Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with Up Regulation of Proteins Involved in Protection against Oxidative Stress
Abstract
:1. Introduction
2. Experimental
2.1. Radiation Source
2.2. Cell Culture Condition and Cell Growth Kinetics
2.3. Senescence-Associated β-Galactosidase (SA-βgal) Assay
2.4. Western Blot Analysis
2.5. Two-Dimensional Polyacrylamide Gel Electrophoresis (2DE)
2.6. MALDI-TOF Mass Spectrometry Analysis and Protein Identification
2.7. Heatmap and Hierarchical Clustering
3. Results and Discussion
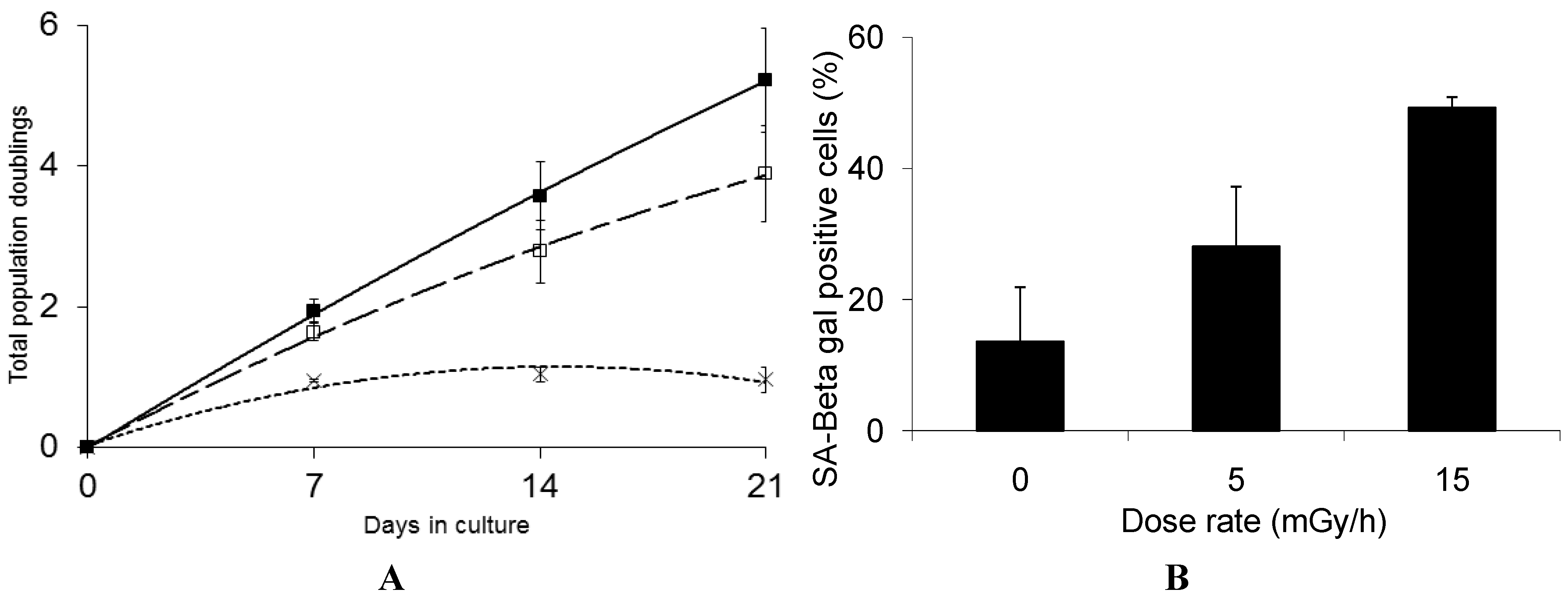
3.1. Radiation-Induced Senescence in Normal Human Fibroblasts
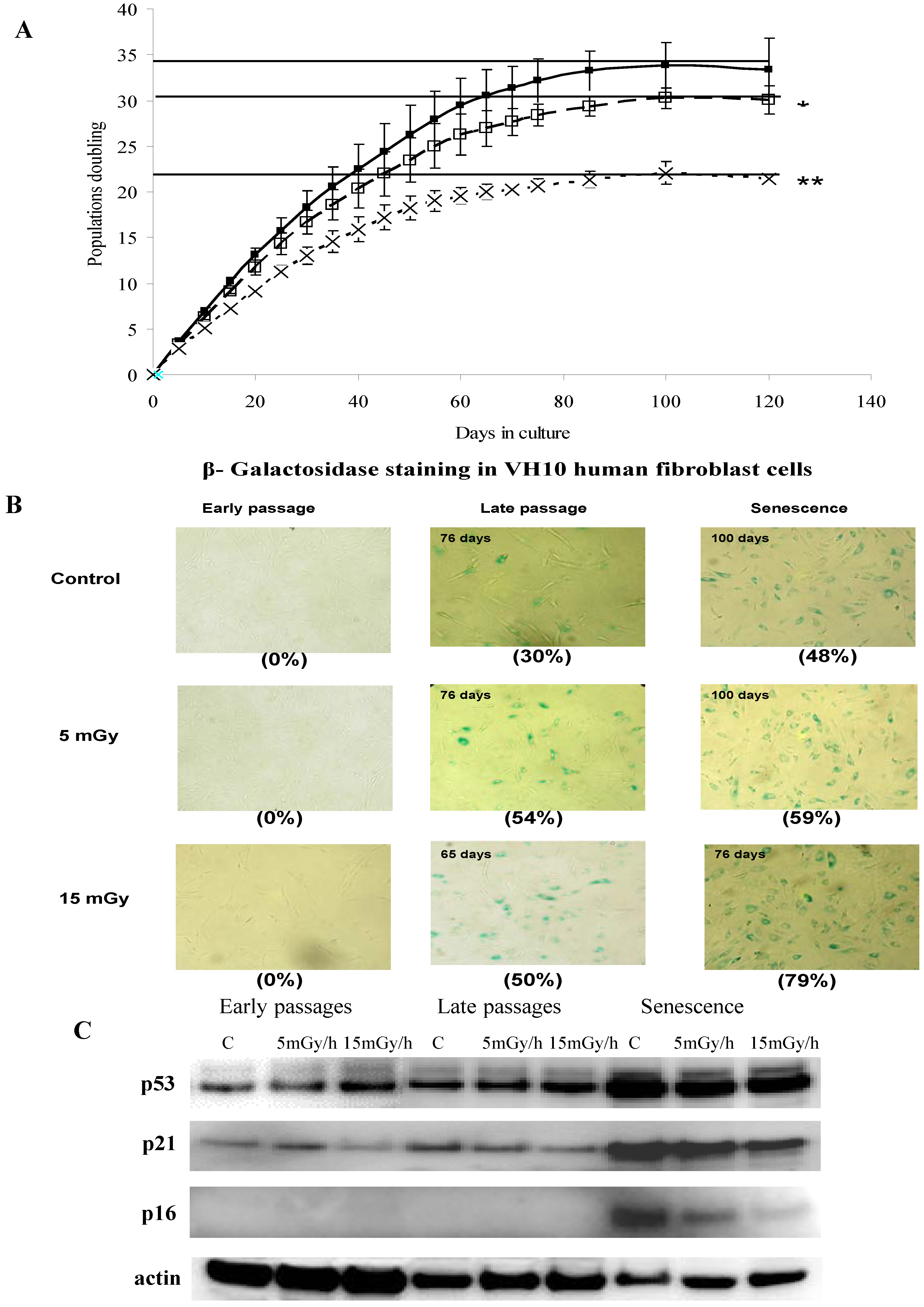
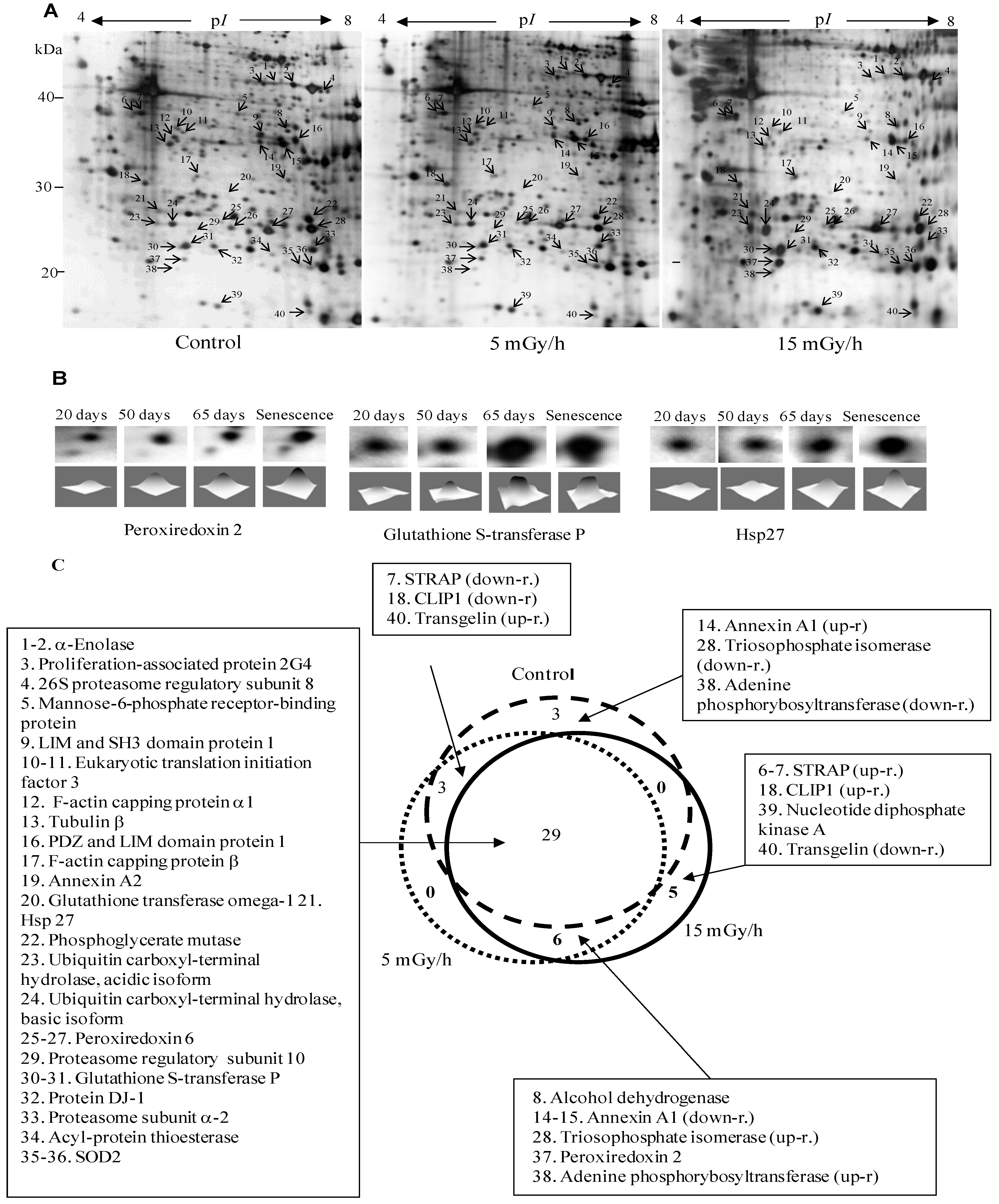
3.2. Effect of Ionizing Radiation on Fibroblast Proteome
| Spot Number | Protein Name | Protein ID | Theoretical pI | Theoretical Mr, kDa | Peptide Matches | Sequence Coverage % | Mascot Score | Protein Function |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Enolase | P06733 | 7.0 | 47.2 | 15 | 36 | 120 | Glycolysis |
| 2 | α-Enolase | P06733 | 7.0 | 47.2 | 20 | 33 | 197 | Glycolysis |
| 3 | Proliferation-associated protein 2G4 | Q9UQ80 | 6.1 | 43.8 | 14 | 27 | 66 | Involved in cell cycle arrest/cell proliferation |
| 4 | 26S protease regulatory subunit 8 | P62195 | 7.1 | 45.6 | 12 | 36 | 85 | Proteasome complex |
| 5 | Mannose-6-phosphate receptor-binding protein 1 | O60664 | 5.8 | 28.1 | 15 | 48 | 141 | Vesicle-mediated transport |
| 6 | Serine-threonine kinase receptor-associated protein | Q9Y3F4 | 5.0 | 38.4 | 9 | 41 | 70 | mRNA processing, regulator of TGFβ pathway, cofactor of p53 |
| 7 | Serine-threonine kinase receptor-associated protein | Q9Y3F4 | 5.0 | 38.4 | 8 | 32 | 65 | mRNA processing, regulator of TGFβ pathway, cofactor of p53 |
| 8 | Alcohol dehydrogenase | P14550 | 6.3 | 36.6 | 13 | 55 | 110 | Glucose metabolic process |
| 9 | LIM and SH3 domain protein 1 | Q14847 | 6.4 | 30.1 | 10 | 31 | 75 | Actin-binding protein |
| 10 | Eukaryotic translation initiation factor 3 | Q13347 | 5.4 | 36.5 | 9 | 30 | 71 | Protein biosynthesis |
| 11 | Eukaryotic translation initiation factor 3 | Q13347 | 5.4 | 36.5 | 10 | 32 | 73 | Protein biosynthesis |
| 12 | F-actin-capping protein α-1 | P52907 | 5.4 | 32.9 | 14 | 67 | 172 | Regulation of cell motility |
| 13 | Tubulin beta | P07437 | 4.8 | 48.7 | 14 | 45 | 132 | Cytoskeleton |
| 14 | Annexin A1 | P04083 | 6.6 | 38.7 | 18 | 52 | 179 | Regulation of apoptosis |
| 15 | Annexin A1 | P04083 | 6.6 | 38.7 | 19 | 62 | 202 | Regulation of apoptosis |
| 16 | PDZ and LIM domain protein 1 | O00151 | 6.6 | 36.5 | 9 | 23 | 78 | Cytoskeleton protein required for actin stress fiber formation |
| 17 | F-actin-capping protein subunit β | P47756 | 5.4 | 31.5 | 9 | 44 | 67 | Actin-binding protein |
| 18 | Chloride intracellular channel protein CLIP1 | O00299 | 5.1 | 27.4 | 16 | 70 | 183 | Chloride ion channel, anti-apoptotic |
| 19 | Annexin A2 | P07355 | 7.6 | 38.6 | 20 | 51 | 210 | Stress response, regulation of apoptosis |
| 20 | Glutathione transferase omega-1 | P78417 | 6.2 | 27.8 | 10 | 29 | 100 | Metabolism of xenobiotics, antioxidant |
| 21 | Heat shock protein β-1 (Hsp27) | P04792 | 6.0 | 22.8 | 8 | 38 | 65 | Involved in stress resistance and actin organization |
| 22 | Phosphoglycerate mutase | P18669 | 6.4 | 26.7 | 18 | 73 | 200 | Glycolysis |
| 23 | Ubiquitin thiolesterase L1, acidic isoforms | P09936 | 5.3 | 24.8 | 14 | 72 | 131 | Processing of ubiquitinated proteins; anti-apoptotic |
| 24 | Ubiquitin thiolesterase L1, basic isoforms | P09936 | 5.3 | 24.8 | 14 | 75 | 171 | Processing of ubiquitinated proteins; anti-apoptotic |
| 25 | Peroxiredoxin 6 | P30041 | 6.0 | 25.0 | 7 | 36 | 57 | Antioxidant |
| 26 | Peroxiredoxin 6 | P30041 | 6.0 | 25.0 | 13 | 64 | 144 | Antioxidant |
| 27 | Peroxiredoxin 6 | P30041 | 6.0 | 25.0 | 14 | 70 | 155 | Antioxidant |
| 28 | Triosephosphate isomerase | P60174 | 6.4 | 26.7 | 15 | 58 | 177 | Carbohydrate metabolism |
| 29 | 26S proteasome subunit 10 | O75832 | 5.4 | 20.4 | 8 | 49 | 58 | Acts as a regulatory subunit of the 26S proteasome |
| 30 | Glutathione S-transferase P | P09211 | 5.4 | 23.6 | 9 | 48 | 94 | Antioxidant, anti-apoptotic |
| 31 | Glutathione S-transferase P | P09211 | 5.4 | 23.6 | 10 | 56 | 146 | Antioxidant, anti-apoptotic |
| 32 | Protein DJ-1 | Q99497 | 6.3 | 19.9 | 8 | 43 | 60 | Redox-sensitive chaperone and a sensor for oxidative stress |
| 33 | Proteasome subunit α type-2 | P25787 | 6.9 | 26.0 | 8 | 44 | 70 | Proteasome complex |
| 34 | Acyl-protein thioesterase 1 | O75608 | 6.3 | 26.7 | 5 | 41 | 56 | De-palmitoylation of signaling proteins |
| 35 | Superoxide dismutase Mn SOD2 | P04179 | 8.3 | 24.7 | 7 | 36 | 69 | Antioxidant, age-dependent response to ROS |
| 36 | Superoxide dismutase Mn SOD2 | P04179 | 8.3 | 24.7 | 8 | 40 | 78 | Antioxidant, age-dependent response to ROS |
| 37 | Peroxiredoxin-2 | P32119 | 5.7 | 21.9 | 9 | 35 | 79 | Antioxidant, anti-apoptotic |
| 38 | Adenine phosphoribosyltransferase | P07741 | 5.8 | 19.6 | 8 | 68 | 91 | Nucleotide metabolism |
| 39 | Nucleoside diphosphate kinase A | P15531 | 5.8 | 17.3 | 8 | 61 | 90 | Synthesis of nucleoside triphosphates other than ATP, tumor suppressor, cofactor of p53 |
| 40 | Transgelin | Q01995 | 8.9 | 22.6 | 13 | 56 | 121 | Actin-binding protein, senescence marker |
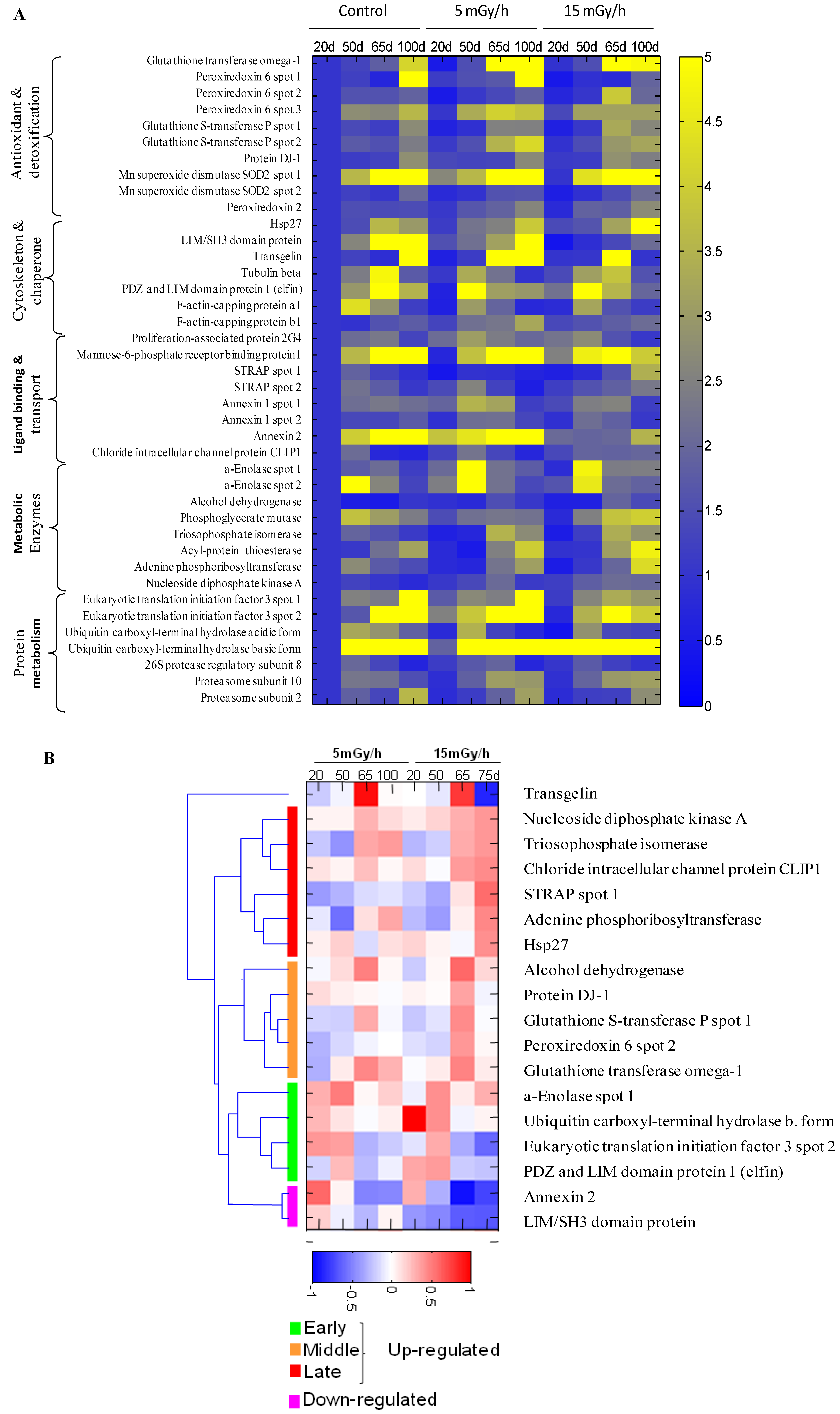
3.3. Radiation Induced Stress Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Little, M.; Tawn, E.J.; Tzoulaki, I.; Wakeford, R.; Hildebrandt, G.; Paris, F.; Tapio, S.; Elliott, P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects and their possible mechanisms. Radiat. Res. 2008, 169, 99–109. [Google Scholar] [CrossRef]
- Chodick, G.; Bekiroglu, N.; Hauptmann, M.; Alexander, B.H.; Freedman, D.M.; Doody, M.M.; Cheung, L.C.; Simon, S.L.; Weinstock, R.M.; Bouville, A.; et al. Risk of cataract after exposure to low doses of ionizing radiation: A 20-year prospective cohort study among us radiologic technologists. Am. J. Epidemiol. 2008, 168, 620–631. [Google Scholar] [CrossRef]
- Hall, P.; Adami, H.O.; Trichopoulos, D.; Pedersen, N.L.; Lagiou, P.; Ekbom, A.; Ingvar, M.; Lundell, M.; Granath, F. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. Br. Med. J. 2004, 328, e19. [Google Scholar]
- Suzuki, K.; Mori, I.; Nakayama, Y.; Miyakoda, M.; Kodama, S.; Watanabe, M. Radiation-induced senescence-like growth arrest requires tp53 function but not telomere shortening. Radiat. Res. 2001, 155, 248–253. [Google Scholar] [CrossRef]
- Yentrapalli, R.; Azimzadeh, O.; Barjaktarovic, Z.; Sarioglu, H.; Wojcik, A.; Harms-Ringdahl, M.; Atkinson, M.J.; Haghdoost, S.; Tapio, S. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013, 13, 1096–1107. [Google Scholar] [CrossRef]
- Yentrapalli, R.; Azimzadeh, O.; Sriharshan, A.; Malinowsky, K.; Merl, J.; Wojcik, A.; Harms-Ringdahl, M.; Atkinson, M.J.; Becker, K.F.; Haghdoost, S.; et al. The pi3k/akt/mtor pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One 2013, 8, e70024. [Google Scholar] [CrossRef]
- Rombouts, C.; Aerts, A.; Quintens, R.; Baselet, B.; El-Saghire, H.; Harms-Ringdahl, M.; Haghdoost, S.; Janssen, A.; Michaux, A.; Yentrapalli, R.; et al. Transcriptomic profiling suggests a role for igfbp5 in premature senescence of endothelial cells after chronic low dose rate irradiation. Int. J. Radiat. Biol. 2014. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell. Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.V.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Ann. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Lu, T.; Finkel, T. Free radicals and senescence. Exp. Cell. Res. 2008, 314, 1918–1922. [Google Scholar] [CrossRef]
- Rai, P.; Onder, T.T.; Young, J.J.; McFaline, J.L.; Pang, B.; Dedon, P.C.; Weinberg, R.A. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc. Natl. Acad. Sci. USA 2009, 106, 169–174. [Google Scholar] [CrossRef]
- Haghdoost, S.; Czene, S.; Näslund, I.; Skog, S.; Harms-Ringdahl, M. Extracellular 8-oxo-dg as a sensitive parameter for oxidative stress in vivo and in vitro. Free Radic. Res. 2005, 39, 153–162. [Google Scholar] [CrossRef]
- Haghdoost, S.; Sjölander, L.; Czene, S.; Harms-Ringdahl, M. The nucleotide pool is a significant target for oxidative stress. Free Radic. Biol. Med. 2006, 41, 620–626. [Google Scholar] [CrossRef]
- Sangsuwan, T.; Haghdoost, S. The nucleotide pool, a target for low-dose gamma-ray-induced oxidative stress. Radiat. Res. 2008, 170, 776–783. [Google Scholar] [CrossRef]
- Njålsson, R. Glutathione synthetase deficiency. Cell. Mol. Life Sci. 2005, 62, 1938–1945. [Google Scholar] [CrossRef]
- Dahl, N.; Pigg, M.; Ristoff, E.; Gali, R.; Carlsson, B.; Mannervik, B.; et al. Missense mutations in the human glutathione synthetase gene result in severe metabolic acidosis, 5-oxoprolinuria, hemolytic anemia and neurological dysfunction. Hum. Mol. Genet. 1997, 6, 1147–1152. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nat. Med. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yan, J.; Wait, R.; Berkelman, T.; Harry, R.A.; Westbrook, J.A.; Wheeler, C.H.; Dunn, M.J. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 2000, 21, 3666–3672. [Google Scholar] [CrossRef]
- Gharahdaghi, F.; Weinberg, C.R.; Meagher, D.A.; Imai, B.S.; Mische, S.M. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: A method for the removal of silver ions to enhance sensitivity. Electrophoresis 1999, 20, 601–605. [Google Scholar] [CrossRef]
- Mass Spectrometry Facility of University of California at San-Francisco. Available online: http://donatello.ucsf.edu (assessed on 1 December 2008).
- Matrix science. Available online: http://www.matrixscience.com (assessed on 8 December 2008).
- Proteinprospector. Available online: http://prospector.ucsf.edu (assessed on 8 December 2008).
- Rockeffeler University. Available online: http://prowl.rockeffeler.edu (assessed on 8 December 2008).
- ExPASy. Available online: http://www.expasy.org (assessed on 12 December 2008).
- HUPO Proteomics Standards Initiative. Available online: http://www.psidev.info (assessed on 15 December 2008).
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Dimri, G.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Suzuki, M.; Boothman, D.A. Stress-induced premature senescence (sips)—Influence of sips on radiotherapy. J. Radiat. Res. (Tokyo) 2008, 49, 105–112. [Google Scholar] [CrossRef]
- Alcorta, D.; Xiong, Y.; Phelps, D.; Hannon, G.; Beach, D.; Barrett, J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (ink4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 1996, 93, 13742–13747. [Google Scholar] [CrossRef]
- Brugarolas, J.; Chandrasekaran, C.; Gordon, J.I.; Beach, D.; Jacks, T.; Hannon, G.J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995, 377, 552–557. [Google Scholar] [CrossRef]
- Dulić, V.; Kaufmann, W.K.; Wilson, S.J.; Tlsty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. P53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced g1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Radomski, N.; Jost, E. Molecular cloning of a murine cdna encoding a novel protein, p38–2g4, which varies with the cell cycle. Exp. Cell Res. 1995, 220, 434–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zhou, H.; Lee, M.; Liu, Z.; Hassel, B.A.; Hamburger, A.W. Alterations in cell growth and signaling in erbb3 binding protein -1 (ebp1) deficient mice. BMC Cell Biol. 2008, 9, e69. [Google Scholar] [CrossRef]
- Dierick, J.; Eliaers, F.; Remacle, J.; Raes, M.; Fey, S.J.; Larsen, P.M. Toussaint O, Stress-induced premature senescence and replicative senescence are different phenotypes, proteomic evidence. Biochem. Pharmacol. 2002, 64, 1011–1017. [Google Scholar] [CrossRef]
- Beckman, K.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar]
- Culotta, V.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar] [CrossRef]
- Asikainen, T.; Huang, T.T.; Taskinen, E.; Levonen, A.L.; Carlson, E.; Lapatto, R.; Epstein, C.J.; Raivio, K.O. Increased sensitivity of homozygous SOD2 mutant mice to oxygen toxicity. Free Radic. Biol. Med. 2002, 32, 175–186. [Google Scholar] [CrossRef]
- Landis, G.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef]
- Wood, Z.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Neumann, C.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; van Etten, R.A. Essential role for the peroxiredoxin prdx1 in erythrocyte antioxidant defence and tumour suppression. Nat. Rev. Mol. Cell Biol. 2003, 424, 561–565. [Google Scholar]
- Wang, Y.; Feinstein, S.I.; Fisher, A.B. Peroxiredoxin 6 as an antioxidant enzyme: Protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J. Cell. Biochem. 2008, 104, 1274–1285. [Google Scholar] [CrossRef]
- Zhou, W.; Freed, C.R. Dj-1 up-regulates glutathione synthesis during oxidative stress and inhibits a53t-synuclein toxicity. J. Biol. Chem. 2005, 280, 43150–43158. [Google Scholar] [CrossRef]
- Andres-Mateos, E.; Perier, C.; Zhang, L.; Blanchard-Fillion, B.; Greco, T.M.; Thomas, B.; Ko, H.S.; Sasaki, M.; Ischiropoulos, H.; Przedborski, S.; et al. Dj-1 gene deletion reveals that dj-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA 2007, 104, 14807–14812. [Google Scholar] [CrossRef]
- Arrigo, A.; Simon, S.; Gibert, B.; Kretz-Remy, C.; Nivon, M.; Czekalla, A.; Guillet, D.; Moulin, M.; Diaz-Latoud, C.; Vicart, P. Hsp27 (hspb1) and b-crystallin (hspb5) as therapeutic targets. FEBS Lett. 2007, 581, 3665–3674. [Google Scholar] [CrossRef]
- Arrigo, A.; Virot, S.; Chaufour, S.; Firdaus, W.; Kretz-Remy, C.; Diaz-Latoud, C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 2005, 7, 414–422. [Google Scholar] [CrossRef]
- Ahmed, E.K.P.C.; Bulteau, A.L.; Friguet, B. Protein oxidative modifications and replicative senescence of wi-38 human embryonic fibroblasts. Ann. N Y Acad. Sci. 2007, 1119, 88–96. [Google Scholar] [CrossRef]
- Jung, H.; Seong, H.A.; Ha, H. Nm23-h1 tumor suppressor and its interacting partner strap activate p53 function. J. Biol. Chem. 2007, 282, 35293–35307. [Google Scholar] [CrossRef]
- Gourlay, C.; Carpp, L.N.; Timpson, P.; Winder, S.J.; Ayscough, K.R. A role for the actin cytoskeleton in cell death and aging in yeast. J. Cell. Biol. 2004, 164, 803–809. [Google Scholar] [CrossRef]
- Gonos, E.; Derventzi, A.; Kveiborg, M.; Agiostratidou, G.; Kassem, M.; Clark, B.F.; Jat, P.S.; Rattan, S.I. Cloning and identification of genes that associate with mammalian replicative senescence. Exp. Cell. Res. 1998, 240, 66–74. [Google Scholar] [CrossRef]
- Assinder, S.; Stanton, J.A.; Prasad, P.D. Transgelin: An actin-binding protein and tumour suppressor. Int. J. Biochem. Cell Biol. 2009, 41, 482–486. [Google Scholar] [CrossRef]
- Littler, D.; Harrop, S.J.; Fairlie, W.D.; Brown, L.J.; Pankhurst, G.J.; Pankhurst, S.; DeMaere, M.Z.; Campbell, T.J.; Bauskin, A.R.; Tonini, R.; et al. The intracellular chloride ion channel protein clic1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004, 279, 9298–9305. [Google Scholar] [CrossRef]
- Zimniak, P. Detoxification reactions: Relevance to aging. Ageing Res. Rev. 2008, 7, 281–300. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef]
- Dalton, T.; Chen, Y.; Schneider, S.N.; Nebert, D.W.; Shertzer, H.G. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004, 37, 1511–1526. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar]
- Ristoff, E.; Larsson, A. Patients with genetic defects in the gamma-glutamyl cycle. Chem. Biol. Interact. 1998, 111–112, 113–121. [Google Scholar] [CrossRef]
- Njålsson, R.; Ristoff, E.; Carlsson, K.; Winkler, A.; Larsson, A.; Norgren, S. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum. Genet. 2005, 116, 384–389. [Google Scholar] [CrossRef]
- Deschavanne, P.; Debieu, D.; Fertil, B.; Malaise, E.P. Re-evaluation of in vitro radiosensitivity of human fibroblasts of different genetic origins. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 279–293. [Google Scholar] [CrossRef]
- Edgren, M.; Révész, L.; Larsson, A. Induction and repair of single-strand DNA breaks after x-irradiation of human fibroblasts deficient in glutathione. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 40, 355–363. [Google Scholar] [CrossRef]
- Nygren, J.; Ristoff, E.; Carlsson, K.; Moller, L.; Larsson, A. Oxidative DNA damage in cultured fibroblasts from patients with hereditary glutathione synthetase deficiency. Free Radic. Res. 2005, 39, 595–601. [Google Scholar] [CrossRef]
- Ristoff, E.; Hebert, C.; Njålsson, R.; Norgren, S.; Rooyackers, O.; Larsson, A. Glutathione synthetase deficiency: Is gamma-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J. Inherit. Metab. Dis. 2002, 25, 577–584. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Dandapat, A.; Singh, S.P.; Siegel, E.R.; Shmookler Reis, R.J.; Zimniak, L.; Zimniak, P. Life span and stress resistance of caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007, 128, 196–205. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Engle, M.R.; Singh, S.P.; Dandapat, A.; Lichti, C.F.; Benes, H.; Shmookler Reis, R.J.; Liebau, E.; Zimniak, P. Lifespan and stress resistance of caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005, 4, 257–271. [Google Scholar] [CrossRef]
Appendix

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loseva, O.; Shubbar, E.; Haghdoost, S.; Evers, B.; Helleday, T.; Harms-Ringdahl, M. Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with Up Regulation of Proteins Involved in Protection against Oxidative Stress. Proteomes 2014, 2, 341-362. https://doi.org/10.3390/proteomes2030341
Loseva O, Shubbar E, Haghdoost S, Evers B, Helleday T, Harms-Ringdahl M. Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with Up Regulation of Proteins Involved in Protection against Oxidative Stress. Proteomes. 2014; 2(3):341-362. https://doi.org/10.3390/proteomes2030341
Chicago/Turabian StyleLoseva, Olga, Emman Shubbar, Siamak Haghdoost, Bastiaan Evers, Thomas Helleday, and Mats Harms-Ringdahl. 2014. "Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with Up Regulation of Proteins Involved in Protection against Oxidative Stress" Proteomes 2, no. 3: 341-362. https://doi.org/10.3390/proteomes2030341
APA StyleLoseva, O., Shubbar, E., Haghdoost, S., Evers, B., Helleday, T., & Harms-Ringdahl, M. (2014). Chronic Low Dose Rate Ionizing Radiation Exposure Induces Premature Senescence in Human Fibroblasts that Correlates with Up Regulation of Proteins Involved in Protection against Oxidative Stress. Proteomes, 2(3), 341-362. https://doi.org/10.3390/proteomes2030341





