Partial-Body Irradiation in Patients with Prostate Cancer Treated with IMRT Has Little Effect on the Composition of Serum Proteome
Abstract
:1. Introduction
2. Experimental Section
2.1 Characteristics of Patient Group
2.2 Profiling of the Low-Molecular-Weight Fraction of Serum Proteome by MALDI-ToF Mass Spectrometry
2.3 Processing of MALDI Spectra and Statistical Analyses
2.4. Identification of Serum Proteins by LC-MS/MS
3. Results
3.1 Profile of Endogenous Serum Peptidome Was Affected in Patients Irradiated Due to Prostate Cancer
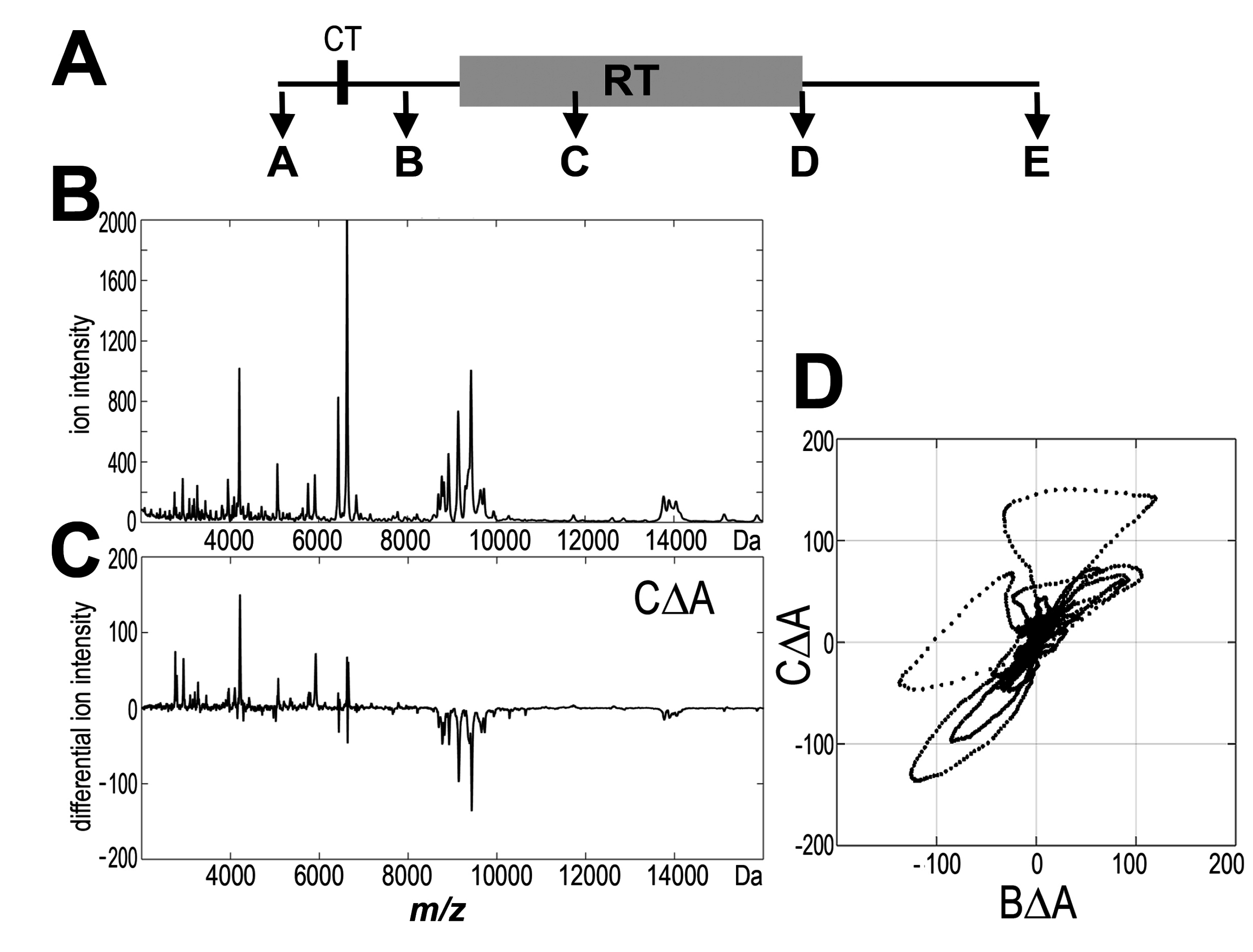
| Change/Patient Group | RT_1 + 2 (n = 126) | RT_1 (n = 60) | RT_2 (n = 66) |
|---|---|---|---|
| AΔB | 12 (92%) | 4 (100%) | 10 (100%) |
| AΔC | 58 (19%) | 57 (19%) | 41 (27%) |
| AΔD | 33 (33%) | 19 (58%) | 18 (61%) |
| AΔE | 31 (35%) | 29 (38%) | 21 (52%) |
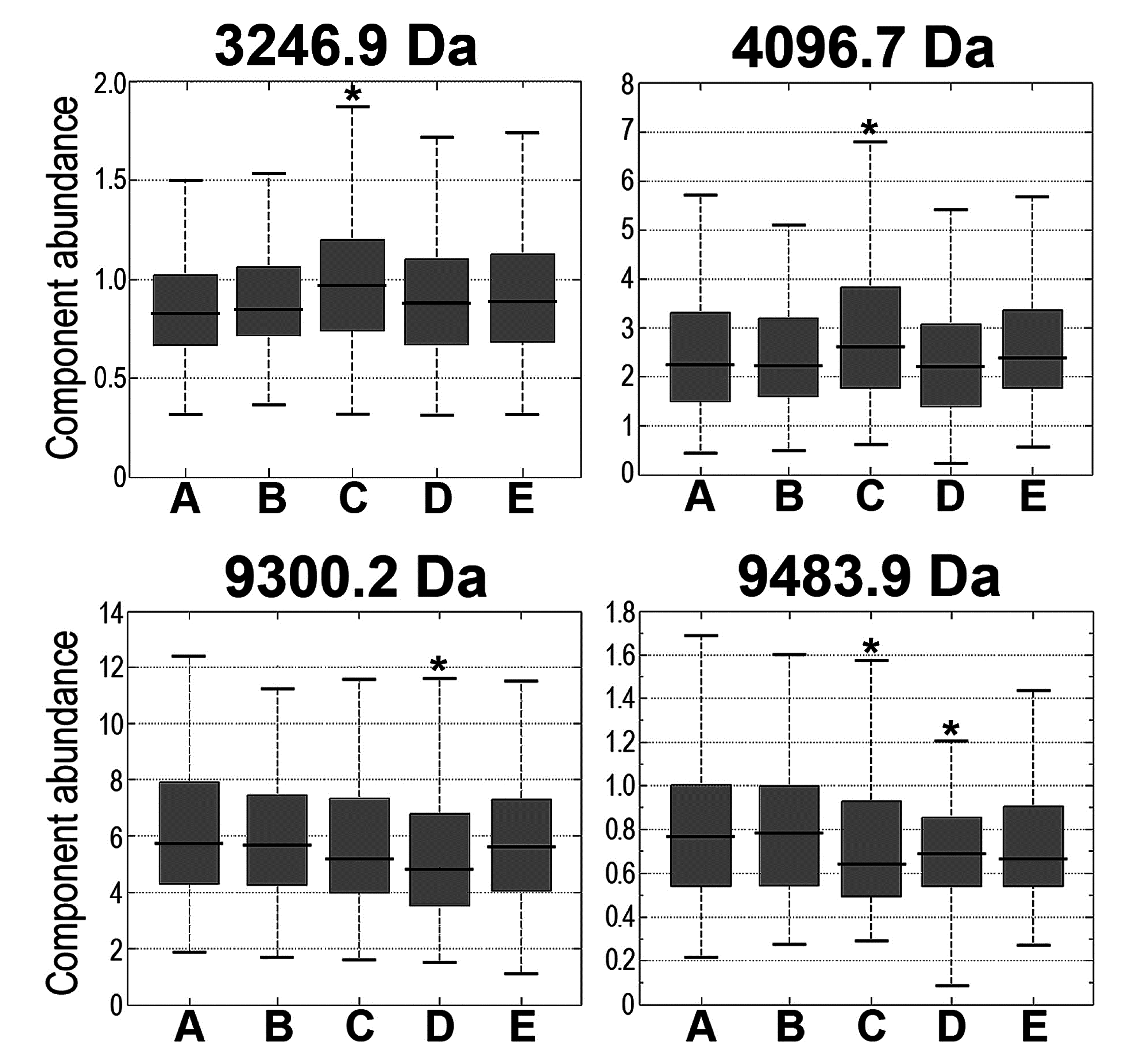
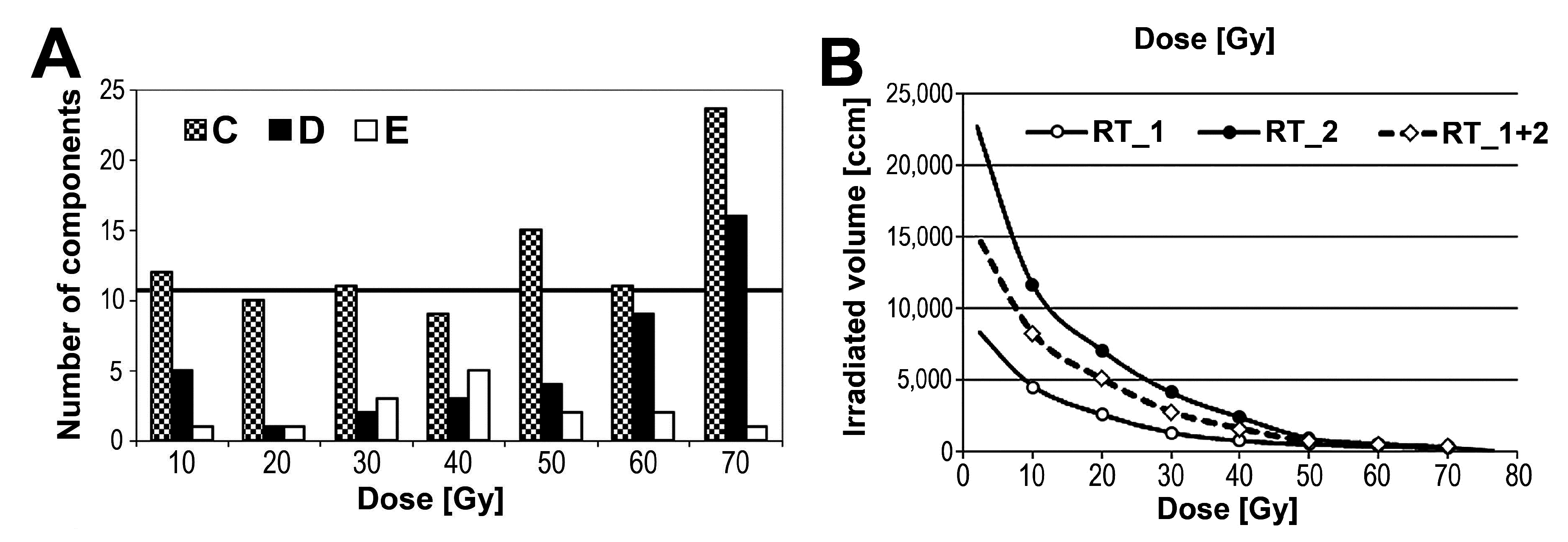
3.2 Changes in Serum Peptidome Were Associated with Neither Volume Nor Toxicity of Irradiated Tissue
3.3 Radiotherapy of Prostate Cancer-Effected Levels of a Few Serum Proteins
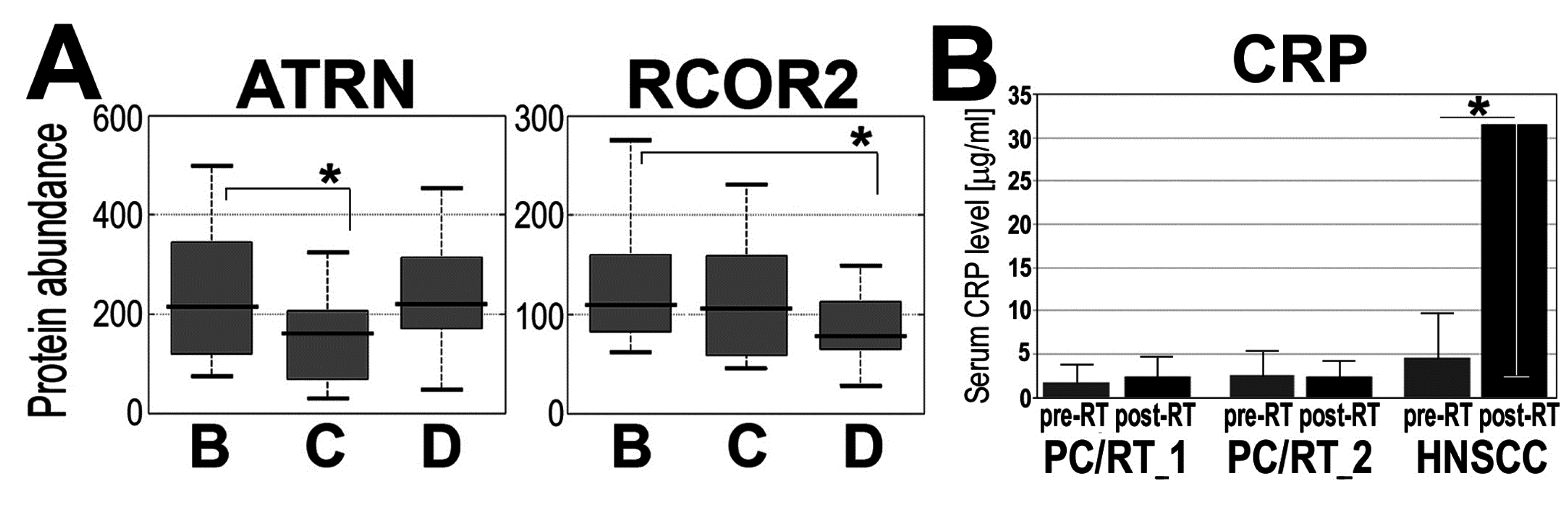
| Protein ID | Protein Name | BΔCΔD Change | BΔC p-Value | BΔD p-Value | CΔD p-Value |
|---|---|---|---|---|---|
| P18428 | Lipopolysaccharide-binding protein | u/d | 0.010 | 0.432 | 0.003 |
| P68871 | Hemoglobin subunit beta (HBB) | u/d | 0.661 | 0.0522 | 0.019 |
| P01767 | Ig heavy chain V-III region BUT | u/d | 0.108 | 0.737 | 0.042 |
| Q68CK4 | Leucine-rich alpha-2-glycoprotein | u/n | 0.064 | 0.035 | 0.217 |
| P13671 | Complement C6 (CO6) | u/n | 0.084 | 0.033 | 0.785 |
| P01598 | Ig kappa chain V-I region EU | n/u | 0.263 | 0.028 | 0.157 |
| O75882 | Attractin (ATRN) | d/u | 0.101 | 0.698 | 0.049 |
| Q8IZ40 | REST corepressor 2 (RCOR2) | d/d | 0.194 | 0.011 | 0.130 |
| P02652 | Apolipoprotein A-II (APOA2) | d/d | 0.145 | 0.049 | 0.550 |
| P02776 | Platelet factor 4 (PLF4) | d/n | 0.007 | 0.015 | 0.765 |
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deasy, J.O.; Fowler, J.F. Radiobiology of imrt. In Intensity Modulated Radiation Therapy: A Clinical Perspective, 1st ed.; Mundt, A.J., Roeske, J.C., Eds.; BC Decker Inc: Hamilton, ON, Canada, 2005; Volume 1, pp. 53–74. [Google Scholar]
- Halperin, E.C.; Perez, C.A.; Brady, L.W. Perez and Brady's Principles and Practice of Radiation Oncology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Brahme, A.; Lind, B.K. A systems biology approach to radiation therapy optimization. Radiat. Environ. Biophys. 2010, 49, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; le Maitre, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Skladowski, K.; Maciejewski, B.; Golen, M.; Tarnawski, R.; Slosarek, K.; Suwinski, R.; Sygula, M.; Wygoda, A. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: Long-term results of phase iii clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Cahlon, O.; Zelefsky, M.J.; Shippy, A.; Chan, H.; Fuks, Z.; Yamada, Y.; Hunt, M.; Greenstein, S.; Amols, H. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Pei, X.; Yamada, J.; Kollmeier, M.A.; Cox, B.; Zelefsky, M.J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 686–692. [Google Scholar] [CrossRef] [PubMed]
- De Neve, W.; de Gersem, W.; Madani, I. Rational use of intensity-modulated radiation therapy: The importance of clinical outcome. Semin. Radiat. Oncol. 2012, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, F.; Swartman, B.; Roeder, F.; Sterzing, F.; Hauswald, H.; Thieke, C.; Weber, K.J.; Huber, P.E.; Schubert, K.; Debus, J.; et al. In vivo measurement of dose distribution in patients’ lymphocytes: Helical tomotherapy versus step-and-shoot imrt in prostate cancer. J. Radiat. Res. 2014, 56, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S. Disease proteomics. Nature 2003, 422, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Wulfkuhle, J.D.; Liotta, L.A.; Petricoin, E.F. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer 2003, 3, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Ferrari, M.; Petricoin, E. Clinical proteomics: Written in blood. Nature 2003, 425, 905. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Cheng, C.H. Oncoproteomics: Current trends and future perspectives. Expert Rev. Proteomics 2007, 4, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Solassol, J.; Jacot, W.; Lhermitte, L.; Boulle, N.; Maudelonde, T.; Mange, A. Clinical proteomics and mass spectrometry profiling for cancer detection. Expert Rev. Proteomics 2006, 3, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, M.; Tiss, A.; Cramer, R. Mass spectrometry in clinical proteomics—From the present to the future. Proteomics Clin. Appl. 2009, 3, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Widlak, P. Maldi-ms-based profiling of serum proteome: Detection of changes related to progression of cancer and response to anticancer treatment. Int. J. Proteomics 2012, 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Coleman, M.A.; Jones, I.M.; Wyrobek, A.J. Candidate protein biodosimeters of human exposure to ionizing radiation. Int. J. Radiat. Biol. 2006, 82, 605–639. [Google Scholar] [CrossRef] [PubMed]
- Guipaud, O. Serum and plasma proteomics and its possible use as detector and predictor of radiation diseases. Adv. Exp. Med. Biol. 2013, 990, 61–86. [Google Scholar] [PubMed]
- Menard, C.; Johann, D.; Lowenthal, M.; Muanza, T.; Sproull, M.; Ross, S.; Gulley, J.; Petricoin, E.; Coleman, C.N.; Whiteley, G.; et al. Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Res. 2006, 66, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Pietrowska, M.; Wojtkiewicz, K.; Rutkowski, T.; Wygoda, A.; Marczak, L.; Marczyk, M.; Polanska, J.; Walaszczyk, A.; Dominczyk, I.; et al. Radiation-related changes in serum proteome profiles detected by mass spectrometry in blood of patients treated with radiotherapy due to larynx cancer. J. Radiat. Res. 2011, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Pietrowska, M.; Polanska, J.; Rutkowski, T.; Jelonek, K.; Kalinowska-Herok, M.; Gdowicz-Klosok, A.; Wygoda, A.; Tarnawski, R.; Skladowski, K. Radiotherapy-related changes in serum proteome patterns of head and neck cancer patients; the effect of low and medium doses of radiation delivered to large volumes of normal tissue. J. Transl. Med. 2013, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Jelonek, K.; Wojakowska, A.; Pietrowska, M.; Polanska, J.; Marczak, Ł.; Miszczyk, L.; Składowski, K. Serum proteome signature of radiation response: Upregulation of inflammation-related factors, and downregulation of apolipoproteins and coagulation factors in cancer patients subjected to radiotherapy—A pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91. [Google Scholar] [CrossRef]
- Nylund, R.; Lemola, E.; Hartwig, S.; Lehr, S.; Acheva, A.; Jahns, J.; Hildebrandt, G.; Lindholm, C. Profiling of low molecular weight proteins in plasma from locally irradiated individuals. J. Radiat. Res. 2014, 55, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hilario, M.; Kalousis, A.; Pellegrini, C.; Muller, M. Processing and classification of protein mass spectra. Mass Spectrom. Rev. 2006, 25, 409–449. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Marczak, L.; Polanska, J.; Behrendt, K.; Nowicka, E.; Walaszczyk, A.; Chmura, A.; Deja, R.; Stobiecki, M.; Polanski, A.; et al. Mass spectrometry-based serum proteome pattern analysis in molecular diagnostics of early stage breast cancer. J. Transl. Med. 2009, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Polanska, J.; Suwinski, R.; Widel, M.; Rutkowski, T.; Marczyk, M.; Dominczyk, I.; Ponge, L.; Marczak, L.; Polanski, A.; et al. Comparison of peptide cancer signatures identified by mass spectrometry in serum of patients with head and neck, lung and colorectal cancers: Association with tumor progression. Int. J. Oncol. 2012, 40, 148–156. [Google Scholar] [PubMed]
- The UniProtKB/Swiss-Prot. Available online: http://web.expasy.org/docs/swiss-prot_guideline.html (accessed on 14 October 2014).
- MaxQuant, version 1.4.1.1. Max Planck Institute of Biochemistry: Martinsried, Germany. Available online: http://141.61.102.17/maxquant_doku (accessed on 6 October 2014).
- Christensen, E.; Pintilie, M.; Evans, K.R.; Lenarduzzi, M.; Ménard, C.; Catton, C.N.; Diamandis, E.P.; Bristow, R.G. Longitudinal cytokine expression during IMRT for prostate cancer and acute treatment toxicity. Clin. Cancer Res. 2009, 15, 5576–5583. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.W.; Shedden, K.; Ao, X.; Davis, M.; Fu, X.L.; Lawrence, T.S.; Lubman, D.M.; Kong, F.M. Plasma proteomic analysis may identify new markers for radiation-induced lung toxicity in patients with non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.J. Radiation treatment effects on the proteome of the tumour microenvironment. Adv. Exp. Med. Biol. 2013, 990, 49–60. [Google Scholar] [PubMed]
- Jelonek, K.; Pietrowska, M.; Ros, M.; Zagdanski, A.; Suchwalko, A.; Polanska, J.; Marczyk, M.; Rutkowski, T.; Skladowski, K.; Clench, M.R.; et al. Radiation-induced changes in serum lipidome of head and neck cancer patients. Int. J. Mol. Sci. 2014, 15, 6609–6624. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Etessami, A.; Wilbault, P.; Lusinchi, A.; Calais, G.; Lapeyre, M.; Pignon, J.P. Altered fractionated radiotherapy in the management of head and neck carcinomas: Advantages and limitations. Curr. Opin. Oncol. 2004, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, K.; Nowicka, E.; Gawkowska-Suwinska, M.; Plewicki, G.; Smolska-Ciszewska, B.; Giglok, M.; Suwinski, R.; Zajusz, A. Early closure of phase ii prospective study on acute and late tolerance of hypofractionated radiotherapy in low-risk prostate cancer patients. Rep. Pract. Oncol. Radiother 2014, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Schubert, M.M. Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis, and management. Oncology 2003, 17, 1767–1779. [Google Scholar] [PubMed]
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wygoda, A.; Maciejewski, B.; Skladowski, K.; Hutnik, M.; Pilecki, B.; Golen, M.; Rutkowski, T. Pattern analysis of acute mucosal reactions in patients with head and neck cancer treated with conventional and accelerated irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Sonis, S. Mucositis: Biology and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Budaus, L.; Bolla, M.; Bossi, A.; Cozzarini, C.; Crook, J.; Widmark, A.; Wiegel, T. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur. Urol. 2012, 61, 112–127. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrowska, M.; Jelonek, K.; Polanska, J.; Wojakowska, A.; Marczak, L.; Chawinska, E.; Chmura, A.; Majewski, W.; Miszczyk, L.; Widlak, P. Partial-Body Irradiation in Patients with Prostate Cancer Treated with IMRT Has Little Effect on the Composition of Serum Proteome. Proteomes 2015, 3, 117-131. https://doi.org/10.3390/proteomes3030117
Pietrowska M, Jelonek K, Polanska J, Wojakowska A, Marczak L, Chawinska E, Chmura A, Majewski W, Miszczyk L, Widlak P. Partial-Body Irradiation in Patients with Prostate Cancer Treated with IMRT Has Little Effect on the Composition of Serum Proteome. Proteomes. 2015; 3(3):117-131. https://doi.org/10.3390/proteomes3030117
Chicago/Turabian StylePietrowska, Monika, Karol Jelonek, Joanna Polanska, Anna Wojakowska, Lukasz Marczak, Ewa Chawinska, Aleksanda Chmura, Wojciech Majewski, Leszek Miszczyk, and Piotr Widlak. 2015. "Partial-Body Irradiation in Patients with Prostate Cancer Treated with IMRT Has Little Effect on the Composition of Serum Proteome" Proteomes 3, no. 3: 117-131. https://doi.org/10.3390/proteomes3030117
APA StylePietrowska, M., Jelonek, K., Polanska, J., Wojakowska, A., Marczak, L., Chawinska, E., Chmura, A., Majewski, W., Miszczyk, L., & Widlak, P. (2015). Partial-Body Irradiation in Patients with Prostate Cancer Treated with IMRT Has Little Effect on the Composition of Serum Proteome. Proteomes, 3(3), 117-131. https://doi.org/10.3390/proteomes3030117








