kepiro, L.; Kovacs, L.; Sonkodi, G.; Kemeny, L.; Gyulai, R. Higher levels of autoantibodies targeting mutated citrullinated vimentin in patients with psoriatic arthritis than in patients with psoriasis vulgaris. Clin. Dev. Immunol. 2013, 2013, 474028. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, A.W.R.; DeGroot, J.; Koeman, R.C.; Sakkee, N.; Baeten, D.L.; Gerlag, D.M.; Tak, P.P. Soluble biomarkers of cartilage and bone metabolism in early proof of concept trials in psoriatic arthritis: Effects of adalimumab versus placebo. PLoS ONE 2010, 5, e12556. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Shen, H.; Pollock, R.A.; Pellett, F.J.; Carty, A.; Cook, R.J.; Gladman, D.D. Soluble biomarkers associated with response to treatment with tumor necrosis factor inhibitors in psoriatic arthritis. J. Rheumatol. 2013, 40, 866. [Google Scholar] [CrossRef] [PubMed]
- Cauza, E.; Hanusch-Enserer, U.; Frischmuth, K.; Fabian, B.; Dunky, A.; Kostner, K. Short-term infliximab therapy improves symptoms of psoriatic arthritis and decreases concentrations of cartilage oligomeric matrix protein. J. Clin. Pharm. Ther. 2006, 31, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Grazio, S.; Razdorov, G.; Erjavec, I.; Grubisic, F.; Kusic, Z.; Punda, M.; Anticevic, D.; Vukicevic, S.; Grgurevic, L. Differential expression of proteins with heparin affinity in patients with rheumatoid and psoriatic arthritis: A preliminary study. Clin. Exp. Rheumatol. 2013, 31, 665–671. [Google Scholar] [PubMed]
- Fiocco, U.; Sfriso, P.; Oliviero, F.; Roux-Lombard, P.; Scagliori, E.; Cozzi, L.; Lunardi, F.; Calabrese, F.; Vezzu, M.; Dainese, S.; et al. Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-alpha blockade in the knee joint. Arthritis Res. Ther. 2010, 12, R148. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.S.; Butt, A.Q.; Gibson, D.S.; Dunn, M.J.; Fearon, U.; van Kuijk, A.W.; Gerlag, D.M.; Pontifex, E.; Veale, D.J.; Tak, P.P.; et al. A clinically based protein discovery strategy to identify potential biomarkers of response to anti-tnf-alpha treatment of psoriatic arthritis. Proteom. Clin. Appl. 2016, 10, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Cretu, D.; Liang, K.; Saraon, P.; Batruch, I.; Diamandis, E.P.; Chandran, V. Quantitative tandem mass-spectrometry of skin tissue reveals putative psoriatic arthritis biomarkers. Clin. Proteom. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Guggino, G.; Ciccia, F.; Di Liberto, D.; Lo Pizzo, M.; Ruscitti, P.; Cipriani, P.; Ferrante, A.; Sireci, G.; Dieli, F.; Fournie, J.J.; et al. Interleukin (il)-9/il-9r axis drives gammadelta t cells activation in psoriatic arthritis patients. Clin. Exp. Immunol. 2016, 186, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Hernandez, B.; Collins, E.; Rooney, C.; Fearon, U.; van Kuijk, A.W.; Tak, P.-P.; Gerlag, D.M.; FitzGerald, O.; Pennington, S.R. Discovery and confirmation of a protein biomarker panel with potential to predict response to biological therapy in psoriatic arthritis. Ann. Rheum. Dis. 2016, 75, 234. [Google Scholar] [CrossRef] [PubMed]
- Cretu, D.; Prassas, I.; Saraon, P.; Batruch, I.; Gandhi, R.; Diamandis, E.P.; Chandran, V. Identification of psoriatic arthritis mediators in synovial fluid by quantitative mass spectrometry. Clin. Proteom. 2014, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kumar, C.; Zhang, Y.; Olsen, J.V.; Mann, M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006, 7, R80. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S.; Porter, D.; Paterson, C.; Hampson, R.; Gaya, D.; Latosinska, A.; Mischak, H.; Schanstra, J.; Mullen, W.; McInnes, I. Urinary proteomics can define distinct diagnostic inflammatory arthritis subgroups. Sci. Rep. 2017, 7, 40473. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Honda, T.; Miyauchi, S.; Yamazaki, K. Th2 cytokines efficiently stimulate periostin production in gingival fibroblasts but periostin does not induce an inflammatory response in gingival epithelial cells. Arch. Oral Boil. 2014, 59, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, Z.; Huo, R.; Xiao, L.; Ouyang, G.; Wang, L.; Sun, Y.; Shen, B.; Li, D.; Li, N. Cyr61 induces il-6 production by fibroblast-like synoviocytes promoting th17 differentiation in rheumatoid arthritis. J. Immunol. 2012, 188, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
| Putative Marker | Biological Source | Biomarker Type | Purpose |
|---|---|---|---|
| S100A8/S100A9 | Serum | Prognostic | Differentiate between mild and severe forms of PsA [15] |
| IL-6 | Serum | Monitoring | Disease activity measure [17] |
| CXCL10 | Serum | Prognostic | Predict the development of PsA in PsC patients [18] |
| CD5L, ITGB5, M2BP, MPO, MMP3, CRP | Serum | Diagnostic | Detect the presence of PsA [20] |
| Anti-MCV | Serum | Prognostic | Differentiate between mild and severe forms of PsA [21] |
| MMP3, MIA | Serum | Predictive | Response to TNFi therapy [22,23] |
| SERPINA11, CFHR5, CRTAC1, F9 | Serum | Prognostic | Differentiate between the onset of RA and PsA [25] |
| IL-1β, IL-1Ra, IL-6, IL-22 | SF | Pharmacodynamic | Degree of response to TNFi treatment [26] |
| ALB, COL4A3, ANXA1, ANXA2 | Synovial tissue | Predictive | Response to anti-TNF-α therapy [27] |
| ITGB5, POSTN | Lesional skin biopsy | Diagnostic | Detect the presence of PsA [28] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahendran, S.M.; Chandran, V. Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers. Proteomes 2018, 6, 5. https://doi.org/10.3390/proteomes6010005
Mahendran SM, Chandran V. Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers. Proteomes. 2018; 6(1):5. https://doi.org/10.3390/proteomes6010005
Chicago/Turabian StyleMahendran, Shalini M., and Vinod Chandran. 2018. "Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers" Proteomes 6, no. 1: 5. https://doi.org/10.3390/proteomes6010005
APA StyleMahendran, S. M., & Chandran, V. (2018). Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers. Proteomes, 6(1), 5. https://doi.org/10.3390/proteomes6010005




